Abstract
Multiple chemical sensitivity (MCS) is an unexplained acquired medical condition that includes multiple, vague, recurrent, and non-specific symptoms in different organs. They are attributed to exposures to various and structurally unrelated environmental chemicals at concentration levels that are well tolerated by the majority of people and normally considered not to have toxic effects in humans. The aim of this review is to examine the multiple explanatory hypotheses for the pathophysiology of MCS: genetic, metabolic, neurological, immunological, and psychological. Several publications suggest a neurological and immunological activation. However, this neurological and immunological hyperresponse is not always observed when performing challenge tests. This suggests that behavioral conditioning could be an important mechanism in the pathogenesis of MCS. Even if psychiatric conditions appear not to be a major cause of MCS, in the case of genuine psychiatric disease, psychotherapeutic therapy is mandatory. Because of the complexity of the pathophysiology, there is no specific drug to treat MCS. However, the use of cognitive behavioral therapy is encouraged, as it has a significant positive impact on patients’ perception of their illness.
Keywords
Multiple chemical sensitivity (MCS), chemical intolerance, environmental exposure, pathophysiology, mechanismIntroduction
Multiple chemical sensitivity (MCS) is an unexplained acquired medical condition that includes multiple, vague, recurrent, and non-specific symptoms in different organs. They are attributed to exposures to various and structurally unrelated environmental chemicals at concentration levels that are well tolerated by the majority of people and normally considered not to have toxic effects in humans [1–4]. MCS is included in the wider spectrum of idiopathic environmental intolerance (IEI), but it has also been described in the literature as chemical intolerance (CI), IEI, or toxicant-induced loss of tolerance (TILT) [5, 6].
The prevalence of this condition is difficult to estimate. Case rates differ when reported by medical staff in comparison with patients’ self-assessments [7]. The self-reported prevalence of MCS varies from 6.5% to 9% in the general population. In contrast, physician-diagnosed MCS was found in only 0.5% of the general population [8, 9].
When exposed to chemicals, people with MCS may experience symptoms that vary from one individual to another, but a typical triad of the most common symptoms, hyperosmia, asthenia, and dyspnea, is observed [1]. Other symptoms that are less frequent can also be present (Table 1).
| Organ system | Symptoms |
|---|---|
| Respiratory | Tachypnea |
| Sense of suffocating | |
| Dyspnea | |
| Asthma | |
| Bronchospasm | |
| Cough | |
| Otorhinolaryngological | Hyperosmia |
| Burning eyes—stinging sensation | |
| Laryngeal burning sensation | |
| Neurological | Cephalgia |
| Migraine | |
| Asthenia | |
| Trembling | |
| Attention deficit | |
| Dizziness | |
| Confusion | |
| Loss of concentration capacity | |
| Sensorial: abnormal perception of odors | |
| Sensorial: visual disturbances | |
| Sensorial: hyperacusis | |
| Insomnia | |
| Dermatological | Pruritus |
| Eczema | |
| Rash | |
| Urticaria/angioedema | |
| Skin photosensitivity | |
| Dermographism | |
| Rheumatological | Arthralgia |
| Myalgia | |
| Cardiovascular | Palpitations |
| Arrhythmia | |
| Tachycardia | |
| Gastroenterological | Nausea |
| Abdominal pain | |
| Irritable colon | |
| Endocrinological | Dysthyroidism |
| Adrenal gland disorders | |
| Pituitary disorders | |
| Psychological/psychiatric | Anxiety |
| Panic attacks | |
| Depression | |
| Bipolar disorder |
The bold symptoms represent the most frequent symptoms in MCS
The symptoms may arise immediately after a single first high-level chemical exposure, or they may gradually appear over time from recurrent low-level chemical exposures to substances that the person previously tolerated [10].
The Cullen criteria (1987) and the Nethercott criteria (1996), with or without the Lacour (2005) extension of the Nethercott criteria, are now the criteria most used to diagnose MCS [11–13]. The Environmental Exposure and Sensitivity Inventory (EESI); its short version, the Quick EESI (QEESI); or the Brief EESI (BREESI) can be used as evaluation forms to assess CIs [14–16].
