Abstract
Asthma is a prevalent chronic respiratory condition in children, often exacerbated by allergic reactions, with house dust mites (HDMs) being a significant trigger. Traditional asthma management primarily involves inhaled corticosteroids and bronchodilators, which do not address the underlying allergic mechanisms. Allergen immunotherapy, including subcutaneous and sublingual immunotherapy (SLIT), has emerged as a strategy to induce immune tolerance to allergens. This review evaluates the efficacy of HDM immunotherapy in paediatric asthma, focusing on reductions in asthma exacerbations, improvements in lung function, decreases in medication use, and enhancements in quality of life (QoL). The review highlights that both subcutaneous immunotherapy (SCIT) and SLIT significantly reduce asthma exacerbations in children, with SCIT showing superior efficacy in lung function improvement. Combination therapies, particularly SCIT with biologics, demonstrate enhanced outcomes, including exacerbations and medication use reductions. SCIT has also been shown to improve lung function more effectively than SLIT, particularly in children with high baseline levels of HDM-specific IgE. In terms of safety, both SCIT and SLIT are generally well-tolerated, though SCIT is associated with more localized and systemic reactions, which can be mitigated by combination with biologics. Furthermore, HDM immunotherapy significantly enhances the QoL in paediatric patients by reducing asthma symptoms and improving sleep, leading to long-lasting benefits. Despite these positive outcomes, there remain gaps in knowledge, particularly regarding the optimal duration of therapy and long-term effects post-treatment. Future research should standardize treatment protocols, explore personalized approaches, and investigate the long-term sustainability of treatment benefits to fully optimize the use of HDM immunotherapy in paediatric asthma.
Keywords
Paediatrics, asthma, immunotherapy, house dust mite (HDM), subcutaneous immunotherapy (SCIT), sublingual immunotherapy (SLIT), quality of life, adverse effectsIntroduction
Asthma is a prevalent chronic respiratory condition affecting millions of children worldwide. It is often exacerbated by allergic reactions, with house dust mites (HDMs) being one of the most common triggers [1]. Traditional management of asthma includes the use of inhaled corticosteroids (ICS) and bronchodilators to control symptoms and prevent exacerbations [2]. However, these treatments do not modify the underlying allergic mechanisms. Allergen immunotherapy (AIT), specifically subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT), has been developed to address this gap by inducing immune tolerance to allergens [3].
Allergic diseases are among the most common chronic conditions worldwide, affecting millions of individuals and posing a significant public health burden [4]. Over the past few decades, there has been a remarkable increase in the prevalence of allergic disorders, including asthma, allergic rhinitis, atopic dermatitis, and food allergies [5]. This trend is particularly pronounced in urbanized and industrialized regions, where lifestyle and environmental changes contribute to heightened exposure to allergens and pollutants [6]. Consequently, the demand for effective and innovative treatments has surged, driving extensive research into the pathophysiology of allergic diseases and the development of novel therapeutic strategies [7].
Asthma exacerbations pose a significant health burden, particularly among paediatric populations, leading to increased healthcare utilization, diminished quality of life (QoL), and potential mortality [8]. Asthma imposes significant burdens including high healthcare utilization, socioeconomic costs, QoL impacts, psychological distress, adverse steroid effects, school absenteeism, physical activity limitations, and the need for intensive medical interventions [9–11]. Children with poorly controlled asthma incur significantly higher healthcare costs, with mean annual total asthma costs being more than twice as high compared to those with better-controlled asthma [12].
HDM allergy is a leading cause of atopic asthma exacerbations, affecting up to 50% of asthma sufferers [13]. HDM-induced allergic asthma significantly disrupts children’s lives, leading to school absenteeism and affecting caregivers’ activities. The Global Initiative for Asthma (GINA) guidelines 2023 suggest HDM immunotherapy for adult and adolescent asthmatics (steps 2–4) with positive sensitization but do not recommend it for children due to limited clinical evidence [14]. The 2019 European Academy of Allergy and Clinical Immunology (EAACI) guideline recommended HDM-SLIT for children with controlled asthma as an adjunct, but the evidence quality for symptom reduction and decreased medication use was poor [15].
