Abstract
Aim:
Eosinophilic granulomatosis with polyangiitis (EGPA) is a vasculitis characterized by eosinophilic inflammation. Patients with EGPA are treated with systemic glucocorticoids and immunosuppressive drugs to induce and maintain remission. However, most patients relapse after tapering glucocorticoids, and there are refractory cases with inadequate response to glucocorticoids. Mepolizumab, a humanized anti-IL-5 antibody, is approved for relapsing or refractory EGPA. Furthermore, recent studies have reported the efficacy of benralizumab, a humanized anti-IL-5 receptor α antibody, in EGPA. Here, we investigate the efficacy of biologics on consecutive cases of EGPA.
Methods:
We retrospectively collected patients with EGPA treated with mepolizumab in addition to glucocorticoids at the Department of Pulmonary Medicine in Kagoshima University Hospital and Imakiire General Hospital. In this study, we compared the effects of biologics on inflammatory parameters between pre- and post-treatment of biologics in patients with EGPA.
Results:
Ten patients were included in the study. All patients were treated with mepolizumab, and one was switched to benralizumab later. Treatment with biologics markedly reduced EGPA relapse from 70% (pre-treatment) to 20% (post-treatment), Birmingham Vasculitis Activity Score from 8.4 to 4.0, peripheral blood eosinophil counts from 470.3 /µL to 40.5 /µL, and glucocorticoid doses from 7.3 mg/dL to 1.6 mg/dL. In contrast, lung function and fractional exhaled nitric oxide levels were not affected by treatment with biologics. Furthermore, the duration of biologics was positively correlated with symptom improvement.
Conclusions:
Treatment with mepolizumab for EGPA was effective in glucocorticoid sparing, symptom reduction, and relapse prevention. Mepolizumab is expected to reduce the risk of glucocorticoid-related adverse events. Therefore, continued administration as well as early intervention with mepolizumab for EGPA might be important to conserve future medical resources and control the disease.
Keywords
Asthma, benralizumab, biologic agent, eosinophilic granulomatosis with polyangiitis, glucocorticoid, mepolizumabIntroduction
Eosinophilic granulomatosis with polyangiitis (EGPA) is characterized by eosinophil-rich and necrotizing granulomatous inflammation frequently affecting the respiratory tract, and necrotizing vasculitis primarily influencing small to medium vessels, involved in the development of asthma and hypereosinophilia [1]. The cornerstone of treatment for EGPA is systemic glucocorticoids, used for remission induction and maintenance therapy [2, 3]. The addition of cyclophosphamide or rituximab is also recommended in cases with severe diseases, such as new-onset organ-threatening or life-threatening diseases. After remission induction, the daily glucocorticoid dosage is decreased to reduce the risk of toxicity and relapse as maintenance therapy [4]. However, relapses frequently occur, and many cases fail to taper or discontinue their daily glucocorticoid dose.
In a phase 3 trial using mepolizumab, a humanized anti-IL-5 monoclonal antibody for relapsing or refractory EGPA, mepolizumab treatment resulted in significantly more weeks in remission and a higher proportion of participants in remission than did placebo, subsequently allowing for reduced glucocorticoid use [5]. Furthermore, in recent studies, treatment with benralizumab, a humanized anti-IL-5 receptor α antibody (IL-5Rα), also led to clinical remission, glucocorticoid-sparing effects, and the induction of eosinophil depletion [6]. Treatment with dupilumab, a humanized anti-IL-4 receptor α antibody, had an effect on EGPA-related nose and throat manifestations [7]. Thus, there is growing real-world data for investigating the efficacy of biologics on patients with EGPA. However, the long-term effect of biologics on patients with EGPA has been insufficiently known. Herein, we retrospectively investigated the efficacy of biologics on consecutive patients with EGPA who were treated with glucocorticoids.