Methods
Data sources and search strategy: The bibliographic research was done in February and July 2023, querying PubMed, ScienceDirect, ResearchGate, and UpToDate using the keywords “MCS”, “multiple chemical sensitivity syndrome”, “idiopathic environmental intolerance”, and “IEI”. The search was restricted to English-language papers and included grey literature.
Pathogenic hypotheses
Explanatory hypotheses have been explored and efforts have been made by scientific teams to understand the pathophysiology of MCS. Below we discuss the few proposed etiological hypotheses (Figure 1) [17, 18].
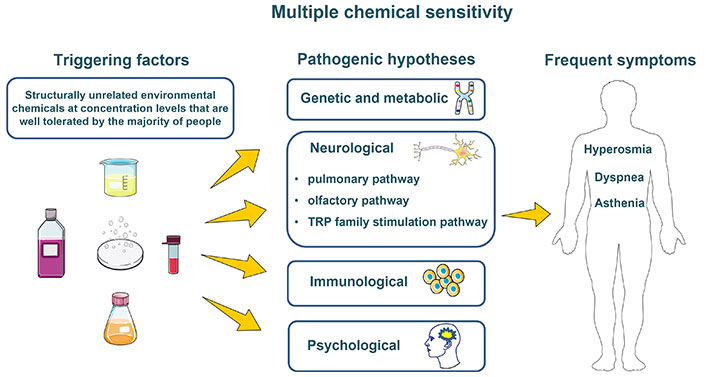
One disease, several hypotheses. Drawn using pictures from Servier Medical Art (https://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/). TRP: transient receptor potential
Genetic and metabolic hypothesis
The level of exposure to air pollution and the ability to eliminate substances have a significant impact on the human body [19]. The biotransformation of foods, xenobiotics, drugs, and heavy metals and their subsequent elimination play a significant role in detoxification [20]. Environmental exposure may produce variations in gene expression and phenotypes and subsequently alter the metabolism of xenobiotics, resulting in toxic effects [21]. Inter-individual differences in enzymatic systems cause differences in detoxification that may explain the various levels of individual susceptibility to xenobiotic compounds [22].
Indeed, several studies have been conducted in order to find out if there is any genetic predisposition for a particular metabolism in MCS patients. Certain genetic polymorphisms modify the rate of chemical metabolism (i.e. drug-metabolizing enzyme polymorphism) and may predispose individuals to MCS. For example, it has been suggested that rapid metabolism for cytochrome P450 2D6 (CYP2D6) and N-acetyl transferase 2 (NAT2) enzymes may elevate the risk of MCS and that the genotypic frequencies of CYP superfamily, UDP-glucuronosyltransferase (UGT), and glutathione S-transferases mu, theta, and pi (GSTM, GSTT, and GSTP) in MCS patients are similar to those in the control population [12, 23]. Moreover, polymorphism of superoxide dismutase 2 (SOD2) and nitric oxide synthase 3 (NOS3) seems to be associated with MCS and increased levels of oxidative stress [24–26].
Furthermore, metabolic markers are modified in MCS patients. These include the production of nitric oxide, accelerated lipid oxidation, and depletion of glutathione, all of which lead to severe oxidative stress [23]. It has been also suggested that N-methyl-D-aspartate receptors might be produced in excess in response to environmental chemicals, with a toxic effect in individuals [27].
These differences in enzyme function might be a convincing hypothesis for differential responses to metals, solvents, and pesticides, but there are no proven correlations between enzyme levels and differences in individuals’ reactivities to chemicals.
Neurological hypothesis
Researchers have explored the theory of air pollution exposure impact on the central nervous system (CNS) [28]. The most common route of exposure to chemicals is inhalation [29]. The chemicals in ambient air include airborne particulate matter (PM) carrying surface-absorbed volatile organic compounds (VOCs), gases (sulfur dioxide, nitrogen oxides), and other substances [28, 30]. PM varies in size but 80–90% is smaller than 0.1 μm [ultrafine PM (UFP)] [30, 31]. Most VOC release occurs indoors from the household cleaning products, air fresheners, floor waxing, carpet and upholstery deodorizers, furniture polishes, and oven and glass cleaners [32].