Based on EAACI guideline 2019, the diagnosis of HDM-driven asthma relies on confirming HDM sensitization (via skin prick testing or specific IgE) alongside a clinical history of asthma exacerbations linked to HDM exposure [15]. HDM provocation testing may further support diagnostic confirmation in selected cases [15]. HDM immunotherapy is typically considered from around age 5; however, selected children aged 2–5 years with clearly defined HDM-driven allergic rhinitis may also be suitable candidates [16].
Evidence on HDM avoidance strategies remains mixed across studies. However, the 2023 GINA guidelines suggest potential clinical benefits for children with HDM-sensitized asthma [14]. A randomized controlled trial (RCT) found that mite-impermeable bedding reduced asthma-related hospital visits but did not lower oral steroid use [17]. Additional strategies reported in the literature include regular hot washing of bedding, carpet removal, humidity control, and the use of high-efficiency particulate air (HEPA) filters [18].
While HDM-SLIT has shown benefits in reducing asthmatic symptoms, lung functions, and QoL, data from various RCTs are inconsistent. Most RCTs lack information on asthma exacerbation rates and primarily target mild-to-moderate asthma groups.
The current knowledge gaps in immunotherapy for HDM-induced asthma include insufficient high-quality evidence regarding its overall impact on asthma exacerbation rates, its efficacy in moderate, severe or uncontrolled asthma cases, and its cost-effectiveness compared to standard treatments. Additionally, there is limited data on the optimal duration of immunotherapy specifically for HDM asthma and the potential long-term effects post-treatment.
This review aims to evaluate the evidence regarding the efficacy of HDM immunotherapy in paediatric asthma, focusing on improvements in asthma exacerbations, lung function, and medication use. Given the current gaps in knowledge, conducting a thorough review is essential to consolidate existing data and identify areas requiring further research. This approach will help to clarify the potential benefits of HDM immunotherapy and guide future clinical practices and research directions in managing paediatric asthma.
Methodology
A comprehensive literature search was conducted using medical databases such as PubMed, Ovid Medline, CINAHL Ultimate, and Embase to identify studies assessing the impact of HDM immunotherapy on paediatric asthma. The inclusion criteria were RCTs, observational studies, and meta-analyses published in peer-reviewed journals. The primary outcomes of interest were the frequency of asthma exacerbations, improvements in lung function tests, annual exacerbation rates, and changes in medication use. Data were extracted and synthesized to provide an overview of the current evidence.
Efficacy of SCIT and SLIT
Asthma exacerbations
The findings from various studies on HDM immunotherapy provide compelling evidence for its efficacy in reducing asthma exacerbations, particularly in paediatric patients (Figure 1). SCIT and SLIT have both been shown to significantly decrease the frequency of asthma exacerbations, which are critical events in the management of asthma. For example, the study by Berce et al. [19] (2024) found that after three years of treatment, the median number of asthma exacerbations dropped from 2 to 0 in children receiving SCIT and from 1 to 0 in those receiving SLIT. This reduction highlights the potential of HDM immunotherapy to provide substantial improvements in clinical outcomes for children with asthma [19]. However, it is important to note that the study by Berce et al. [19] was not placebo-controlled, limiting the strength of the evidence.
