Abstract
Asthma is a complex inflammatory airway disease affecting a significant global population, spanning from childhood through adulthood. Despite advances in treatment modalities, a significant subset of patients, approximately 10%, grapple with severe asthma, characterized by increased healthcare utilization and diminished quality of life. Tezepelumab, a monoclonal antibody targeting thymic stromal lymphopoietin (TSLP), offers promising therapeutic potential. TSLP is a protein released by a variety of cells, with a predominance of epithelial cells, in reaction to plenty of stimuli, such examples as viruses, aeroallergens, and others. Its action is upstream and pertains to initiating numerous subsequent innate and adaptive immune reactions, contributing to the continuation of asthma pathophysiological processes. Tezepelumab’s unique efficacy spans diverse severe asthma phenotypes, significantly reducing exacerbation rates across eosinophilic and non-eosinophilic subtypes. Its favorable safety profile and clinically meaningful improvements in asthma control, accompanied by reductions in cytokine levels and baseline biomarkers, underscore its broad impact on asthma inflammation. Its efficacy, irrespective of type 2 (T2) endotype, reinforces the idea that TSLP blockade broadly inhibits pathways crucial to asthma pathophysiology, rather than narrowly focusing on individual downstream factors, as previous biological treatments have. This review discusses the rationale for TSLP blockade and the efficacy of tezepelumab in severe asthma using data from key trials.
Keywords
Asthma, severe asthma, anti-TSLP, tezepelumab, T2-low asthma, T2-high asthmaIntroduction
Asthma is a chronic, non-communicable, airway inflammatory disease that presently impacts 262 million people globally [1]. Data suggests that between 5% and 10% of all asthma patients have severe asthma [2], a heterogeneous disease state characterized by its diverse clinical presentation, necessitating the use of multiple medications at high doses to manage the condition effectively. Nevertheless, despite receiving high doses of medication as indicated in therapeutic step 5 of the Global Initiative for Asthma (GINA), some patients encounter challenges in effectively managing their condition, known as severe uncontrolled asthma (SUCA) [3]. In such patients, health-related quality of life (HRQoL) is considerably impacted due to severe symptoms, frequent and life-threatening exacerbations, and the need for high levels of medication [4]. Severe asthma is also frequently associated with comorbidities such as allergic rhinitis, chronic rhinosinusitis, nasal polyposis (NP), gastroesophageal reflux disease, and bronchiectasis that can exacerbate or mimic asthma symptoms, contributing to poor disease control [5, 6]. Moreover, patients with severe asthma receive increased levels of oral and systemic corticosteroids, which is linked to various side effects, including infections, as well as complications related to cardiovascular, metabolic, ocular, psychiatric, and bone health [7–11]. For patients identified as having SUCA, a condition often exacerbated by insufficient standard therapeutic options, there emerges a promising intervention in the form of tezepelumab. Tezepelumab, an anti-thymic stromal lymphopoietin (TSLP) monoclonal antibody (mAb), has garnered attention for its potential to address unmet needs in asthma management [3].
Asthma endotyping and phenotyping
Despite the progress in therapy, a considerable number of asthma patients still lack adequate control. A possible explanation for this fact is that asthma is a condition encompassing a variety of pathophysiological mechanisms. This variety, evident through numerous phenotypes (clinical presentations) and distinguishing endotypes (molecular mechanisms), is now the target of therapies. In the level of phenotypes, heterogeneity is demonstrated by the existence of various, overlapping phenotypes (e.g., young atopic, obese middle aged, and elderly) (Figure 1). These phenotypes present with the same clinical manifestations, which are wheezing, breathlessness leading to coughing, chest tightness, and variable airflow obstruction [12]. As for endotypes, inflammatory mechanisms observed in severe asthma may manifest as either persistent eosinophilic, known as type 2 (T2) high endotype [which is characterized by increased levels of fraction of exhaled nitric oxide (FeNO) and/or allergic comorbidities], or non-eosinophilic, incorporating neutrophilic (sputum neutrophils > 40–60%) and paucigranulocytic (normal sputum levels of both eosinophils and neutrophils), known as T2 low endotype [12–14]. Regarding eosinophilic inflammation, a primary role is played by T helper 2 (Th2) lymphocytes and group 2 innate lymphoid cells (ILC2s), by producing interleukins 4 (IL-4), 5, 9, and 13 [12–16]. Νon-eosinophilic asthma, known as T2-low endotype, including neutrophil inflammation and paucigranulocytic subtype, is linked to the activation of Th1 and/or Th17 cells and dysregulation in the balance of Th17 and T regulatory lymphocyte (Treg) cells, particularly in cases of neutrophilic asthma [12]. Furthermore, Th17 cells release IL-17, whereas Th1 cells release tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) [16]. In this pathobiological framework, the bronchial epithelium plays a crucial role by releasing three innate cytokines, known as alarmins, which consist of TSLP, IL-33, and IL-25 [17, 18]. These alarmins act as upstream regulators of both T2 high asthma, by activating both the innate (ILC2) and adaptive (Th2) immunity [19], and T2 low asthma by promoting the differentiation of Th17 cells, with the central memory T cell phenotype [20]. Among anti-alarmins, anti-TSLP, and especially tezepelumab, is the most studied mAb in clinical trials [21–23]. Owing to its upstream mode of action, which encompasses both the T2 high and T2 low endotypes, tezepelumab is a promising drug.
