Abstract
Aim:
Evaluation of real-world data regarding the use of omalizumab on lung function, asthma control, exacerbations, and oral corticosteroid (OCS).
Methods:
The single-centre, retrospective study included data from adult patients with severe allergic asthma treated with omalizumab for at least five years to ten years to evaluate its long-term efficacy. The primary outcome parameters were lung function (FEV1), the asthma control test (ACT) score, the number of exacerbations, and OCS use.
Results:
Data from 74 adults (mean age 51 years, 61% females, median IgE 276 kU/L), with severe allergic asthma, due to perennial allergens, who were treated for at least 5 years with omalizumab in one centre could be evaluated up to 10 years. The mean improvement in FEV1 from baseline was 13.4% in the first year and constantly remained high throughout the duration of the treatment. The ACT improved from baseline (12.4 points) to 16.4 in the first year and reached 18.8 after 5 years, followed by values nearly reaching 20 (19.2 in year 8). The rate of exacerbations decreased from 3.3 events in the last 12 months before omalizumab initiation to 0.4 in the first year and remained low (e.g., 0.2 after 5 years). The mean OCS use was 20.9 mg/day in 44/74 patients before the first injection of omalizumab and decreased to 5 mg/day in the same patients within the first year. Following 6 years of omalizumab treatment, OCSs were used by 22 patients, and by 12 patients after 8 years.
Conclusions:
The consistent improvement in lung function, asthma control, reduction in exacerbations, and OCS use throughout a minimum of five up to ten years confirms that omalizumab remains effective for many years. There were no signs of tolerance or tachyphylaxis against the biologic.
Keywords
Exacerbations, long-term effectiveness, lung function, omalizumab, real-world evidence, severe allergic asthma, tachyphylaxis, oral corticosteroidIntroduction
Severe asthma is classified as a subtype of uncontrolled asthma despite the use of high dose long-acting beta-2-agonists (LABA)/inhaled corticosteroids (ICS) and treatment of contributory factors. Current guidelines recommend considering biologic therapies as an additional therapy step if the patient meets the requested criteria [1]. For patients with severe allergic asthma, omalizumab is one of the appreciated biologics for the severe asthma.
Omalizumab, an anti-IgE monoclonal antibody, was the first biologic approved for patients aged six years and older with severe persistent allergic asthma which is not under control with a high dose ICS treatment. Omalizumab reduces asthma exacerbations and hospitalization rates, while improving quality of life (QoL) and asthma daily symptoms, partially up to 8 years of use [2–4].
Randomized clinical trials (RCTs) were performed under optimized conditions and stringent inclusion and exclusion criteria, which do not reflect necessarily medical practice in the real-world setting. By including a narrow subset of patients this limits the extrapolation of the findings to the more heterogeneous patient population encountered in routine clinical practice. The effectiveness of omalizumab in the real-world was demonstrated by Bousquet et al. [5] in a meta-analysis.
The Allergy Centre-Charité in Berlin, Germany, started to use omalizumab in adults with severe allergic asthma according to the approved criteria in 2006. As a single centre real-life study, we aimed to assess the efficacy of omalizumab treatment in adult asthma patients over a very long period, at least five years to ten years. We were also interested to see whether tachyphylaxis would develop over so many years of use.
Materials and methods
Study design
This retrospective study used data from the Institute of Allergology, Charité Universitätsmedizin Berlin, Germany, outpatient clinic. Study subjects were adult patients who must have had severe allergic asthma due to a perennial allergen despite treatment with high dose ICS/LABA in order to initiate treatment with omalizumab, between 2006 and 2012.
Initial injections were given at the outpatient clinic, later injections were either continued at the clinic or self-administered (in a minority of patients) via pre-filled syringes by the patients themselves. Only the patients who completed at least 5 years of therapy were included in the study analysis. The data were taken from the available patient files which have been collected in a standardized form by the same doctor in the centre since 2006.