Materials and methods
Patients
We reviewed the medical records of patients with EGPA who visited the Department of Respiratory Medicine at Kagoshima University Hospital and Imakiire General Hospital between September 2019 and December 2023 and who were administered biologics [mepolizumab (100 mg or 300 mg every 4 weeks), benralizumab (30 mg every 8 weeks with the first three doses administered every 4 weeks), and dupilumab (300 mg every 2 weeks)]. Patients with a follow-up less than three months after induction of biologics were excluded. The diagnoses and severity of EGPA were determined according to criteria for EGPA issued by the Japanese Ministry of Health, Labour and Welfare [8]. Patient background (age, gender, age at onset, treatment duration of biologics, other treatments, presence of asthma and other tissue injury), lung function, fractional exhaled nitric oxide (FeNO) levels and laboratory findings [blood eosinophil counts and frequencies, C-reactive protein (CRP) level, IgE level, rheumatoid factor/anti-neutrophil cytoplasmic antibody (ANCA) positivity, and Birmingham Vasculitis Activity Score (BVAS)] were assessed and compared at diagnosis and before and after the initiation of biologics, retrospectively [9]. The primary outcomes were in relapse rate and glucocorticoid doses. Relapse was defined as “worsening or relapse of clinical symptom”, “an increase in the dose of glucocorticoids”, and “the administration of intravenous immunoglobulin (IVIg)”.
Enzyme-linked immunosorbent assay (ELISA)
IL-5 levels in serum were measured with a Human IL-5 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Serums for measurement of IL-5 levels were collected on the day of biologic administration for pre-treatment procedure and at least one month after induction for post-treatment procedure.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical differences among groups were analyzed using the Wilcoxon signed rank test to compare between pre- and pro-biologic stages. Correlations between the percentage of improvement in BVAS and the duration of illness and treatment were analyzed using Spearman’s rank correlation. Data were analyzed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). P < 0.05 was considered statistically significant.
Results
Patient characteristics
Background characteristics and parameters are shown in Table 1. Ten patients were included, consisting of two men and eight women. The mean age at EGPA diagnosis was 53.3 years (21–84 years). The mean duration from diagnosis to biologics initiation was 39.9 months (0–136 months). The duration of treatment with biologics was 37.4 months (3–82 months). All patients were treated with mepolizumab [100 mg (n = 3) or 300 mg (n = 7) every 4 weeks], but one patient treated with mepolizumab 100 mg was switched to benralizumab (30 mg every 8 weeks with the first three doses administered every 4 weeks) later due to uncontrolled asthma. On the other hand, no patients received dupilumab. All patients had asthma, nine had neuropathy, and seven had skin conditions. Specifically, two were positive for ANCA. With regard to treatment for EGPA, all patients were administered glucocorticoids, and five had been treated with methylprednisolone pulse therapy. Furthermore, one was treated with azathioprine, and two patients received intravenous cyclophosphamide pulse therapy. Three were treated with IVIg to control neuropathy.
Characteristics of the patients
| Characteristic | n = 10 (mean ± SEM) |
|---|---|
| Age, year | 59.3 ± 6.1 |
| Age at diagnosis, year | 53.3 ± 6.4 |
| Duration of illness, year | 6.0 ± 1.2 |
| Sex, male/female, n | 2/8 |
| Biologic agents, n† | Mepolizumab, 10Benralizumab, 1Dupilumamb, 0 |
| Duration to biologic initiation, month | 39.9 ± 12.6 |
| Duration of treatment with biologics, month | 37.4 ± 7.8 |
| Clinical manifestations | |
| Asthma, n | 10 |
| Asthma severity (GINA)Step1/2/3/4/5, n | 0/0/2/2/6 |
| Neuropathy, n | 9 |
| Cutaneous, n | 7 |
| Cardiomyopathy, n | 2 |
| Cerebrovascular disease, n | 1 |
| Fever, n | 2 |
| Positive for ANCA, n | 2 |
| Treatment | |
| Corticosteroids, n | 10 |
| Corticosteroid doses at baseline, mg/day | 24.7 ± 4.9 |
| Methylprednisolone pulse therapy, n | 5 |
| Immunosuppressive agents, n | 1 |
| Intravenous cyclophosphamide pulse therapy, n | 2 |
| IVIg, n | 3 |
| EGPA severity | |
| Severe, n | 10 |
GINA: Global Initiative for Asthma; ANCA: anti-neutrophil cytoplasmic antibody; IVIg: intravenous immunoglobulin; SEM: standard error of the mean; † As one patient received both mepolizumab and benralizumab, the sum of patients was set to 11
Effects of biologic agents on EGPA
The study had two primary outcomes. The first primary outcome was the relapse rate. The relapse rate was 70% (7/10 patients) at the pre-biologic stage and 20% (2/10 patients) at the post-biologic stage (Figure 1A). The second primary outcome was glucocorticoid doses. Treatment with biologic agents resulted in a reduction of glucocorticoid doses (mean ± SEM: 7.3 ± 1.6 mg/dL vs. 2.4 ± 0.83 mg/dL, P = 0.002) (Figure 1B). Furthermore, 40% (4/10) of patients were able to completely discontinue glucocorticoids. BVAS at the initiation of biologic agents have already been reduced more than those at diagnosis (data not shown). Moreover, the administration of biologic agents induced a significant reduction in BVAS from 8.40 to 4.00 (mean ± SEM: 8.4 ± 1.8 vs. 4.5 ± 1.3, P = 0.0078) (Figure 1C). Notably, one patient had a BVAS of “0”, which is indicative of symptom-free remission. On the other hand, almost all patients were left neuropathy after the induction of biologic agents, and one was treated with IVIg for exacerbations.