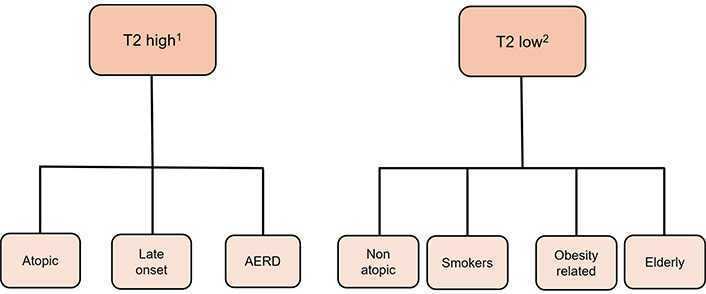
Endotypes and phenotypes of asthma. 1 eosinophilic asthma; 2 non-eosinophilic asthma. T2: type 2; AERD: aspirin-exacerbated respiratory disease
Current management of severe asthma
According to GINA, maintenance therapy for SUCA typically involves combining high-dose inhaled corticosteroids (ICS) with a high-dose long-acting β2-adrenergic agonist (LABA), along with an additional controller medication, such as a long-acting muscarinic antagonist (LAMA), which implies tiotropium or glycopyrronium, and/or biologic therapy. Short courses of oral corticosteroids (OCS) may be also needed in these patients [3].
More precisely, the specific therapy will be determined following an assessment of the patient’s inflammatory phenotype (T2 high or T2 low asthma), as well as considering the patient’s comorbidities [3]. In clinical practice the identification of T2 inflammation is achieved by detecting the following biomarkers: baseline blood eosinophil count (BEC), FeNO level, and serum specific immunoglobulin E (IgE) levels [3]. Given the fact that T2 biomarkers are suppressed by OCS, the tests for their detections should be conducted before initiating OCS, or 1–2 weeks after completing a course of OCS, or while on the lowest possible OCS dose [3]. Furthermore, systemic corticosteroids may also decrease IgE levels by suppressing the synthesis of IL-4 and IL-13, which are necessary for IgE production [24].
If T2 inflammation is not detected, options include add-on LAMA, low dose azithromycin, anti-IL-4A receptor (anti-IL-4AR), add-on OCS, bronchial thermoplasty, and anti-TSLP (tezepelumab) [3]. If T2 inflammation is present, one should consider the use of biologic therapy if it is accessible and affordable [3]. Current available biologic agents for the treatment of severe asthma are anti-IgE (omalizumab), anti-IL-5 (mepolizumab, reslizumab), anti-IL-5R (benralizumab), anti-IL-4R (dupilumab, blocking IL-4 and IL-13), anti-TSLP (tezepelumab), and mAbs. In the key trials of the biologics currently approved, the decrease in exacerbation rates relative to placebo ranged from 47% to 59% with the most effective dosage regimen [25–29]. Along with that, the biologics mentioned above show significant improvement in conventional indicators of asthma control, i.e., forced expiratory volume in the 1st second (FEV1), Asthma Quality of Life Questionnaire (AQLQ), and Asthma Control Questionnaire (ACQ) scores [30–32], as well as OCS sparring effects [33]. It is worth noting, however, that in a meta-analysis comparing the efficacy of tezepelumab with other biologics in reducing exacerbations, tezepelumab ranked highest in lowering exacerbation rates [34]. A possible explanation of that could be the fact that biologics targeting T2 mediators act on downstream levels of the pathophysiology of asthma. On the contrary, anti-TSLP drugs act at a higher level of the cascade, making them promise broad-spectrum action across the entire spectrum of asthma pathophysiology. Tezepelumab is currently the only approved add-on treatment for severe asthma that targets TSLP [3].
Biological role of TSLP
TSLP is a cytokine that triggers inflammatory responses, produced mainly by epithelial cells found in various parts of the body, including the skin, gastrointestinal system, respiratory system, and thymus gland [35]. It is classified as a part of the IL-2 family, and it was first described as a stimulator of murine thymocytes to induce B cell proliferation and development [36]. Along with IL-25 and IL-33 is categorized as an alarmin, which is a separate group of mediators responsible for adapting the immune system to various antigens [37].
TSLP is crucial in managing immune responses, especially regarding allergic inflammation and overall immune system regulation. In genetically predisposed individuals, TSLP is produced by epithelial cells as a response to a variety of environmental stimuli such as allergens, bacteria, viruses, toxins, or tissue trauma [38]. The protein manifests its role by binding to cells that carry a specific heterodimeric receptor consisting of TSLP receptor (TSLPR) and IL-7Rα [39]. On its own, the TSLPR has a low affinity for the cytokine, but in combination with IL-7Rα, they form a high-affinity complex, which is found on several cell types [36]. The presence of this heterodimer in several cells such as dendritic cells (DCs), hematopoietic cells, mast cells, airway smooth muscle cells (ASMCs), and others, demonstrates the importance of the range of TSPL’s effectiveness [38].
TSLP is a protein that has a central role in physiological and pathological immune-related situations [36]. It is involved in the preservation of DC-mediated CD4+ T cell homeostasis [40] and its expression by thymus regulates the capacity of DCs and the development of Tregs induced by the myeloid DCs and plasmacytoid DCs [41]. It is also responsible for maintaining the homeostasis in gut, by regulating the role of non-inflammatory gut DCs [36]. Moreover, in inflammatory conditions, research indicates that TSLP stimulates a T2 immune response by the upregulation of OX40 ligand in DCs and by directly triggering the production of IL-4 in naive CD4+ T cells [42]. Exposure to allergens, especially aeroallergens, induces a T2 cytokine response leading to excess production of inflammatory ILs IL-4, IL-5, IL-13. This overproduction contributes to the activation and recruitment of the eosinophils and the release of IgE [17]. Additionally, DCs also trigger naive CD4+ T cells to differentiate into T follicular helper cells. This leads to the secretion of IgG and IgE by lymphocytes [43].