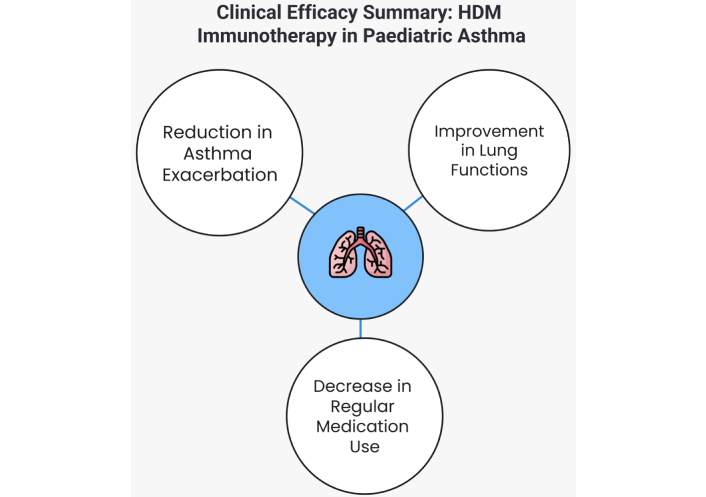
Clinical efficacy summary of house dust mite (HDM) immunotherapy in paediatric asthma
Moreover, the combination of SCIT with biologic agents like omalizumab has shown even greater efficacy. Long et al. [20] (2024) reported that adding omalizumab to SCIT resulted in a more significant reduction in asthma exacerbations compared to SCIT alone. However, the study lacked a placebo control and was limited to a 48-week treatment duration, which may constrain the generalizability of its findings. This suggests a potential synergistic effect, where the biologic’s ability to target IgE-mediated pathways enhances the immunomodulatory impact of SCIT, leading to better control over asthma symptoms and fewer exacerbations. This combination therapy could be particularly beneficial for patients with severe asthma or those who do not respond adequately to immunotherapy alone.
There is a lack of studies directly comparing omalizumab alone versus its combination with HDM-SCIT in paediatric populations. In an adult study, Bożek et al. [21] (2023) reported a significant reduction in annual asthma exacerbation rates across all treatment groups—omalizumab alone, HDM-SCIT alone, and the combination—with the greatest reduction observed in the combination group. In clinical practice, omalizumab is frequently used alongside SCIT to mitigate the risk of adverse reactions [22].
Long-term benefits of HDM immunotherapy are also supported by studies like that of Nogami et al. [23] (2022), which showed that children who received SCIT had sustained reductions in annual exacerbation rates over time. This finding is particularly important as it suggests that HDM immunotherapy contributes to long-term disease modification, potentially reducing the overall burden of asthma as children grow older. This study is also limited by small sample size, lack of a control group, follow-up period likely too short to assess long-term lung decline, and variability in lung function classifications.
Finally, a meta-analysis by Zheng et al. [24] (2023) further reinforced the effectiveness of SCIT in reducing asthma exacerbations by pooling data from multiple studies. The meta-analysis provided evidence that SCIT consistently lowers the frequency of exacerbations across diverse paediatric populations, adding weight to the argument for its widespread use in clinical practice [24]. However, In Zheng et al. [24]’s meta-analysis, only five of the 21 trials assessing the efficacy of SCIT in children with HDM-induced asthma were double-blind, placebo-controlled (DBPC), limiting the strength of causal inferences. While the pooled data from all RCTs showed significant reductions in short-term asthma symptoms [standardised mean difference (SMD) –1.19; 95% CI: –1.87, –0.50] and medication scores (SMD –1.04; 95% CI: –1.54, –0.54), the analysis did not stratify outcomes based on placebo versus non-placebo designs. Notably, no long-term efficacy data (≥ 1 year post-treatment) were available.
In the recent Mite Asthma Paediatric Immunotherapy Trial (MAPIT), HDM-SLIT tablets did not significantly reduce annual asthma exacerbations (0.18 per year) compared to placebo (0.21 per year) in paediatric cohorts aged 5 to 17 years, nor did they influence nocturnal awakenings requiring short acting beta agonist (SABA) use [25]. However, these results may have been impacted by the COVID-19 pandemic, which was associated with a general decrease in asthma exacerbations across the UK, potentially affecting the baseline rates in the study.
The EfficAPSI real-world study of over 445,000 allergic rhinitis patients found that personalized SLIT-liquid significantly reduced asthma onset and progression [26]. Compared to symptomatic treatment, SLIT-liquid lowered new asthma event risk by 36%, prevented asthma onset by one-third in those without prior asthma, and reduced disease worsening by a third in those with existing asthma, while increasing the likelihood of treatment step-down.