There are three pathways that can overpass the host defense mechanisms that are supposed to block the effects of these chemicals.
The pulmonary pathway
Inhaled UFPs escape broncho-mucociliary actions and carry the VOCs into regions of the lungs they may not normally reach, where the toxic substance is released while the majority of PM is expelled. Thus, the VOCs attain direct contact with alveolar cell plasma membranes and simply diffuse through them into cell bodies, inducing oxidative stress and tissue injury [33, 34]. Some of these UFPs are further distributed in the systemic circulation, potentially penetrating the intact blood-brain barrier (BBB) in mice and reaching the brain tissue, resulting in the extrapulmonary effects of air pollution [35–37].
The small vessels within the brain parenchyma constitute the BBB. Compared with the peripheral vessels, cerebral microvessels are not leaky because they have tight junctions that severely restrict the passage of many substances. Their diameter is between 3 μm and 8 μm, and they are a chemical and physical barrier to toxins, small organic drugs, ions, and macromolecules, protecting the brain from external damaging factors [38–42].
Compared with other organs, the brain is more vulnerable to oxidative stress because its high consumption of oxygen generates large amounts of reactive oxygen species (ROS) and its lower antioxidant enzyme (glutathione peroxidase, catalase) activity and richness in polyunsaturated fatty acids (PUFAs) makes it highly susceptible to lipid peroxidation [43].
The olfactory pathway
In regard to the direct effects of inhaled UFPs on the CNS, studies on rats showed that approximately 20% of the UFPs deposited on the olfactory mucosa can be translocated to the olfactory bulb (trans-synaptic transport) and along the olfactory bulb, possibly reaching certain brain regions [44]. Humans have a lower olfactory deposition efficiency (the olfactory epithelium covers only 5.5% of the nasal cavity in humans, but 40% in rats). Also, in humans, the size of the inhaled particle has an impact on the quantity of particles translocated into the olfactory bulb: 1% of inhaled 1–2-nm particles are deposited in the olfactory bulb, but this decreases to 0.01% for 100-nm particles [28, 45].
The transient receptor potential family stimulation pathway
This large family of membrane proteins that function as ion channels (Ca2+ influx) can be found in almost every tissue of the body, even in central and peripheral neurons. These chemosensory and temperature-sensitive receptors play a role in nociception, being activated by heat and capsaicin in the peripheral nervous system. They have a large variety of activation mechanisms (G-protein coupled receptor dependent, ligand binding).
Transient receptor potential (TRP) receptors are part of the TRP ankyrin 1 (TRPA1) and TRP vanilloid 1 (TRPV1) subfamily of channels [46]. Together they may contribute to chemical hypersensitivity and reactive airway dysfunction syndrome (RADS), sensorial irritation being a common response to inhaled VOCs [47, 48].
The transient sensitization of airway neurons is thought to be a protective mechanism that eliminates inhaled irritants. In contrast, persistent sensitization of airway neurons increases the sense of airway irritation and nasal sensitivity. Being exposed to high levels of TRPA1 agonists (chlorine, aldehydes) might induce RADS [49], with some patients developing asthma-like symptoms such as cough, wheezing, dyspnea, and chest tightness. Administration of TRP-channel antagonists may reduce sensory irritation and eventually prevent long-term neurogenic inflammation [47].
Chronic activation of the TRP receptor can lead to sensitization (increased responsiveness of the receptor) and subsequently to a lowered threshold for activation and stronger cellular response to a substance [50, 51]. Almost half of patients with MCS have migraines when exposed to scented products [52].
Stimulation of these receptors that play a role in nociception could alter the functional status of neurons. Affected neurons may present a reduced activation threshold. Amplification of normal sensory input by the CNS is called central sensitization [53]. Stimulus perception might no longer be accurately coupled with the intensity and duration of noxious peripheral stimuli [54].