In addition to its effects on DCs and T cells, TSLP can also influence the activity of other immune cells such as basophils, mast cells, and eosinophils, which are involved in allergic responses. Eosinophils express both TSLPR and IL-7Rα, thus TSLP influences them directly, mainly by decreasing the apoptosis [38] but also in an indirect way, by stimulating the production of cytokines and chemokines responsible for eosinophil proliferation and differentiation [44]. Mast cells when triggered by TSLP release T2 cytokines and chemokines to progenerate eosinophil production, but studies have shown that mast cells can also produce TSLP after cross-linking with IgE, or when combined with IL-4, proving that TSLP is not produced only by epithelial cells (Table 1) [40, 45, 46]. Besides allergic reactions, TSLP has an important role in non-allergic responses, by triggering ILC2s. The combination of the alarmins stimulates ILC2s to produce T2 proinflammatory cytokines like IL-5, IL-13, and IL-9 promoting eosinophil inflammation [38].
Cells that produce thymic stromal lymphopoietin (TSLP) and the cell targets of TSLP
| Cells that produce TSLP [36, 46] | Targets of TSLP [38] |
|---|---|
| Epithelial cells of the respiratory system, gastrointestinal system, and skin | Dendritic cells |
| Dendritic cells | Hematopoietic progenitor cells |
| Mast cells | Eosinophils |
| Basophils | Basophils |
| Human CD68+ macrophages | Mast cells |
| Fibroblasts | Airway smooth muscle cells |
| Keratinocytes | Group 2 innate lymphoid cells |
| Lymphocytes | |
| Monocytes/macrophages |
Role of TSLP in asthma
TSLP is a dominant mediator in asthma. A study from Ying et al. [47] establishes that numerous TSLP m-RNA expressing cells are present in respiratory epithelium in asthma patients in comparison to healthy individuals. Airway structural cells seem not only to be targets of TSLP activity but also to produce TSLP and that indicates that TSLP is responsible for airway remodeling [48]. Dysregulation of airway structure cells results in characteristic structure alterations, like ASMC hypertrophy, sub-epithelial fibrosis, and goblet cell hyperplasia [38]. These changes contribute to persistent airflow limitation and worsening of asthma. TSLP is present in bronchoalveolar lavage fluid (BAL) and in samples from sputum in asthma patients [40, 49]. This protein is a part of both allergic and non-allergic subtypes of asthma. Other studies demonstrated that the elevation of TSLP levels correlates with disease severity [36], and the presence of TSLP and IL-33 in asthma is linked to ongoing airway inflammation and impaired lung function, even with corticosteroid treatment. Consequently, they are imperative mediators in severe asthma [50].
Allergic asthma
Regarding allergic asthma, TSLP is believed to have a significant impact on both the pathophysiology and the level of exacerbations. As mentioned before, TSLP is responsible for the maturation and activation of DCs to promote the differentiation of naive T cells to Th2 cells (Figure 2). Furthermore, it stimulates Th2 cells to produce proinflammatory cytokines like IL-4, IL-5, and IL-13 that play a crucial role in orchestrating an allergic inflammation [51, 52]. IL-4 mediates the induction of IgE by B lymphocytes and upregulates the IgE receptors on cells’ membranes, enhancing the allergic reaction [53], whereas IL-5 activates the eosinophils and is involved in their maturation [52]. IL-13 is the dominant cytokine of Th2 mediated immune response, which regulates mucus production, bronchoconstriction, airway hyperresponsiveness (AHR), remodeling, and fibrosis [54]. It has been also observed that TSLP prompts basophil responses to function as antigen presenting cells in allergen induced Th2 response.
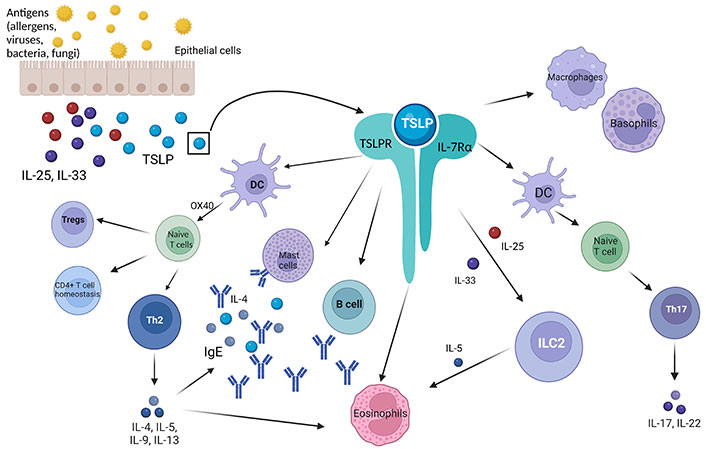
Role of TSLP and TSLPR-IL-7Rα complex. TSLP: thymic stromal lymphopoietin; TSLPR: TSLP receptor; Tregs: T regulatory lymphocytes; Th2: T helper 2; DC: dendritic cell; IgE: immunoglobulin E; ILC2: group 2 innate lymphoid cell; IL-25: interleukin 25; IL-7Rα: IL-7 receptor α; OX40: tumor necrosis factor receptor superfamily, member 4 (TNFRSF4, also known as CD134 and OX40). Created in BioRender. Papaiakovou, G. (2024) BioRender.com/s26z522
Non-allergic asthma
In non-allergic asthma, the antigens that stimulate TSLP production include viruses, parasites, bacteria, smoke, and others [55]. In this subtype, there is limited literature regarding the role of TSLP. It is known that in the non-allergic eosinophilic subtype, TSLP stimulates ILC2 to produce IL-5, which is responsible for the production of eosinophils. Studies also demonstrate the significant role of alarmins in ILC2 regulation and their correlation with steroid resistant asthma, thus it is suggested that, while dexamethasone can reduce the IL-33 driven inflammatory effect of ILC2s, TSLP may provide steroid resistance to ILC2s [40, 56].