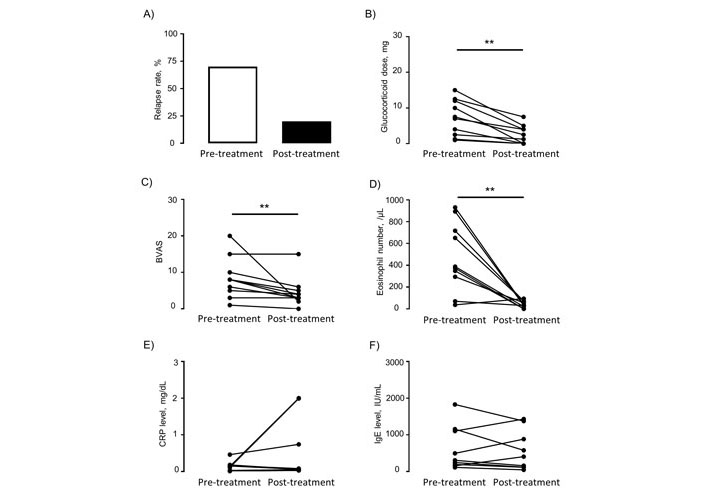
Changes in clinical parameters of EGPA between pre- and post-treatment with biologic agents. (A) Relapse rate; (B) glucocorticoid dose; (C) BVAS; (D–F) clinical indicators. BVAS: Birmingham Vasculitis Activity Score; CRP: C-reactive protein; ** P < 0.01 as measured by the Wilcoxon signed rank test
With regard to eosinophil, the numbers of peripheral eosinophils at the initiation of biologic agents were significantly reduced due to previous treatment with glucocorticoids (mean ± SEM: 7481.9 ± 2332.7 /µL vs. 540.8 ± 90.2 /µL, P = 0.0039) (Figure S1A). Treatment with biologic agents resulted in the further reduction of eosinophil numbers to the normal range (mean ± SEM: 470.3 ± 99.7 /µL vs. 40.5 ± 9.8 /µL, P = 0.0059) (Figure 1D). For CRP and IgE, pre-treatment with glucocorticoids also tended to decrease the levels of CRP (mean ± SEM: 0.89 ± 0.23 mg/dL vs. 0.13 ± 0.04 mg/dL, P = 0.0117) and IgE (mean ± SEM: 1048.5 ± 392.7 IU/mL vs. 622.2 ± 200.4 IU/mL, P = 0.25), while treatment with biologic agents did not contribute to reduced levels of CRP (mean ± SEM: 0.13 ± 0.04 vs. 0.51 ± 0.26, P = 0.4063) and IgE (mean ± SEM: 622.2 ± 200.4 vs. 568.6 ± 181.4, P = 0.6523) (Figure 1E and F; Figure S1B and C). Furthermore, we investigated the correlation between a period of illness, the duration from EGPA diagnosis to biologic agent initiation, and the improvement in BVAS. However, correlation was not observed (Figure S2A and B). By contrast, the duration of treatment with biologics was significantly correlated with decreased BVAS (Figure 2).