The cerebral dysfunction in MCS: Researchers have tried to explore MCS more objectively, leaving behind the chemical/perfume challenges that have not used common or similar methodological criteria for measuring neurological dysfunction. A study from 1999 produced EEG findings by demonstrating through the level of limbic activation differences between normal and vulnerable individuals by exposing them to low levels of environmental chemicals, supporting the neural sensitization hypothesis [55]. The earlier single-photon emission computed tomography (SPECT) studies observed functional changes in the olfactory cortex [56, 57]. Pre- and post-exposure studies were conducted. When exposed to chemical substances and experiencing symptoms, MCS subjects, compared with healthy controls, displayed stronger deactivation in the olfactory cortex, left hippocampus area, rolandic area, and right thalamus. They also demonstrated hypoactivity in the parieto-temporal cortex, which is involved in processing attention and, together with the hippocampus, in memory formation. This could also influence the neurocognitive deficits in these patients [57].
On functional magnetic resonance imaging (fMRI), MCS patients have been shown to display more significantly increased signal intensity in the limbic system immediately after being exposed to nasal inhalation of 5 ppb, 10 ppb, or 25 ppb of toluene and 10 ppm of phenylethyl alcohol (PEA) as fragrant substances, compared with a control group [58].
In contrast, a study in which 12 patients with IEI and 17 healthy controls underwent cerebral F-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography (PET) found no consistent pathological cognitive performance and functional imaging pattern in MCS patients [59].
Immunological hypothesis
An immunological hypothesis was also raised so possible dysregulation of the immune system was investigated.
In 1992 it was demonstrated that a group of 289 subjects with workplace chemical exposure had quantitative and qualitative alterations at the cellular level (T and B lymphocytes and NK cells), such as abnormal T4/T8 ratios, elevated T cell (CD3+) and B cell (CD19+) numbers, activated T cells (CD10+, CD15+, CD26+, CD38+), suppressed T cell and B cell function, and abnormal NK cell cytotoxic activity. This study also investigated if chemical exposure can induce autoimmunity. They detected the presence of rheumatoid factor, immune complexes, anti-nuclear antibodies, and anti-myelin basic protein antibodies, so they concluded that autoimmunity might also be involved in their chemical-exposed study population [60].
A controlled study investigating possible altered patterns of cytokine expression enrolled 133 Italian MCS patients and 218 healthy controls. MCS patients show increased plasma inflammatory cytokines such as interleukin-8 (IL-8), IL-10, interferon (IFN)-gamma, monocyte chemoattractant protein-1 (MCP-1), platelet derived growth factor BB (PDGF-BB), and vascular endothelial growth factor (VEGF) [23]. Another study, on a Danish population, showed that TNF alpha, IL-1b, IL2/4/6, and IL-4/IL-13 are increased in individuals with MCS in comparison with healthy controls [61]. Furthermore, cytokines might be involved in communication between the immune system and the CNS and induce neuropsychological manifestations, as hypersecretion of cytokines plays a role in the onset and maintenance of depressive illness [62].
Many of the immunological methods and tests that have been used in the previous studies have not been standardized for evaluating MCS.
Psychological hypothesis
MCS has attracted the attention and efforts of specialists in the fields of allergy, immunology, neurology, and toxicology, but also in psychiatry and psychology, as the clinical manifestations seem to be frequently intertwined with psychological and psychiatric factors.
Some authors consider the main component and origin of MCS to be psychological [63]. Some consider MCS to be similar to panic disorder, with a possible chemical phobia that instills in certain individuals’ irrational beliefs about the toxicity of environmental chemicals [64]. In 2006, Pavlovian conditioning was described as contributing to the development of symptoms in some individuals and the authors suggested that MCS is similar to the learned reflex: The perception of unpleasant odors triggers the symptoms of MCS [65].