On the other hand, in the neutrophilic type of asthma, TSLP drives DCs to stimulate the production of neutrophil activating Th17 lymphocytes [37]. More specifically, IL-17, a cytokine produced by Th17, is highly expressed in patients with moderate to severe asthma when compared to healthy individuals. It is suggested that a combination of Th2, Th1, and neutrophilic inflammation orchestrated by Th17 cells, is involved in the pathogenesis of severe asthma. A study conducted by Tanaka et al. [20] suggests that DCs activated by TSLP and Toll-like receptor 3 (TLR3) ligands, encourage the differentiation of Th17 cells, particularly in conditions that typically favor the development of Th2 cells. Additionally, these Th17 cells exhibit characteristics of central memory T cells [20]. Overall, TSLP is pivotal in initiating and intensifying allergic inflammation in asthma, as well as the development of non-allergic asthma by promoting airway inflammation, hyperresponsiveness, and remodeling, making it a promising target for therapeutic intervention in various subtypes of asthma including severe T2 low subtype.
Clinical trials evaluating tezepelumab’s efficacy
Key trials assessing asthma exacerbation rates, lung function, and symptom control
PATHWAY (NCT02054130), a phase 2, multicenter, randomized, and double-blind study, compared the efficacy of subcutaneous (SC) tezepelumab administered at three dose levels over placebo during a 52-week treatment period [57]. The participants of the study were nonsmokers, 18 to 75 years of age, and had SUCA despite receiving medium or high doses of ICS along with LABA. Other criteria for the enrollment were the following: FEV1 between 40–80%; a history of ≥ 2 asthma exacerbations in the past year that required the use of systematic glucocorticoids or hospitalization; a score on the six-item ACQ (ACQ-6) of at least 1.5 during screening [56]. The participants of the trial were randomly allocated (in a 1:1:1:1 ratio) to four subgroups to receive one of three different doses (70 mg, 210 mg, or 280 mg) of SC tezepelumab or placebo [57]. The primary endpoint of the study was to evaluate how tezepelumab affects the annualized asthma exacerbation rate (AAER) at week 52. Results showed that AAER was markedly lower in tezepelumab groups versus placebo by 62% (low-dose group), 71% (medium-dose group), and 66% (high-dose group) [57]. This reduction in AAER occurred independently of eosinophil blood counts. Thus, the drug’s action was demonstrated across T2 high and T2 low inflammation. In addition to this, data from post hoc analyses of the PATHWAY study on AAER revealed the following: tezepelumab decreased AAER by 66–78%, versus placebo, in patients with any perennial allergy [58]; the drug decreased AAER across all seasons compared with placebo [59]. As for secondary endpoints, the prebronchodilator FEV1 was greater in the low-dose, medium-dose, and high-dose tezepelumab groups than in the placebo group by 120 mL, 130 mL, and 150 mL, respectively [57]. The drug also decreased significantly ACQ-6 score in all three tezepelumab groups and AQLQ[S] + 12 ([standardized] for patients 12 years of age or older), score only in the high-dose group, at the 52nd week of treatment [57].
NAVIGATOR (NCT03347279), a phase 3, multicenter, randomized, double-blind, placebo-controlled trial enrolled patients aged 12 to 80 years old, randomly assigned to receive 210 mg tezepelumab or placebo, SC, every 4 weeks (Q4W) for a 52 week treatment period. Patients were also diagnosed with asthma by a physician and were under treatment with a medium- or high-dose ICS (daily dose of ≥ 500 μg of fluticasone propionate or equivalent) for at least 12 months before screening, in combination with not less than one other controller medication, with or without OCS, for at least 3 months prior to their enrollment [60]. Moreover, asthma had been diagnosed ≥ 12 months, and patients had had a minimum of 2 exacerbations in the preceding 12 months, in advance of agreeing to participate [60]. The primary outcome measure in this study, consistent with that of the previous one, was AAER measured in patients with BEC both more and less than 300 cells per microliter [60]. Secondary endpoints included change in the prebronchodilator FEV1 from the baseline (minimum clinically important difference 0.1 L), ACQ-6, AQLQ[S] + 12, and the weekly mean asthma symptom diary (ASD) score. Concerning the primary endpoint, the rate of AAER was notably lower in the tezepelumab group compared to the placebo group [60]. In a pre-specified exploratory analysis tezepelumab reduced the AAER versus placebo through all seasons in both baseline BEC subgroups (≥ 300 eosinophils/μL and ≤ 300 eosinophils/μL), whereas in the placebo group, there were seasonal variations in the AAER, with a peak in winter [61]. In addition, tezepelumab reduced AAER by week 52 by 58–68% in patients with proof of allergic inflammation (perennial aeroallergen sensitivity, confirmed symptomatic allergy, and omalizumab-eligible patients) [62]. Especially, among patients appropriate for receiving omalizumab, AAERs were decreased regardless of baseline BEC and FeNO levels [62]. Regarding secondary outcomes, a difference of 0.13 liters in the pre-bronchodilator FEV1 was noticed between the groups from the first time of examination (week 2) and maintained until the end of the trial. As for the scores mentioned above, they were all remarkably improved in the tezepelumab group [60]. Patients both with and without perennial allergy, experienced improvement in lung function (FEV1 increase) and patient-reported outcomes [62].