Statistical analysis
Descriptive statistics for the study population were derived from mean values and standard deviation (SD) or 95% confidence intervals (CI) for continuous variables. Median and interquartile range (IRQ, lower and upper quartile) were used for non-normally distributed variables, and numbers and percentages for categorical variables. The normality of data distribution was checked using the Shapiro-Wilk test. For normally distributed variables, a paired samples t-test was used. For non-normally distributed variables, a Wilcoxon signed-rank was applied to the test baseline measurements acquired at year one and year five. For both tests, a P value of < 0.05 was considered to indicate statistical significance. A notable aspect of our study was the attrition of participants over time, decreasing from 74 patients initially, to 41 at year 6, and further down to 8 by year 10. Therefore, the data from the patient group from year 6 onwards is only presented descriptively.
Demographics, medication, and comorbidities
This study included 74 patients and their demographics are shown in Table 1.
Characteristics of patients
| Characteristics | Baseline | Year 1 | Year 5 | Year 6 | Year 7 | Year 8 | Year 9 | Year 10 |
|---|---|---|---|---|---|---|---|---|
| Patients, n | 74 | 74 | 74 | 41 | 32 | 18 | 11 | 8 |
| Age (year) mean [standard deviation (SD)] | 51.3 (11) | 51.3 (11) | 51.3 (11) | 50.6 (11.1) | 50.5 (10.2) | 50.7 (6.8) | 52.3 (7.3) | 52 (8.5) |
| Female, n (%) | 45 (60.8) | 45 (60.8) | 45 (60.8) | 26 (63.4) | 22 (68.8) | 12 (66.7) | 7 (63.6) | 6 (75) |
| Male, n (%) | 29 (39.2) | 29 (39.2) | 29 (39.2) | 15 (36.6) | 10 (31.3) | 6 (33.3) | 4 (36.4) | 2 (25) |
| Weight (kg), mean (SD) | 76.3 (13.1) | 76.3 (13.1) | 76.3 (13.1) | 77.5 (14.2) | 76.6 (13.5) | 73.7 (11.2) | 75.6 (10.8) | 71.6 (9.9) |
| BMI (kg/m2) | 26.3 | 26.3 | 26.3 | 26.7 | 26.4 | 25.4 | 26 | 25.9 |
| Smoking status, n (%) | ||||||||
| Never, n (%) | 47 (63.5) | 47 (63.5) | 47 (63.5) | 28 (68.3) | 22 (68.8) | 14 (77.8) | 9 (81.8) | 6 (75) |
| Current, n (%) | 5 (6.8) | 5 (6.8) | 5 (6.8) | 3 (7.3) | 3 (9.4) | 0 (0) | 0 (0) | 0 (0) |
| Previous, n (%) | 22 (29.7) | 22 (29.7) | 22 (29.7) | 10 (24.4) | 7 (21.9) | 4 (22.2) | 2 (18.2) | 2 (25) |
| Use of oral corticosteroids (OCSs) | ||||||||
| Patients, n | 44 | 44 | 44 | 26 | 22 | 12 | 7 | 5 |
| OCS (mg), mean (SD) | 20.9 (21.3) | 5 (7.2) | 3.9 (6.3) | 5 (9.1) | 6.5 (12.9) | 4.2 (4.8) | 5.4 (6.3) | 6.5 (5.4) |
On the day of the first omalizumab injection, all demographic data were checked and corrected if necessary. The number of asthma exacerbations [the use of oral corticosteroid (OCS) for at least three consecutive days or doubling of an existing OCS dose for at least three days] within the last 12 months was asked and documented.
Medication (on the day of 1st injection of omalizumab): all patients received on a regular basis high doses of inhaled steroid (800 µg/day to 1200 µg/day), long acting betamimetic and short acting betamimetic, if needed. They received repeated controls of their ability of correct inhalation techniques. The type and dose of inhalational medication have been adapted over the years to the current needs of the patients. The actual national and international recommendations for the treatment of severe asthma and the medication of comorbidities were taken into account. ENT (ear, nose, and throat) diseases were treated by ENT specialists from the Charité.
The dose of OCS shown in Table 1 represents the amount of OCS reported by the patients at a visit to the center in the previous seven days.
The spirometry measurements were carried out with the EasyOne spirometer (ndd Medical Technologies Inc., Andover, MA, USA) while standing. Asthma control was assessed using the asthma control test (ACT) at each patient’s visit.