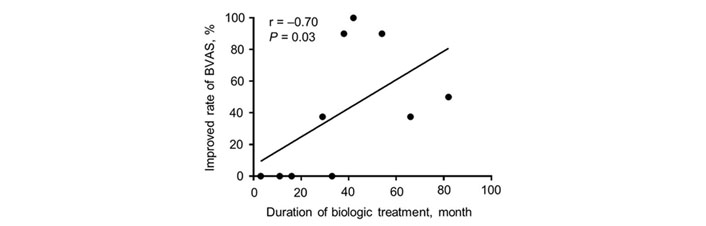
Correlation between a percentage of improvement in BVAS and the duration of treatment with biologics. BVAS: Birmingham Vasculitis Activity Score; P < 0.05 as measured by Spearman’s rank correlation
On the other hand, one patient treated with mepolizumab 100 mg was switched to benralizumab later due to uncontrolled asthma. After receiving benralizumab, asthma control was achieved, and glucocorticoids could be temporarily discontinued. However, frequent treatment with IVIg was performed due to uncontrolled neurological symptoms, eventually, low-dose glucocorticoid therapy was resumed (Figure 3).
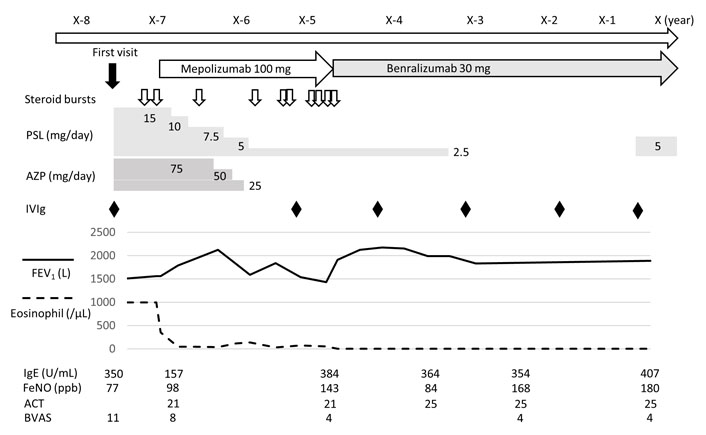
Clinical course of patients treated with both mepolizumab and benralizumab. ACT: asthma control test; AZP: azathioprine; BVAS: Birmingham Vasculitis Activity Score; FeNO: fraction of exhaled nitric oxide; FEV1: forced expiratory volume in one second; IVIg: intravenous immunoglobulin; PSL: prednisolone
These findings suggest that treatment with biologics for EGPA has a glucocorticoid-sparing effect as well as a reduction in systematic symptoms and peripheral eosinophil numbers and a prevention of relapse. It also has been shown that the longer biologics are used, the more they tend to improve the symptoms of EGPA.
Effects of biologic agents on asthma complicated with EGPA
In this study, all patients had asthma complications. Among them, six patients had uncontrolled asthma even with high-dose inhaled glucocorticoid therapy prior to the diagnosis of EGPA. Therefore, we investigated whether treatment with biologic agents affects the control of asthma complicated with EGPA. Because the Asthma Control Test (ACT) was not evaluated in most of the patients, we evaluated the degrees of wheeze on the BVAS instead of the ACT. Although neurological symptoms remained unchanged as described above, most patients had BVAS values of “0” for asthma symptoms after treatment with biologic agents (Figure 4A). Additionally, FeNO and forced expiratory volume in one second were not different before and after the administration of biologic agents (Figure 4B–E). These findings suggest that although treatment with biologic agents improves asthma symptoms of EGPA, no improvement in long-term lung function was seen with mepolizumab and benralizumab for severe asthma.