Clinical trials evaluating the efficacy of tezepelumab are shown in Table 2.
Summary of the effects of tezepelumab on clinical results
| Studies, dose | AAER* | ACQ-6 score† | AQLQ[S] + 12 score† | ASD score† | FEV1† (liters) | |
|---|---|---|---|---|---|---|
| PATHWAY (NCT02054130) [57] | ||||||
| 70 mg | 0.27 | –1.17 | 1.12 | - | 0.07 | |
| 210 mg | 0.20 | –1.20 | 1.17 | - | 0.08 | |
| 280 mg | 0.23 | –1.22 | 1.32 | - | 0.1 | |
| Placebo | 0.72 | –0.91 | 0.97 | - | –0.06 | |
| NAVIGATOR (NCT03347279) [60] | ||||||
| 210 mg | 0.93 | –1.55 ± 0.05 | 1.49 ± 0.05 | –0.71 ± 0.03 | 0.23 ± 0.02 | |
| Placebo | 2.10 | –1.22 ± 0.05 | 1.15 ± 0.05 | –0.59 ± 0.03 | 0.09 ± 0.02 | |
| DESTINATION (NCT03706079) [75, 79] | ||||||
| 210 mg | PS SOURCE | 0.42 | - | - | - | - |
| PS NAVIGATOR | 0.61 | - | - | - | - | |
| Placebo | - | - | - | - | - | |
| NOZOMI (NCT04048343) [76] | 0.11 | –0.98 | - | - | 0.075 ± 0.266 | |
*: events per patient-year-trough week 52; †: least-squares mean change from baseline at week 52; -: not measured. AAER: annualized asthma exacerbation rate; ACQ-6: six-item Asthma Control Questionnaire; AQLQ[S] + 12: Asthma Quality of Life Questionnaire [standardized] for patients 12 years of age or older; ASD: asthma symptom diary; FEV1: forced expiratory volume in the 1st second; PS: parent study
Efficacy in OCS-dependent severe asthma
As mentioned previously, a portion of patients with SUCA receive daily maintenance OCS (mOCS), for a period longer than 6 months (chronic use), which is linked to adverse events (AEs) on many aspects of health. In the NAVIGATOR study, 100 of the 1,062 randomized patients were receiving mOCS [63]. In the mOCS subgroup, treatment with tezepelumab reduced numerically AAER and resulted in substantial progress in asthma symptoms, quality of life, and lung function compared to placebo [63].
SOURCE (NCT03406078) was an OCS-sparing, phase 3, multicenter, randomized, double-blind, placebo-controlled trial, that enrolled 150 participants from 18 to 80 years old, diagnosed with asthma by a physician, undergoing treatment with medium-dose or high-dose ICS and a history of ≥ 1 exacerbation the previous 12 months before the screening day [64]. After an 8-week OCS optimization, participants were randomly assigned to receive SC tezepelumab 210 mg or placebo, Q4W, for 48 weeks [65]. The study primarily aimed to investigate whether the medication would reduce and to what extend the daily mOCS dose from baseline to week 48 while maintaining asthma control. Although the decrease in daily mOCS did not reach the level of statistical significance, there was indeed a numerical reduction in the group of tezepelumab, in comparison to the placebo group, at week 48 [65].
Mechanistic studies
Both UPSTREAM (NCT02698501) [66] and CASCADE (NCT03688074) [67, 68] aimed to assess tezepelumab effects on AHR. UPSTREAM was a randomized, double-blind, placebo-controlled, phase 2 trial. Patients included were adults, non-smokers, aged between 18 and 75 years, having SUCA and AHR to inhale mannitol [provoking dose of mannitol causing a 15% reduction in FEV1 (PD15) ≤ 315 mg] while receiving stable doses of ICS [66]. Patients were randomly allocated into two subgroups (1:1) to receive SC either 700 mg tezepelumab or placebo, Q4W for a 12-week treatment period [66]. Regarding the primary outcome, at week 12 tezepelumab did not significantly change PD15 in doubling doses (DD) from baseline, indicating no remarkable reduction in AHR to mannitol. However, the proportion of patients without AHR to mannitol (at week 12) was significantly greater [66]. Alongside this, tezepelumab led to a remarkable reduction in the number of eosinophils in the sub-epithelium, as well as in the BAL and in the number of airway tissue mast cells (secondary outcome) [66].