Lung function tests
The efficacy of HDM immunotherapy in improving lung function in paediatric asthma has been well-documented, particularly with SCIT (Figure 1). According to Berce et al. [19] (2024), SCIT significantly enhanced lung function, evidenced by an 8% increase in forced expiratory volume in one second (FEV1) after three years of treatment in children with HDM-induced asthma. In contrast, SLIT did not demonstrate a significant improvement, with a slight decrease in FEV1 by 1%, suggesting that SCIT may be more effective in this regard. Additionally, Long et al. [20] (2024) found that combining SCIT with omalizumab resulted in even greater improvements in lung function compared to SCIT alone, indicating that biologics can potentiate the benefits of immunotherapy by better-controlling airway inflammation.
Further supporting these findings, Nogami et al. [23] (2022) highlighted the long-term benefits of HDM-SCIT on lung function, particularly in paediatric patients with higher baseline levels of HDM-specific IgE and eosinophil counts. These subgroups experienced sustained improvements in lung function, suggesting that certain patients may derive more significant benefits from SCIT based on their immunological profile [23]. Zheng et al. [24] (2023) also provided a broader perspective through a meta-analysis, confirming that SCIT generally leads to improvements in lung function, as measured by FEV1 and peak expiratory flow (PEF), although the degree of improvement can vary across different studies. This meta-analysis reinforces the overall efficacy of SCIT in enhancing lung function in children with HDM-induced asthma, further validating its role as a key therapeutic option in managing paediatric asthma. While SLIT remains beneficial in other aspects of asthma management, its impact on lung function appears to be less pronounced compared to SCIT [24].
Impact on maintenance therapy
The efficacy of HDM immunotherapy in reducing medication needs in paediatric asthma has been supported by several studies, particularly in the context of both SCIT and SLIT (Figure 1). For instance, Berce et al. [19] (2024) found that after three years of treatment, daily controller therapy could be withdrawn or significantly reduced in 56.3% of children receiving SCIT and 65.6% of those receiving SLIT. This reduction in medication reliance indicates that both forms of immunotherapy can lead to sustained improvements in asthma control, allowing patients to reduce their dependence on ICS and other asthma medications [19].
In the phase III MATIC study, one year of HDM-SLIT treatment led to significant improvements in asthma outcomes compared to placebo, including reduced daily symptom scores, fewer nocturnal awakenings, decreased weekly SABA use, and an increase in SABA-free days [27].
Further evidence from an adult study, Bozek et al. [28] (2024) showed that combining SCIT with omalizumab, a biologic agent, resulted in an even more pronounced reduction in medication use compared to SCIT alone. This study highlighted that the combination therapy may potentially allow a more significant decrease in daily ICS doses, with some patients able to discontinue ICS entirely [28]. However, further evidence is needed to confirm these findings in the paediatric population.
Moreover, a real-world study by Jutel et al. [29] (2024) reinforced these findings by showing that SCIT significantly reduced the use of asthma medications, including both ICS and bronchodilators, throughout treatment.
Safety and adverse effects
The safety and adverse effects of HDM immunotherapy in paediatric asthma have been extensively studied, with both SCIT and SLIT generally being well-tolerated by children. According to Tempels-Pavlica et al. [30] (2024), SLIT, particularly in the form of HDM tablets, has a favourable safety profile, with the most common adverse effects being mild and including symptoms such as oral itching or mild gastrointestinal discomfort. These side effects are typically transient and do not necessitate discontinuation of therapy, making SLIT a suitable option for children, especially those with a lower tolerance for injections.
SCIT, while effective, is associated with a higher incidence of localized reactions, such as redness and swelling at the injection site, as noted in the study by Bulbul and Nursoy [31] (2024). Systemic reactions, although rare, can occur and include symptoms such as generalized urticaria, mild asthma exacerbations, or in very rare cases, anaphylaxis [32]. These reactions are generally manageable with appropriate pre-treatment precautions and monitoring during and after administration. The study emphasized the importance of identifying patients at higher risk for systemic reactions, such as those with high baseline IgE levels or a history of multiple allergies, to enhance safety during SCIT [32]. Currently, evidence remains limited regarding the identification of risk factors for systemic reactions in paediatric populations undergoing AIT.