IgE and IgE antibodies were determined from blood samples of all study subjects using fluorescent enzyme immunoassay (Thermo Fisher Diagnostics GmbH, D-79111 Freiburg, Germany). Table 2 summarizes the results of total IgE and specific IgE antibody levels in the study subjects. Present comorbidities are listed in Table 3.
IgE and specific IgE-antibody against perennial allergens in 74 subjects
| Allergen | Number of sensitized patients |
|---|---|
| House dust mites | 43 |
| Cat | 33 |
| Dog | 19 |
| Horse | 3 |
| Rabbit | 4 |
| Aspergillus fumigatus | 17 |
| Alternaria | 8 |
| Cladosporium | 1 |
Baseline: IgE, median: 283 kU/L; mean value: 850 kU/L; range: 25–24533 kU/L
Comorbidities (known on the day of 1st injection of omalizumab)
| Disease | Number of patients |
|---|---|
| Allergic rhinoconjunctivitis | 68 |
| Nasal polyposis | 8 |
| Dermatitis | 3 |
| Diabetes | 5 |
| Coronary heart disease | 4 |
| Rheumatoid arthritis | 9 |
Results
Lung function
At the start of the omalizumab therapy, the patients had a forced expiratory volume in 1s (FEV1) of about 53% and showed a remarkable increase, approximately 13%, in the first year of omalizumab use. A plateau followed for many years to a value of approximately 68%, with a slight decrease in the years 6, 7, and 8. The few patients in years 9 and 10 increased their FEV1 (Figure 1).
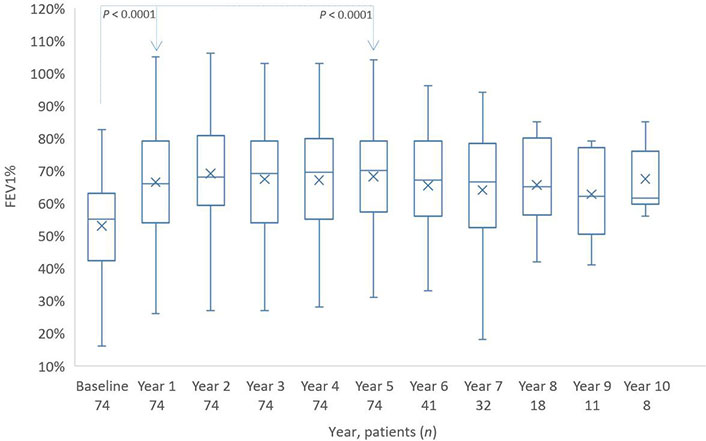
Distribution of FEV1%-values from baseline to year 10. The boxes indicate the median, the 25th and 75th percentiles, whereas the extremes represent the minimum and maximum values. The cross represents the mean value. The P value for baseline/year 1 and baseline/year 5 is based on a Wilcoxon signed rank test
ACT
With a median of approximately 12.5 points, the ACT shows a clear lack of asthma control at the start of omalizumab therapy which then increased during the first year of therapy by 4 points. In the next years, there was a constant increase to a mean of 19 points (Figure 2).
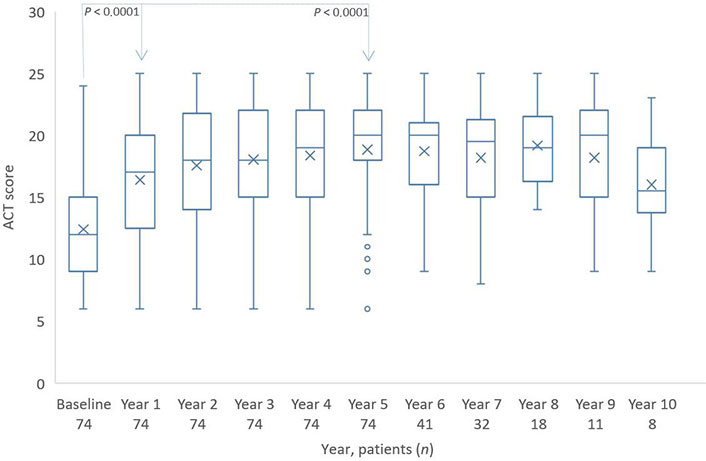
Distribution of ACT scores from baseline to year 10. The boxes indicate the median, the 25th and 75th percentiles, whereas the extremes represent the minimum and maximum values. The cross represents the mean value. Individual points are outliers. The P value for baseline/year 1 and baseline/year 5 is based on a Wilcoxon signed rank test
Exacerbations
The patients had multiple exacerbations in the last 12 months before starting omalizumab therapy. With an average of 3.3 events, and the severity of the disease was also documented.