CASCADE (NCT03688074) was an exploratory, phase 2, randomized, double-blind, placebo-controlled trial, that evaluated how tezepelumab impacts inflammatory cells, remodeling, and hyperresponsiveness of the airways in patients with moderate-to-SUCA [67, 68]. Participants aged 18–75 years with moderate-to-SUCA under treatment with medium-to-high dose ICS with a minimum of one additional asthma controller medication, with or without OCS, and were randomized (1:1) to receive SC either 210 mg tezepelumab or placebo, Q4W, for 28 weeks [67]. The patients were sectioned into subgroups according to baseline BEC as follows: 26% had less than 150, 34% had 150 to less than 300, and 40% had 300 cells/μL or more [67]. The trial mainly investigated (primary endpoint) how the drug affected the levels of the airway submucosal inflammatory cells (i.e., T cells, mast cells, neutrophils, and eosinophils), compared with the baseline levels, at week 28, as observed in bronchoscopic biopsies [67]. Secondarily, it evaluated whether there was a reduction in the reticular basement membrane (RBM) thickening and whether epithelial integrity increased, by week 28 [67]. Another secondary objective was the exploration of the primary outcome measures in patients having any of the two endotypes (T2 high or T2 low). As for the primary objective, tezepelumab markedly reduced submucosal eosinophils versus placebo, regardless of baseline BEC [68]. Speaking of secondary outcomes, no significant differences were observed between the groups in RBM thickening and airway epithelial integrity. As mentioned in an exploratory analysis, AHR to mannitol was significantly higher reduced in the drug group, rather than in the placebo group [68].
As mentioned previously both UPSTREAM and CASCADE studies assessed the effectiveness of tezepelumab in AHR. Following that, a proteomics analysis elucidated the potential mechanisms of tezepelumab’s effects, by determining which proteins alter the drug in the serum of PATHWAY participants [69]. The analysis noted that at baseline, periostin and MMP-10 (which are indicators of matrix remodeling) were significantly related to BEC and FeNO levels [69]. Besides that, it was also noted that the levels of MMP-10 and periostin, IL-5Rα, pregnancy-associated plasma protein-A (PAPP-A, which are eosinophil-related), IgE, and thymus and activation regulated chemokine (TARC) proteins were remarkably reduced in tezepelumab group versus the placebo group, at week 52 [69]. These data suggest that anti-TSLP blockade has further mechanisms besides the impediment of airway inflammation. Exploratory findings of the NAVIGATOR study supported the previous ones, identifying a linear association between MMP-10, MMP-3, and BEC, as well as a significant decrease in MMP-10 and MMP-3 levels in tezepelumab arm compared to the placebo [70].
Impact on biomarkers
Regarding biomarkers in the PATHWAY trial, remarkable and enduring reductions in BEC and FeNO levels were observed from week 4 in all tezepelumab groups, after treatment initiation [57]. Decreases from baseline in BEC and FeNO levels were noticed by week 2 and maintained during the treatment duration in patients who received tezepelumab in the NAVIGATOR study [60]. In both PATHWAY and NAVIGATOR, total serum IgE gradually reduced in all drug treatment groups [57, 60]. The data mentioned above indicate that tezepelumab impacts the entire spectrum of asthma inflammation.
A post hoc analysis of the PATHWAY study revealed supplementary information on how biomarkers and AAER are related to each other [71]. This analysis indicated a positive correlation between the indicators of T2 inflammation (i.e., IL-5, IL-13, periostin, BEC, and FeNO) at baseline [71]. Additional results were the reduction of all biomarkers by baseline at week 52 in the tezepelumab versus the placebo group and a reduction by 55–83% in the AAER regardless of the baseline levels of BEC, FeNO or serum total IgE, IL-5, IL-13, periostin, TARC, and TSLP [71]. Furthermore, another post hoc analysis revealed that the baseline IL-5 and IL-13 were notably greater in patients with SUCA of the PATHWAY study than in the ones who participated in a separate cohort of age-matched healthy individuals [72]. This study also demonstrated that following treatment of 52 weeks, tezepelumab reduced IL-5 and IL-13 levels in patients having SUCA to the extent they were comparable to those observed in the healthy group [72]. As for NP profile and biomarker levels, participants treated with tezepelumab 210 mg demonstrated greater reductions in BEC and levels of FeNO, IL-5, and IL-13 than the ones treated with placebo, regardless of NP status [73]. In a post hoc analysis of the NAVIGATOR trial, the efficacy of tezepelumab on biomarker levels was assessed in groups of patients of different baseline biomarker levels [74]. Results showed that at week 52, tezepelumab reduced BEC, FeNO levels, and serum total IgE levels in those with elevated biomarkers at baseline, with more pronounced decreases observed in patients who initially had higher levels of the specific biomarker [74].
In a subgroup analysis of the SOURCE study, tezepelumab demonstrated a greater decrease in daily OCS dose in patients with baseline BEC of ≥ 150 cells/μL and ≥ 300 cells/μL, compared to the placebo group [65]. Conversely, in patient groups with ≤ 150 cells/μL and ≤ 300 cells/μL, a higher reduction was observed in the placebo group [65].
As discussed earlier, inflammatory biomarkers were additionally observed to decrease with the administration of tezepelumab in the CASCADE trial. Tezepelumab exhibited more substantial reductions compared to placebo in BEC, FeNO levels, IL-5, IL-13, and plasma eosinophil-derived neurotoxin at the end of the treatment period and during the follow up [68]. Similar results were revealed by the UPSTREAM study, as tezepelumab remarkably reduced FeNO and eosinophil levels in BAL, sputum, and blood, in comparison with the placebo [66]. All the data noted above, confirm the anti-inflammatory role of TSLP-blockade in patients with SUCA.