Additionally, the combination of SCIT with biologics like omalizumab has been shown to mitigate some of the adverse effects associated with immunotherapy. For instance, Long et al. [20] (2024) reported that the addition of omalizumab to SCIT reduced the incidence of systemic reactions, potentially making the treatment safer for paediatric patients with more severe or complex asthma profiles. This combination approach highlights the potential for biologics to enhance the safety and tolerability of HDM immunotherapy.
In summary, HDM immunotherapy, both SCIT and SLIT, is safe and generally well-tolerated in paediatric asthma patients, with most adverse effects being mild and manageable (Figure 2). However, careful patient selection, ongoing monitoring, and the use of combination therapies with biologics can further enhance safety in some evidence, making HDM immunotherapy a potential and effective option for managing paediatric asthma. As always, the benefits of therapy must be weighed against the potential risks, particularly in children with more severe asthma or multiple allergies.
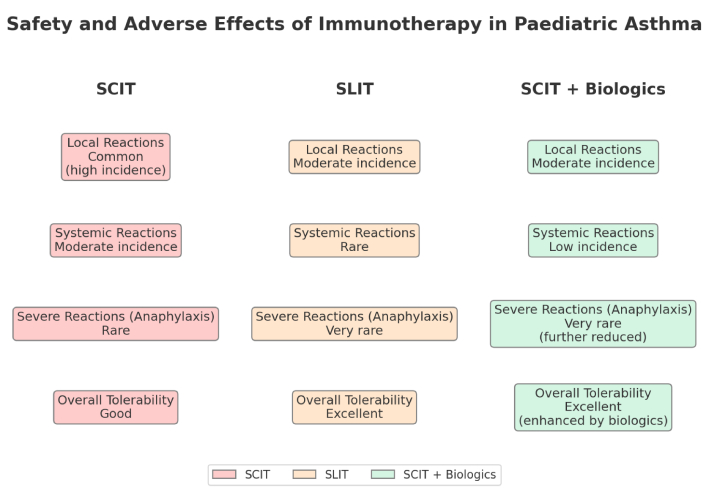
Safety and adverse effects of immunotherapy in paediatric asthma. SCIT: subcutaneous immunotherapy; SLIT: sublingual immunotherapy
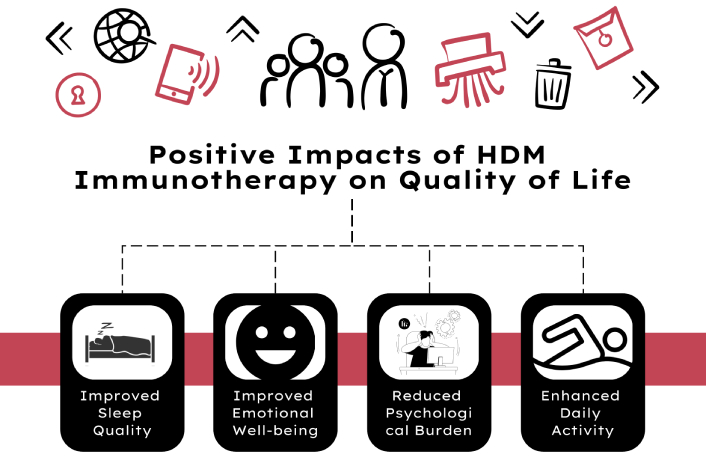
Quality of life
The impact of HDM immunotherapy on the QoL in paediatric asthma patients has been positively highlighted in several studies, showing substantial improvements in various aspects of daily living. Trusova et al. [32] (2023) conducted a comprehensive study over five years, which included three years of HDM subcutaneous SCIT followed by a two-year follow-up. The study demonstrated that children undergoing SCIT experienced significant improvements in their overall QoL, particularly in reducing the impact of asthma symptoms on their daily activities and emotional well-being. These improvements were sustained even one year after the completion of the treatment, indicating the long-lasting benefits of immunotherapy in enhancing the QoL for these patients [32].