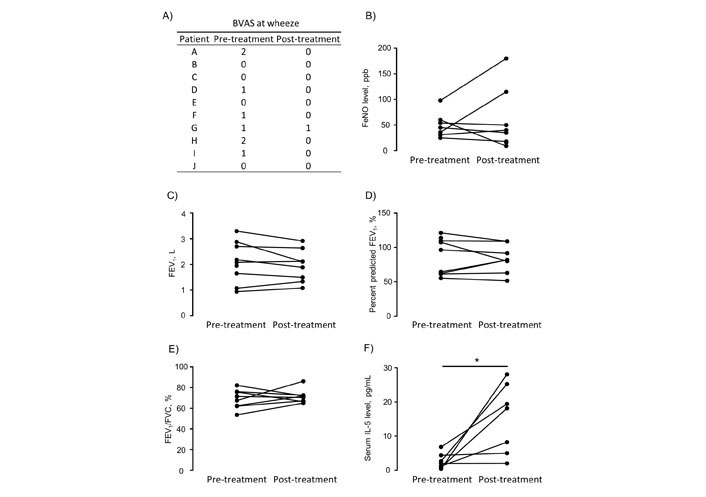
Changes in asthma parameters between pre- and post-treatment with biologic agents. (A) BVAS at wheeze; (B) FeNO level; (C–E) lung function; (F) serum IL-5 level. Percent predicted FEV1 is the ratio of FEV1 to predicted FEV1 to be used to assess the degree of airway obstruction. FEV1/FVC is used to diagnose obstructive lung disease such as asthma. BVAS: Birmingham Vasculitis Activity Score; FeNO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; * P < 0.05 as measured by the Wilcoxon signed rank test
Effect of biologic agents on serum IL-5 levels
Finally, we investigated the influence of biologics, such as mepolizumab and benralizumab, on serum IL-5. Pre-treatment serum was collected on the day of biologic administration and post-treatment serum was collected more than one month after treatment induction. Levels of serum IL-5 were significantly higher post-treatment than pre-treatment (mean ± SEM: 2.6 ± 0.86 pg/mL vs. 15.2 ± 3.85 pg/mL, P = 0.0156) (Figure 4F). This finding suggests that biologics bind to IL-5 or IL-5Rα to inhibit IL-5 acting on eosinophils, resulting in the persistent release of IL-5 into the blood.
Discussion
This real-world study demonstrated that treatment with mepolizumab for EGPA was effective in glucocorticoid sparing, symptom reduction, and relapse prevention. These results were consistent with the previous randomized controlled study investigating the efficacy of mepolizumab on EGPA [5]. We searched for studies that investigated the efficacy of mepolizumab on EGPA in PubMed after the randomized controlled study [5]. As a result, twelve studies were found [5, 7, 10–19], showing that mepolizumab treatment results in a reduction in symptoms, glucocorticoid dose, and relapse rate, so that 9–48% of patients could discontinue glucocorticoid treatment (Table 2). Our study was also consistent with these studies in that treatment with mepolizumab resulted in a reduction in glucocorticoid doses, relapse rates, and improved symptoms.
Summary of studies investigating the efficiency of mepolizumab on EGPA patients
| Study | Cases, n | Mepolizumabdose | Observationperiod | OCS doses | OCS discontinuation | Symptom | Remission | Relapse |
|---|---|---|---|---|---|---|---|---|
| Wechsler ME et al. [5] | 136 | 300 mgq4w | 52 weeks | ↓ | - | ↓ (ACQ-6) | 28% | 56% |
| Kitamura N et al. [10] | 6 | None stated | 12 weeks | ↓ (29%) | - | ↓ | - | - |
| Ueno M et al. [11] | 16 | 300 mgq4w | 12 months | ↓ (62%) | 25% | ↓ (BVAS) | 75% | - |
| Canzian A et al. [12] | 51 | 100 or 300 mgq4w | 12 months | ↓ (61%) | - | ↓ (BVAS) | 82% | - |
| Ríos-Garcés R et al. [13] | 11 | 100 or 300 mgq4w | 12 months | ↓ (45%) | 9% | ↓ | - | 27% |
| Nakamura Y et al. [14] | 13 | 100 mgq4w | 12 months | - | - | ↓ (VAS) | - | - |
| Bettiol A et al. [15] | 191 | 100 or 300 mgq4w | 24 months | ↓ | 42% | ↓ (BVAS) | - | 25% |
| Özdel Öztürk B et al. [16] | 25 | 100 or 300 mgq4w | 12 months | ↓ (67%) | - | ↓ | - | - |
| Yamane T et al. [17] | 27 | None stated | 48 weeks | ↓ (80%) | 48% | ↓ (VDI) | - | 4% |
| Nolasco S et al. [7] | 49 | 100 or 300 mgq4w | 24 months | ↓ (75%) | 28% | ↓ | 57% | 30% |
| Matsuno O [18] | 20 | 300 mgq4w | - | ↓ (61%) | 40% | → (ACT) | - | - |
| Ishii T et al. [19] | 118 | 300 mgq4w | 96 weeks | ↓ (71%) | 38% | ↓ | - | 5% |
OCS: oral corticosteroid; ACQ-6: asthma control questionnaire-6; BVAS: Birmingham Vasculitis Activity Score; VAS: visual analogue scale; VDI: vasculitis damage index; ACT: asthma control test; q4w: every 4 weeks; ↓: reduction; →: no change; -: no data