Safety of tezepelumab
Combined safety information regarding the utilization of tezepelumab in patients with SUCA was established by the polling analysis of NAVIGATOR and SOURCE trials, as well as the long-term-extension (LTE) DESTINATION [75] and the NOZOMI [76] study. According to pooling estimates, tezepelumab and placebo arm did not exhibit significant differences in the percentage of AEs during the treatment (75% and 77% respectively) [77]. Nasopharyngitis, upper respiratory tract infection, headache, and asthma, were the most common AEs in both groups [77]. Pharyngitis, arthralgia, and headache were more common in tezepelumab group with an incidence of 3% or above [78], while sinusitis and asthma were reported with greater frequency among the placebo patients [77]. In recipients with arthralgia, severity/intensity was mild or moderate in all the patients and one individual receiving tezepelumab withdrew from the study, due to arthralgia [77]. At baseline, daily OCS use was reported in 24% of patients receiving tezepelumab and 0% of those receiving placebo, which is a possible explanation for the higher incidence in tezepelumab arm [77]. Among patients with back pain, daily OCS use was similar in both groups, while severity/intensity was mild or moderate in all recipients. Only one patient who was receiving placebo reported severe back pain, even though the study drug was not halted [77]. Overall, the proportions of patients reporting serious AEs (SAEs) during the treatment, were reported for 9% and 13% in the tezepelumab and placebo groups, respectively, with only 0.8% versus 0.3% reporting cardiac SAEs, respectively [77]. Additionally, tezepelumab and placebo group presented with similar incidences of severe infection and neoplasms (benign and malignant) and there were no reported anaphylactic reactions related to tezepelumab [77]. 4% of patients in tezepelumab group and 3% in the placebo group experienced injection-site reactions. In general, the discontinuation rates of treatment were about 2% for the tezepelumab arm and 3% for the placebo arm (1% and 2%, respectively, owing to SAEs) [77]. As for deaths, none was reported during the treatment period, whereas two deaths were reported during the safety follow-up period [77].
DESTINATION (NCT03706079) was a phase 3, LTE study in adults (18–80 years old) and adolescents (12–17 years old) with SUCA who completed the treatment period of NAVIGATOR and SOURCE studies [75]. The main outcome of this trial was to evaluate the tolerability and long-term safety of them in a 104-week treatment period (including the treatment period of the previous study) [75]. A secondary objective was the assessment of the continuous influence of tezepelumab on AAER [75]. Regarding both primary and secondary outcomes, DESTINATION revealed consistent results with those from NAVIGATOR and SOURCE, after treatment with tezepelumab for 2 years, showing safety, as indicated by its minimal side effects, and efficacy, indicated by prolonged reduction in AAER irrespectively of baseline T2 inflammation biomarkers [79].
NOZOMI (NCT04048343) was a phase 3, open-labeled, and single-arm study that mainly assessed the safety and tolerability of tezepelumab in 65 Japanese patients [76]. Participants aged 12 to 80 years old, diagnosed with SUCA and using medium to high doses of ICS and a minimum of one supplementary controller medication, with or without OCS, received 210 mg SC tezepelumab, Q4W, for a 52-week period. Among these 65 patients, 60% experienced 94 different AEs, most of them identified as mild or moderate [76]. The dominant ones were nasopharyngitis, pharyngitis, back pain, herpes zoster, and upper respiratory tract inflammation with an incidence of > 3%. Two individuals underwent AEs deemed to be drug related (injection site erythema of mild intensity) and four noted one SAE (atrial fibrillation, viral gastroenteritis, lung abscess, tonsilitis) [76]. All the SAEs were unrelated to the drug and did not lead to a fatal outcome. During the study, no cases of immune complex disease, anaphylactic reactions, or hypersensitivity were recorded [76]. In terms of exploratory outcomes, tezepelumab was associated with a low AAER (0.11/patient-year), a mean change of 0.075 L in pre-bronchodilator FEV1, and a change in ACQ-6 score of −0.98. It is worth mentioning that tezepelumab demonstrated safety and tolerability in patients with T2 low inflammation at baseline, who constituted 60% of the patients in the NOZOMI study [76].
Mechanistic studies evaluating the efficacy, safety, and tolerability of tezepelumab in asthma are shown in Table 3.