Anxiety disorder, depression, and somatization disorder have increased prevalence in MCS patients [66]. This somatization may in some cases reflect sequelae of childhood physical and sexual abuse and may play an important role in the occurrence of CI in female patients [67]. However, the psychophysiological electroencephalographic patterns of women with CI and no sexual abuse differ from those of sexually abused women without CI [68]. Negative affect (as measured by level of anxiety and neuroticism) might be a possible risk factor for MCS, and anxiety contributes to the maintenance of the CI via somatic attributions [69, 70]. IEI/MCS patients are also reported to have a higher prevalence of personality disorders in comparison with a normal population [71].
In 2003 in a randomly selected sample of 1,582 residents of Atlanta, 3.1% (n = 49) were diagnosed with MCS. Among patients with MCS, 1.4% declared depression, anxiety, and other emotional disturbances before the appearance of chemical hypersensitivity but, in contrast, 37.7% declared they developed these symptoms after the emergence of their condition, with 27.5% taking medication for these emotional problems [72].
The role of provocation tests in understanding pathophysiology
In a 1997 provocation test study, 15 patients with MCS symptoms underwent open challenges with products they deemed to cause distressing symptoms (nail polish remover, hair spray, disinfectant, perfume, bathtub sealant) and were observed clinically. Pre- and post-challenge pulmonary function tests were performed [pO2, pCO2, oxygen saturation (SaO2)]. In the 11 patients who reproduced their typical symptoms upon challenge testing, hyperventilation was induced in every case, with a rapid fall in the pCO2 in blood and no change or a rise in pO2 or SaO2, while pulmonary function remained unchanged. The authors concluded that hyperventilation is, at least in part, one of the mechanisms by which MCS patients develop their symptomatology [73].
Another placebo-controlled challenge (1996) with perfume in patients with asthma-like symptoms was performed. Nine patients known to have respiratory symptoms after being exposed to nonspecific irritating stimuli were provoked with perfume or a placebo (saline). The patients were tested in a special exposure chamber and a nasal clamp was used in order to avoid scent detection, so they breathed through the mouth during the provocations. The peak expiratory flow (PEF), pulse rate, SaO2, respiratory rate, CO2 in exhaled air, and symptom scores were measured before and after the exposure. Excepting one patient, all patients had a stronger reaction to perfume than to saline. Perfume can provoke symptoms suggesting hyperreactivity of the respiratory tract (dyspnea, coughing, hoarseness) without the presence of bronchial obstruction. When provoked with perfume with or without a carbon filter mask to evaluate if symptoms could be prevented in this manner, no differences in symptoms were noted. PEF values, pulse rate, SaO2, respiratory rate, and CO2 in the expired air were normal throughout the provocations with both saline and perfume. Since the patients had nasal clamps and could not smell during the provocation test, the authors concluded that the symptoms are not always transmitted via the olfactory nerve but might be induced by a trigeminal reflex through the respiratory tract or by the eyes [74].
Bornschein et al. [75] conducted a double-blind placebo-controlled study with an exemplary methodology. In a challenge “climate chamber” for chemical provocations, 20 subjects with MCS and 17 control subjects were exposed to a solvent mixture or a placebo (clean air) during six different exposure sessions. The solvent mixture consisted of 6 hydrocarbons (toluene, xylene, ethylacetate, heptan, decan, and undecan) of the 15 solvents that are the most common in houses. High-toxicity substances such as benzene and formaldehyde or strong-smelling compounds such as higher esters and monoterpenes were avoided in this study. The hypothesis that patients with MCS can distinguish reliably between solvents and placebo was not confirmed. There were no significant differences in neuropsychological parameters such as cognitive performance between the groups when exposed to an active agent vs. a placebo. The authors acknowledged as a study limitation the low recruitment rate of patients with MCS, as many of them refuse to participate in these studies due to fear of health risks [75].
In patients with MCS, near-infrared spectroscopy (NIRS) imaging revealed that odor processing was associated with increased regional cerebral blood flow (rCBF) in the pre-frontal area during olfactory stimulation. MCS patients were found to have an intensified perception of the intensity and unpleasantness of odors [76].