Already in the first year of omalizumab therapy, the number of exacerbations was reduced to approximately 0.4 per year and remained very low, approximately 0.3 to 0.2, in the following years (Figure 3).
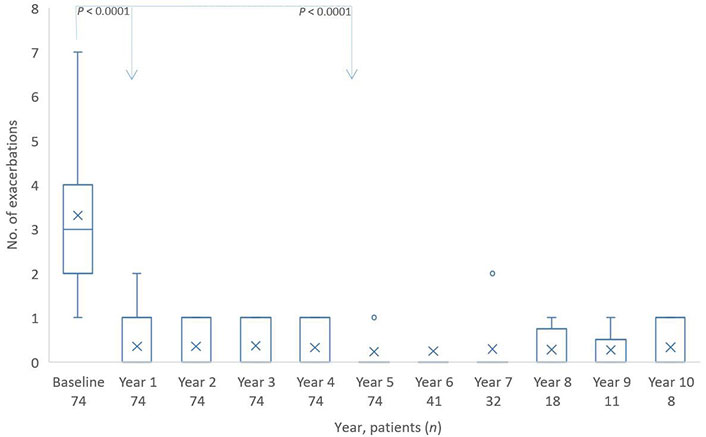
Distribution of exacerbations from baseline to year 10. The boxes indicate the median, the 25th and 75th percentiles, whereas the extremes represent the minimum and maximum values. The cross represents the mean value. Individual points are outliers. The P value for baseline/year 1 and baseline/year 5 is based on a Wilcoxon signed rank test
OCS use
Over half of the patients (44/74) used oral steroids at a mean dose of 20 mg prednisolone daily before the biologic therapy. During the first year, the number of patients using OCS could not be reduced, but the dose was reduced to 5 mg daily (Figure 4). The number of patients using OCS remained constant for the next few years.
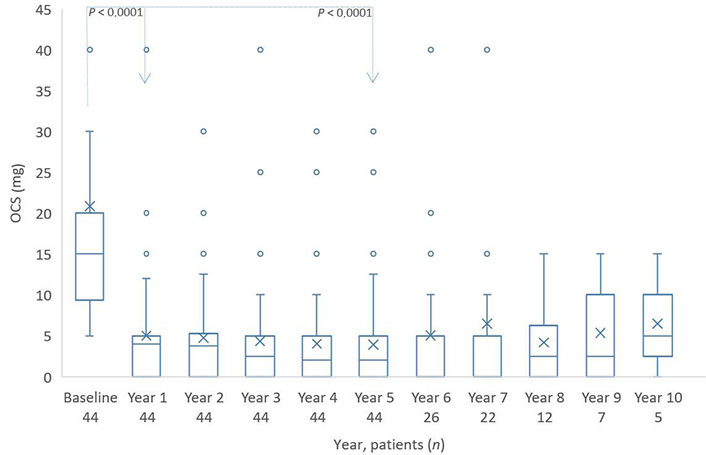
Distribution of OCS (mg) from baseline to year 10. The boxes indicate the median, the 25th and 75th percentiles, whereas the extremes represent the minimum and maximum values. The cross represents the mean value. Individual points are soutliers. The P value for baseline/year 1 and baseline/year 5 is based on a Wilcoxon signed rank test. OCS: oral corticosteroid
The type of comorbidities had a clear influence on the further intake of OCS. Of the 9 patients with rheumatoid arthritis, 6 patients continued taking OCS—albeit at low doses. Two patients with severe nasal polyposis used OCS, as well.