Moreover, Demoly et al. [26] (2024) reported that HDM-SLIT tablets significantly improved not only asthma symptoms but also associated issues such as sleep quality, which is a crucial component of overall QoL. The study highlighted that better control of allergic symptoms led to fewer night-time awakenings and more restful sleep, contributing to improved daytime functioning and emotional health in children [26].
Further evidence from the QUALI study (Garrido-Fernández et al. [33], 2024) supports these findings, indicating that adherence to SLIT over a minimum of six months led to marked improvements in the QoL of children with HDM-induced asthma. This study emphasized that consistent use of SLIT not only reduced the physical symptoms of asthma but also lessened the psychological burden on both children and their families, leading to a more significant overall enhancement in QoL [33].
In summary, HDM immunotherapy, through both SCIT and SLIT, has been shown to improve the QoL in paediatric asthma patients (Figure 3). These improvements encompass not only physical health, by reducing asthma symptoms and improving sleep, but also emotional and psychological well-being, thereby offering a holistic benefit to children suffering from asthma. The sustained effects of these therapies post-treatment further underscore their value in long-term asthma management, making them an essential component of paediatric asthma care.
Cost-effectiveness analysis
Farraia et al. [34] (2022) conducted a cost-effectiveness analysis of HDM AIT in children with allergic asthma using a 10-year Markov model based on the Portuguese healthcare system. Both SCIT and SLIT were found to be cost-effective compared to standard pharmacotherapy, with incremental cost-effectiveness ratios (ICERs) of €1,281 and €7,717 per quality-adjusted life year (QALY) gained, respectively—well below the national threshold of €18,482.80. SCIT demonstrated greater health gains and cost savings than SLIT, primarily due to lower acquisition costs and better adherence. These results highlight the economic and clinical value of HDM immunotherapy—particularly SCIT—in managing paediatric allergic asthma.
Current evidence on the optimal duration of immunotherapy
The duration of AIT, particularly for HDM-induced asthma, remains a topic of active research and debate. Traditionally, both SCIT and SLIT have been administered for periods ranging from three to five years, based on the premise that prolonged exposure is necessary to induce long-term immunological tolerance [35]. However, the evidence regarding the optimal duration for achieving sustained benefits and the mechanisms underlying these effects is still evolving.
A three-year course of immunotherapy is commonly recommended and has been supported by several studies. For instance, Trusova et al. [32] (2023) conducted a five-year prospective study, which included three years of HDM-SCIT followed by a two-year follow-up period. The study showed that children who completed the full three-year course experienced significant improvements in their QoL and asthma control, with these benefits persisting for at least one year after treatment cessation [32].
While the three-year duration is widely accepted, some studies suggest that extending the duration of treatment might offer additional benefits, particularly in more severe or highly sensitized patients. Nogami et al. [23] (2022) explored the long-term trajectory of lung function in children who underwent HDM-SCIT for more than three years. The study found that children who continued treatment beyond three years had more sustained improvements in lung function, particularly in those with high baseline levels of HDM-specific IgE and eosinophil counts [26]. This suggests that certain subgroups of patients may benefit from a longer duration of therapy, potentially leading to more durable remission of asthma symptoms.
However, a study by Stelmach et al. [36] (2012) compared the efficacy of 3-year versus 5-year HDM-SCIT and found no significant differences between the two groups.