Tezepelumab clinical trials in asthma
| Phase study nameNCT number | Design | Patient populationKey inclusion criteria (N) | Treatment dose | Primary objective |
|---|---|---|---|---|
| Phase 2PATHWAYNCT02054130 | Randomized, placebo-controlled, double-blind, multiple-dose trial to evaluate safety and efficacy | Adults with severe, uncontrolled asthma and a history of exacerbations during the year prior to trial entry (550) | 70 mg or 210 mg SC Q4W or 280 mg SC or placebo Q2W | Assess the effect of tezepelumab on asthma exacerbations at week 52 [57] |
| Phase 3NAVIGATORNCT03347279 | Randomized, placebo-controlled, double-blind trial to evaluate efficacy and safety | Adults and adolescents with severe, uncontrolled asthma on medium- to high-dose ICS and ≥ 1 additional asthma controller medication with/without OCS (1,059) | 210 mg SC or placebo Q4W | Assess the effect of tezepelumab on asthma exacerbations at week 52 [60] |
| Phase 3SOURCENCT03406078 | Randomized, placebo-controlled, double-blind trial to evaluate efficacy and safety | Adults with OCS-dependent asthma; a stable daily dose of OCS 7.5 mg to 30 mg for ≥ 1 month; 35% with eosinophils ≥ 300 cells/µL (150) | 210 mg SC or placebo Q4W | Assess the effect of tezepelumab in reducing the prescribed OCS maintenance dose at week 48 while not losing asthma control [64, 65] |
| Phase 3DESTINATIONNCT03706079 | Randomized, placebo-controlled, double-blind extension trial to evaluate safety and tolerability | Adults and adolescents with severe, uncontrolled asthma; previously enrolled in NAVIGATOR or SOURCE trial | 210 mg SC or placebo Q4W | Assess a long-term safety and tolerability of tezepelumab over 104 weeks [75, 79] |
| Phase 2UPSTREAMNCT02698501 | Randomized, placebo-controlled, double-blind trial to evaluate AHR | Adults with uncontrolled asthma and AHR to inhale mannitol despite any stable dose of ICS (40) | 700 mg IV or placebo Q4W | Assess the effect of tezepelumab on AHR to mannitol at 12 weeks [66] |
| Phase 2CASCADENCT03688074 | Randomized, placebo-controlled, double-blind trial to evaluate airway inflammation | Adults with inadequately controlled moderate-to-severe asthma on ICS and ≥ 1 additional asthma controller (116) | 210 mg SC or placebo Q4W | Assess the effects of tezepelumab on change from baseline to week 28 in airway submucosal inflammatory cells [67, 68] |
| Phase 3NOZOMINCT04048343 | Open-label, single-arm trial to evaluate safety and tolerability | Adults and adolescents with severe uncontrolled asthma on medium- to high-dose ICS and ≥ 1 additional asthma controller, with/without OCS, and a history of ≥ 1 exacerbation during the year prior to trial entry (65) | 210 mg SC tezepelumab Q4W | Assess a long-term safety and tolerability of tezepelumab over 52 weeks [76] |
SC: subcutaneous; Q4W: every 4 weeks; ICS: inhaled corticosteroids; OCS: oral corticosteroids; AHR: airway hyperresponsiveness; IV: intravenous
Note. Adapted with permission from “Targeting TSLP in Asthma” by Parnes JR, Molfino NA, Colice G, Martin U, Corren J, Menzies-Gow A. J Asthma Allergy. 2022;15:749–65 (https://doi.org/10.2147/JAA.S275039). CC BY-NC.
Conclusions
Asthma is now considered as an umbrella diagnosis that consists of various phenotypes and distinct endotypes, all of them presenting with the same clinical manifestations [12]. Despite the improvement observed with biologic agents, some patients treated with them, particularly those lacking T2 inflammation, continue to experience symptoms and asthma exacerbations, indicating that they would possibly benefit from biologics targeting different parts of asthma pathophysiology. TSLP, as an upstream mediator, plays a pivotal role in the pathogenesis of asthma, by initiating and amplifying both T2 high inflammation [53–56] and T2 low inflammation [20, 39], involving in airway remodeling [48] and correlating with disease severity [36] and steroid resistance [40, 56]. Thus, targeting TSLP using an mAb, tezepelumab, has been studied by a plethora of research studies that have consistently validated tezepelumab’s safety [74–76] and effectiveness in reducing exacerbations, improving respiratory function, controlling symptoms [57, 62], biomarker levels [57, 60, 66, 68, 71–74], AHR, and remodeling [66–70]. These significant findings have been observed across various asthma phenotypes, encompassing eosinophil-low asthma [57, 61, 62, 68, 69]. Moreover, tezepelumab ranks first in the reduction of exacerbation rates among other biologics and its effects are evident not only in patients with high levels of inflammatory biomarkers at baseline but also in those with low levels. Thus, it is currently used for all patients with severe asthma, regardless of their endotype or phenotype. In conclusion, tezepelumab represents a well-tolerated and efficacious treatment that may address the requirements of patients with uncontrolled severe asthma, paving the way for personalized and effective asthma management strategies in the future. However, further research, including larger-scale investigations and head-to-head comparisons with other biologics, is warranted to fully elucidate the theoretical superiority of tezepelumab in asthma management.
Abbreviations
| AAER: | annualized asthma exacerbation rate |
| ACQ: | Asthma Control Questionnaire |
| AEs: | adverse events |
| AHR: | airway hyperresponsiveness |
| AQLQ: | Asthma Quality of Life Questionnaire |
| BAL: | bronchoalveolar lavage fluid |
| BEC: | blood eosinophil count |
| DCs: | dendritic cells |
| FeNO: | fraction of exhaled nitric oxide |
| FEV1: | forced expiratory volume in the 1st second |
| ICS: | inhaled corticosteroids |
| IgE: | immunoglobulin E |
| IL-4: | interleukin 4 |
| IL-4AR: | interleukin 4A receptor |
| ILC2s: | group 2 innate lymphoid cells |
| mAb: | monoclonal antibody |
| mOCS: | maintenance oral corticosteroids |
| NP: | nasal polyposis |
| OCS: | oral corticosteroids |
| Q4W: | every 4 weeks |
| SAEs: | serious adverse events |
| SC: | subcutaneous |
| SUCA: | severe uncontrolled asthma |
| T2: | type 2 |
| Th2: | T helper 2 |
| TSLP: | thymic stromal lymphopoietin |
| TSLPR: | thymic stromal lymphopoietin receptor |
Declarations
Author contributions
AK, GP, AB, NA, EK, ET, and MS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. NR: Conceptualization, Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
Nikoletta Rovina who is the Editorial Board Member and the Guest Editor of Exploration of Asthma & Allergy had no involvement in the decision-making or the review process of this manuscript. The other authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.