Discussion
In 2005 a new era began for patients with severe allergic asthma. The approval of the first biologic, omalizumab, for severe allergic asthma meant that many sufferers had the opportunity to lead a new life. Patients and their doctors were enthusiastic about the reduction in symptoms and the possibilities of reducing or stopping the unpopular OCS. Avoiding asthma exacerbations and maintaining better lung function were the viable basis for an active life and asthma could be brought under control.
Omalizumab is suitable and approved for the severe allergic asthma phenotype that occurs in patients who have suffered from childhood allergic asthma, often associated with allergic rhinitis and conjunctivitis. After a symptom-free interval that can sometimes last for decades, asthma can recur in adulthood, triggered by sensitization to house dust mites, animal hair, or mold.
Some patients and doctors wondered whether the great effectiveness of the biologic would last, or whether its effectiveness would decrease with prolonged use, and whether tachyphylaxis would occur. The use of omalizumab over three years showed no signs of tachyphylaxis [6], but there was no data on possible tachyphylaxis for longer periods of time. Patients under omalizumab treatment could answer best the question of tachyphylaxis of this biologic because of its use for nearly 20 years now.
We examined the data of our adult patients with severe allergic asthma who were treated with omalizumab for at least 5 years to 10 years in continuation in one center. This allowed for a truly real-world evaluation because the patients were seen by the same doctor. Different perspectives, e.g., assessing symptoms and therapy characteristics in different centers are excluded by the evaluation in one center.
Only the patients whose therapy with omalizumab was not interrupted were included in the analysis. Those patients who received omalizumab only as a booster were not included because continuous use of omalizumab is more effective than boosting [7].
The most frequent allergic triggers were house-dust mites—a known trigger for severe allergic asthma, and animal allergens, mostly cat allergens. The patients had symptoms despite inhaling high doses of steroids and long acting betamimetics, and were checked for their ability to inhale correctly.
Already in the first year of using omalizumab, the FEV1 increased to 65–68% in most patients. They even achieved a clinically important increase of around 13%, and a slightly further increase in the next years. A third of the patients achieved an increase of 20% or more. Therefore, most patients reached the minimal clinically important difference in FEV1, which is determined as 0.23 L or 10.4% change from baseline [8]. Pennock et al. [9] estimated thresholds for acute significant changes in FEV1 of 11%. The American Thoracic Society estimates changes in FEV1 ≥ 15% in long-term trials (i.e. ≥ 1 year) to be confident that a clinically meaningful change has occurred [10].
This improvement in lung function, once achieved, is maintained over the coming year—and in some cases, increased again in the next years to come.
The ACT is an accepted tool for assessing asthma control [11]. In our long-term study, the ACT showed significant and sustained improvement, reaching the minimum clinically important difference of three points [12] at two years and then further increasing. Approximately half of all patients achieved a normal score of at least 20 points. This emphasizes that long-term use of omalizumab has a great stabilizing effect on asthma control and thus on the QoL of patients.
The therapeutic value of a biologic in severe asthma must be measured in particular by reducing the number of exacerbations which has been shown using omalizumab in controlled clinical trials [13–14] and in real-world studies [5].
Akenroye et al. [15] used a hypothetical randomized trial using electronic health records from a large US-based academic health care system to compare the effectiveness of omalizumab, mepolizumab, and dupilumab in individuals with moderate-to-severe asthma for reduction of exacerbations over 12 months of follow-up. Dupilumab reduced the exacerbations (0.46 exacerbations per person year) which was slightly better than omalizumab (0.93 exacerbations per person year), and mepolizumab (1.32 exacerbations per person year).
In our study, we have seen a very stable positive effect on the reduced number of exacerbations over ten years. The greatest changes occurred within the first year of treatment. Patients had an average of more than three exacerbations in the 12 months prior to the first injection. This number was reduced dramatically, to about half an exacerbation per year, and remained consistently extremely low. This number of exacerbations is similar to the efficacy of dupilumab in the study of Akenroye et al. [15]. There was no change in exacerbation avoidance over the entire ten-year period, but an almost exacerbation-free future was achieved for the patients since the first injection. Even patients who experienced 4, 5, or even 16 cortisone-depending exacerbations per year before omalizumab were protected.