Current evidence on combination therapy
Recent studies have highlighted the potential benefits of combining HDM immunotherapy with biologics, such as omalizumab, for the treatment of paediatric asthma [20]. This combination therapy has been shown in some studies to enhance asthma control more effectively than either treatment alone, particularly in reducing asthma exacerbations and improving lung function. For instance, patients receiving both SCIT and omalizumab experienced significant improvements in FEV1 and greater reductions in daily ICS use compared to those on SCIT alone [20]. These findings suggest that biologics may synergize with the immunomodulatory effects of HDM immunotherapy, leading to better clinical outcomes and a reduction in the need for daily controller medications, which is especially beneficial in specific paediatric populations.
However, a study of eighteen weeks of omalizumab plus tree pollen SCIT reduced symptoms in patients with seasonal allergic rhinitis and allergic asthma during treatment, with no sustained effect post-omalizumab [37]. A modest FEV1 improvement suggests potential long-term respiratory benefits warranting further investigation [37].
Moreover, the safety profile of this combination therapy appears favourable, with studies indicating no significant increase in adverse effects compared to immunotherapy alone. However, while the short-term results are promising, there remains a need for further long-term studies in combined therapy with HDM immunotherapy to fully assess the safety and durability of these benefits.
Future research
Long-term outcomes and durability of immunotherapy effects
While several studies have demonstrated the benefits of a three-year course of HDM immunotherapy, the long-term durability of these effects post-treatment remains inadequately explored. Current evidence suggests that benefits such as improved lung function, reduced asthma exacerbations, and decreased medication use can persist for some time after discontinuation of therapy. However, the duration of these benefits varies, and the factors influencing long-term remission need further investigation. Future research should focus on longitudinal studies that track paediatric patients for 5–10 years post-immunotherapy to better understand the longevity of treatment benefits. Additionally, identifying biomarkers or patient characteristics that predict long-term responders versus those who may relapse after treatment cessation is essential to optimize treatment outcomes.
Combination therapies
Recent studies have begun exploring the potential benefits of combining HDM immunotherapy with other treatments, such as biologics (e.g., omalizumab). These combinations may enhance efficacy, particularly in patients with more severe asthma or multiple allergies. To further this understanding, RCTs comparing the efficacy of HDM immunotherapy alone versus in combination with biologics in paediatric populations are necessary. Additionally, mechanistic studies that investigate how combination therapies alter the immune response compared to monotherapy could help identify the best candidates for such treatments, thus advancing the field of personalized asthma care.
Comparison of immunotherapy and prevention strategies
Comparative evidence between HDM immunotherapy and allergen avoidance is currently lacking but represents an important gap in paediatric asthma management. Immunotherapy targets long-term disease modification through immune tolerance, whereas allergen avoidance provides the reduction in allergen exposure. Direct comparisons evaluating combined approaches may determine their relative efficacy and identify which patients may benefit most from each approach. Incorporating allergen avoidance into clinical practice remains important as part of a comprehensive, individualized management plan for children with HDM-sensitized asthma.
Conclusions
HDM immunotherapy, both SCIT and SLIT, has demonstrated benefits in managing paediatric asthma by reducing exacerbations, improving lung function, decreasing medication use, and enhancing QoL. Despite these promising findings, there are limitations and biases in the current evidence that must be addressed through well-designed, long-term studies. Future research should focus on standardizing outcome measures, improving patient adherence, and exploring the impact of geographical and environmental factors on treatment efficacy. Overall, HDM immunotherapy represents a valuable addition to the management of paediatric asthma, offering a potential disease-modifying approach that goes beyond symptom control.
This comprehensive review synthesizes the current evidence on the efficacy and safety of HDM immunotherapy in paediatric asthma, highlighting key findings and identifying areas for future research.
Abbreviations
| AIT: | allergen immunotherapy |
| FEV1: | forced expiratory volume in one second |
| HDMs: | house dust mites |
| ICS: | inhaled corticosteroids |
| QoL: | quality of life |
| RCT: | randomized controlled trial |
| SABA: | short acting beta agonist |
| SCIT: | subcutaneous immunotherapy |
| SLIT: | sublingual immunotherapy |
Declarations
Author contributions
MLQ: Conceptualization, Investigation, Writing—original draft, Writing—review & editing.
Conflicts of interest
The author declares that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.