In our study, one case was treated with benralizumab to control EGPA. Benralizumab treatment for relapsing-refractory EGPA patients was reported to increase the rate of remission and freedom from relapse, as well as having glucocorticoid-sparing effects and reducing BVAS (Table 3) [20–22]. Recently, a phase 3 comparative study showed that benralizumab was not inferior to mepolizumab in the rate of remission and relapse and that benralizumab was superior to mepolizumab in glucocorticoid withdrawal. These results suggest that benralizumab provides efficacy and utility for EGPA treatment [23]. In our case, before the initiation of benralizumab, mepolizumab 100 mg was used for severe uncontrolled asthma. However, the effect of mepolizumab 100 mg on asthma symptoms and neuropathy in EGPA was poor. We considered an increased dose of mepolizumab for EGPA, but we finally selected benralizumab for severe asthma because the patient preferred to receive other medications. Consequently, switching to benralizumab resulted in an improvement in asthma control, including a reduction in the frequencies of exacerbation and attack requiring glucocorticoid administration, although it did not control neuropathy sufficiently.
Summary of retrospective studies on the efficacy of benralizumab in patients with EGPA
| Study | Cases, n | Benralizumabdose | Observationperiod | OCS doses | OCS discontinuation | Symptom | Remission | Relapse |
|---|---|---|---|---|---|---|---|---|
| Cottu A et al. [20] | 68 | 30 mgq4–8w | - | - | 38% | - | 11% | 49% |
| Bettiol A et al. [21] | 121 | 30 mgq4–8w | 12 months | - | - | - | 19% | 46% |
| Nanzer A et al. [22] | 70 | 30 mgq4–8w | 24 months | ↓ (75%) | 68% | ↓ (BVAS) | 15% | 67% |
OCS: oral corticosteroid; BVAS: Birmingham Vasculitis Activity Score; q4–8w: every 8 weeks (first three doses given 4 weeks apart); ↓: reduction; -: no data
In the present study, treatment with biologic agents resulted in reduced BVAS, indicating improvement in EGPA-caused symptoms. However, only one case had a BVAS of “0” and most remaining patients had residual neurological symptoms. This may be due to the slow rate of peripheral nerve regeneration and a decrease in peripheral nerve regenerative capacity correlated with aging [24, 25]. In contrast, a study with six patients with EGPA showed mepolizumab improved the sensory nerve conduction amplitude of the sural nerve as well as clinical symptoms, BVAS, and peripheral eosinophil counts [10]. Although we hypothesized that the morbidity period and the duration between EGPA diagnosis and induction of biologic agents might be negatively correlated with a poor improvement in BVAS, no correlation was found. On the other hand, there was a positive correlation between the duration of treatment with biologics and the improvement rate of BVAS. Previous studies in patients with severe asthma have demonstrated the efficacy and safety of mepolizumab treatment for up to 4.5 years and benralizumab treatment for up to 5 years [26, 27]. Thus, long-term treatment with biologics may lead to future improvement in EGPA symptoms, including neurological symptoms, and further investigations regarding long-term treatment with biologics are required.
IL-5 plays a crucial role in the development, maturation, and activation of eosinophils [28]. As compared to asthma patients, patients with active EGPA have a more significant airway type-2 response to induce eosinophilia through the local production of IL-5. Additionally, increased levels of IL-5 in serum or plasma at the baseline have been shown to be correlated with EGPA severity and exacerbation [28, 29]. On the other hand, changes in serum IL-5 levels before and after treatment with biologics have not been reported so far. In our study, treatment with biologics resulted in elevated serum IL-5 levels (Figure 3F). This suggests that, as a consequence of biologics treatment, IL-5 is released into the blood and cannot bind to eosinophils, as described above. Thus, the withdrawal of biologics targeting IL-5 may result in an increased risk of recurrence or the exacerbation of EGPA.