Also, of particular importance when evaluating a biologic is its ability to stop or reduce OCS use. Starting from an average dose of 20 mg per day, the OCS could be reduced to approximately 5 mg. A part of those patients who continued to use doses around 5 mg had comorbidities such as rheumatic arthritis and needed a small dose to suppress some pain in the joints.
In a randomized open-label study, Domingo et al. [16] evaluated the addition of omalizumab to the standard of care in patients with severe asthma receiving OCSs. The withdrawal of 75% OCS versus 7.7% (P = 0.001) OCS was observed in the omalizumab and control group, respectively, which was similar to our results. A recent meta-analysis [17] including omalizumab use for > 5 years in adults with asthma documented that the mean daily maintenance OCS dose significantly decreased by > 75% [18] but only approximately half of the patients receiving maintenance OCS at baseline discontinued OCS therapy during omalizumab treatment [19]. Therefore, it seems that omalizumab is less effective in stopping OCS compared to other biologics—possibly because it does not reduce the eosinophils in blood that keep asthmatic inflammation going.
The number of patients after the 6th year of therapy was small and we could not evaluate how the patients who were no longer in the study behaved regarding OCS. Therefore, we have shown further OCS use from the 6th to the 10th year of therapy, but did not subject these results to analysis.
Conclusions
Omalizumab is the biologic with the longest stable positive results in severe allergic asthma over 10 years. In this first, single-centre real-world study over an extended period, describing omalizumab in severe allergic asthma in Germany with 5 years up to 10 years of follow-up, we demonstrated the long-term maintenance of efficacy of the biologic with no signs of diminishing tachyphylaxis.
Improvements in lung function and asthma control were maintained in patients for up to 10 years. Exacerbations were impressively and permanently avoided. The use of OCS could be significantly reduced for use in asthma therapy; in patients with comorbidities, e.g., rheumatic diseases, residual amounts were retained.
Abbreviations
| ACT: | asthma control test |
| FEV1: | forced expiratory volume in 1s |
| ICS: | inhaled corticosteroids |
| OCS: | oral corticosteroid |
Declarations
Acknowledgments
We thank our patients for their contribution and willingness to use their data in the manuscript.
Author contributions
KCB: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. TH and KS: Writing—review & editing. SK: Data Curation, Formal analysis, Visualization. TZ: Writing—review & editing, Supervision.
Conflicts of interest
Karl-Christian Bergmann and Torsten Zuberbier who are the Associate Editors of Exploration of Asthma & Allergy had no involvement in the decision-making or the review process of this manuscript. KCB gives lectures at ALK, AstraZeneca, Bencard, Berlin-Chemie, GSK, HAL, Leti, Sanofi, Stallergenes. TZ reports industry consulting, research grants and/or honoraria from AImmune, Ajanta Pharma, AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, Bio Cryst, Celldex, FAES, HAL, Henkel, Kryolan, Leti, Lofarma, L’Oreal, Meda, Medi Wound, Menarini, Merck, MMV Medicines for Malaria Venture, MSD, Novartis, PCM Scientific, Pfizer, Sanofi, Sanoflore, Stallergenes, Takeda, Teva, and UCB. He is a member of the Initiative “Allergic Rhinitis and its Impact on Asthma” (ARIA), Member of the Board for German Society for Allergy and Clinical Immunology (DGAKI), Chairman of the Board of European Centre for Allergy Research Foundation (ECARF), President of Global Allergy and Asthma European Network (GA2LEN), and member of Committee on Allergy Diagnosis and Molecular Allergology for World Allergy Organisation (WAO). All other authors declare no conflicts of interest.
Ethical approval
The monocentric study was approved by the Charité ethics committee (permit number EA 1/098/18) and complied with the Declaration of Helsinki.
Consent to participate
Informed consent to participate was obtained from relevant participants.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservations, to any qualified researcher.
Funding
Exploration and statistic evaluation of data was financially supported by Novartis GmbH, Germany. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2024.