In 2006, Das-Munshi et al. [77] published a systematic review of provocation studies of individuals reporting MCS. In 37 studies conducted on 547 healthy controls and 784 individuals reporting MCS, blinding was inadequate in most studies, as odors of the chemical substances were apparent. In most controlled provocation studies no difference was found between MCS and control groups, suggesting that reactions to chemicals seem to be related to expectations and prior beliefs [77].
Treatment of MCS
There are no generally accepted therapeutic principles. The social and emotional problems of these patients should be addressed in a compassionate manner [78]. If the patient has a genuine psychiatric disease, psychotherapeutic therapy is mandatory. As a means to express care for these patients, the Canadian Human Rights Commission has set a policy on environmental sensitivities in the workplace, highlighting the importance of reducing the use of chemicals and enforcing scent-free policies [79, 80].
Cognitive behavioral therapy
Cognitive behavioral therapy (CBT) for MCS is encouraged; this is similar to the therapeutic approach to patients with somatoform disorders where behavior therapy is applied successfully. A therapeutic goal would be to abandon the chemical substance avoidance behavior, leading to reintegration into work and society. Treating this disease by avoiding chemical substances or by body detoxification is not encouraged and is considered dangerous. Patients may learn how to increase their own well-being by relaxation training so they can regain a sense of control and have an influence over their own state of health [71, 77].
Mindfulness-based cognitive therapy
Mindfulness-based cognitive therapy (MBCT) combines cognitive behavioral techniques with mindfulness strategies in order to help individuals better understand and manage their thoughts and emotions. In a 2015 study on 69 subjects, this therapy resulted in no improvement in the overall illness status but had a significant positive impact on illness perception [81].
Mind-body transformations therapy
Mind-body transformations therapy (MBT-T) is a naturalistic approach using hypnosis and is based on the capacity of natural biological rhythms of the body to activate inner mind-body healing processes. This therapy has been shown to be beneficial in reducing the perceived level of stress, stimulating resilience, and increasing effective coping, and can even be used to induce analgesia and anesthesia [82].
Conclusions
The pathophysiology of MCS appears to be complex. Some interpretations of etiological mechanisms may be more objective than others.
To explain this large variety of symptoms, it could be hypothesized that the penetration of chemical substances through the nose and bronchi could induce a stimulation of the peripheral and CNS, bypassing the BBB and/or ionic channels like TRPs. This could explain the activation observed on SPECT and fMRI during acute exposure of MCS patients to chemical substances.
Moreover, an observed increase in cytokines might be involved in communication between the immune system and the CNS and further play a role in the onset and maintenance of depressive illness. However, this neurological and immunological hyperresponse is not always observed when performing challenge tests, since in some studies subjects with MCS did not show different results compared with healthy subjects with respect to odor detection thresholds or in distinguishing between active and control stimuli when exposed to subthreshold levels of odors. This suggests that behavioral conditioning could be an important mechanism in the pathogenesis of MCS.
Even if psychiatric conditions appear not to be the major cause of MCS, in the case of genuine psychiatric disease, psychotherapeutic therapy is mandatory.
Because of the complexity of the pathophysiology, there is no specific medication against MCS. However, CBT is encouraged, as it has a significant positive impact on the perception of illness.
Abbreviations
| BBB: | blood-brain barrier |
| CI: | chemical intolerance |
| CNS: | central nervous system |
| EESI: | Environmental Exposure and Sensitivity Inventory |
| IEI: | idiopathic environmental intolerance |
| IL-8: | interleukin-8 |
| MCS: | multiple chemical sensitivity |
| PM: | particulate matter |
| SaO2: | oxygen saturation |
| TRP: | transient receptor potential |
| UFP: | ultrafine particulate matter |
| VOCs: | volatile organic compounds |
Declarations
Acknowledgments
Acknowledgments for the figurines in Figure 1: The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Author contributions
CEL: Conceptualization, Writing—original draft, Writing—review & editing, Validation. NM: Methodology, Writing—review & editing, Supervision, Validation. FdB: Conceptualization, Writing—original draft, Writing—review & editing, Validation, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.