In ANCA-associated vasculitis, including EGPA, treatment with high-dose glucocorticoids and immunosuppressants has been reported to be associated with adverse events. Infections were the most common adverse event, and patients who developed infections were characterized by frequent pulses of methylprednisolone and hemodialysis [30]. Two patients had recurrent airway infections, which may be related to long-term treatment with glucocorticoids. To prevent such common treatment-related adverse events in patients with vasculitis, shortened duration of glucocorticoid administration is recommended [30]. We now believe that the early introduction of biologics after vasculitis symptoms have been controlled with glucocorticoids should address concerns about glucocorticoid-related complications.
This study has some limitations. Our study has a small number of cases and is a retrospective study. Furthermore, at EGPA diagnosis, some cases had been treated with low doses of glucocorticoids for uncontrolled asthma. Although a low dose of glucocorticoids prior to EGPA diagnosis might not directly affect the main results of this study, parameters such as peripheral eosinophil numbers at diagnosis may have been underestimated. In our study, two cases developed neurological and cutaneous symptoms during treatment with low-dose glucocorticoids for severe asthma and were eventually diagnosed with EGPA. Asthma guidelines state that EGPA is listed in the differential of asthma with hypereosinophilia [31]. However, EGPA presents a diagnostic difficulty that the median duration between the onset of asthma and EGPA diagnosis is 5 years (range 0–40 years) [32].
In conclusion, this study demonstrated that the initiation and continuation of mepolizumab for EGPA result in a reduction in glucocorticoid doses and symptom relapse rates. Also, similar effects were observed in one patient treated with benralizumab. The use of mepolizumab is expected to reduce the risk of adverse events associated with treatment with glucocorticoids. Therefore, the early initiation and continuation of mepolizumab for EGPA could be considered important to conserve future medical resources.
Abbreviations
| ACT: | Asthma Control Test |
| ANCA: | anti-neutrophil cytoplasmic antibody |
| BVAS: | Birmingham Vasculitis Activity Score |
| CRP: | C-reactive protein |
| EGPA: | eosinophilic granulomatosis with polyangiitis |
| ELISA: | enzyme-linked immunosorbent assay |
| FeNO: | fractional exhaled nitric oxide |
| IL-5Rα: | IL-5 receptor α antibody |
| IVIg: | intravenous immunoglobulin |
Supplementary materials
The supplementary figures for this article are available at: https://www.explorationpub.com/uploads/Article/file/100958_sup_1.pdf.
Declarations
Acknowledgments
We thank Minako Muraji and Mizuki Morishita for their assistance with data collection, and Tammy Bicket for English language editing.
Author contributions
TM: Conceptualization, Investigation, Methodology, Writing—original draft. HM and YD: Investigation, Resources, Writing—review & editing. MO: Visualization. K Tsuruzono, HU, SY, TS, JI and K Machida: Resources. KK and K Mizuno: Project administration. K Takagi and K Tanaka: Writing—review & editing. HI: Project administration, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
HI reports research/educational grants from Boehringer-Ingelheim, GlaxoSmithKline, and OMRON, and payment or honoraria for lectures/advisory committees from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Kyorin, Novartis and Sanofi. K Tanaka reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chugai Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Daiichi Sankyo, Eli Lilly, Merck, Pfizer, Takeda Pharmaceutical, MSD, Bristol Myers Squibb and Novartis. However, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript for this study. The other authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Institutional Review Board of Kagoshima University (No.190118). The study was also conducted in accordance with the ethical principles of the Declaration of Helsinki.
Consent to participate
The informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The datasets for this manuscript are not publicly available because of ethical reasons. Requests for accessing the datasets should be directed to the corresponding author Dr. Hiromasa Inoue, inoue@m2.kufm.kagoshima-u.ac.jp.
Funding
Not applicable.
Copyright
© The Author(s) 2024.