Abstract
In the context of severe asthma, one of the causes of poor disease control has been identified in mucus plugs, which are real obstacles to the physiological flow of air in the bronchi. This clinical case will present the efficacy of dupilumab, as measured by respiratory function and chest computed tomography (CT) imaging, in reducing mucus plugs with consequent improvement in symptoms and function.
Keywords
Mucus plug, severe asthma, dupilumab, anti IL-4R, remodeling, epitheliumIntroduction
Asthma is a common inflammatory airway disease, affecting more than 300 million people worldwide [1]. Its severe form was recorded in about 5% of the whole asthmatic population, nevertheless becoming a critical burden in terms of its impact on health, quality of life, and pharmacoeconomic [2]. For people with severe asthma, in the last decades, biological drugs were developed, marketed with encouraging results in terms of efficacy in reducing exacerbations and the use of oral corticosteroids (OCS), and in improving lung function and disease control, lastly, the challenging concept of clinical remission [3].
Recent studies have highlighted the growing importance of mucus plugs in asthma management, owing to their influence on respiratory function and pathogenesis [4–6].
Computed tomography (CT) has the potential to demonstrate significant changes in the extension of mucus plugs before and after treatment with biologic drugs, while spirometry allows simultaneous improvements in the respiratory function.
This case report aims at showing the effects on mucus plugs and lung function of biological switching in a patient with severe asthma.
Case report
We present the case of a 68-year-old man suffering from nasal polyps and has a late onset of severe asthma. He is a never smoker patient with a history of work-related exposure to pollutants for 35 years. Together with the nasal and bronchial pathology, the patient reports an intolerance to aspirin, a comorbidity often associated with the previous mentioned two pathologies [7]. In his past life symptoms more severely affected the upper airways, characterized by complex, debilitating chronic rhinosinusitis and nasal polyposis, necessitating four surgical interventions, the most recent in 2017. In 2009, the patient underwent fibrobronchoscopy for the presence of diffuse pulmonary nodulations, which were detected on a chest CT scan. These nodular formations were identified as non-neoplastic, but rather dense mucous lacunae not removable with broncholavage and chronically filling the patient’s bronchiectasis. In subsequent years, to control asthma symptoms, the patient was treated with the maximum dose of inhaled therapy, including inhaled corticosteroids (ICS), long-acting β2-adrenoceptor agonists (LABA), long-acting muscarinic antagonists (LAMA), and montelukast. Nevertheless, there was a need for systemic steroids, at least four times a year, to control respiratory symptoms and asthma exacerbations. In 2019, when the patient came to our attention, new functional tests, the search for exhaled nitric oxide, and a blood eosinophilic count (Table 1) were performed. In addition, given the presence of bronchiectasis repleted with mucus, what was necessary to rule out a form of allergic bronchopulmonary aspergillosis (ABPA) was done and, given the eosinophil levels (800 cells/μL), a parasitological examination of stools and their antibodies on blood were requested, all of which proved negative.
Variation of mucus plug score, lung function, and markers, in the year of dupilumab administration
| T0 | T6 | T12 | R2 | P-value | |
|---|---|---|---|---|---|
| Mucus plug score [8] | 15 | 14 | 11 | 0.92 | 0.18 |
| Lung function | |||||
| FEV1 % | 53 | 69 | 72 | 0.87 | 0.24 |
| FEV1 (L) | 1.62 | 2.02 | 2.03 | 0.77 | 0.32 |
| FVC % | 70 | 81 | 88 | 0.98 | 0.08 |
| FVC (L) | 2.78 | 3.08 | 3.24 | 0.97 | 0.11 |
| Markers | |||||
| FeNO | 57 | 9 | 8 | 0.77 | 0.32 |
| Eosinophils (μL) | 800 | 200 | 200 | 0.75 | 0.33 |
T0 means before dupilumab administrations, T6 after 6 months of therapy, T12 after one year of administration. P-value was calculated between T12 and T0 (statistical analysis used was linear regression done with Jamovi vers 2.6.23). FEV1: forced expiratory volume in one second; FeNO: fractional exhaled nitric oxide
A biologic for asthma was therefore added to the patient’s therapy from January 2020, with a sudden improvement of the patient’s symptoms and resulting in an excellent asthma control. The introduction of this drug precluded further disease exacerbations, which previously required steroid therapy, and allowed to obtain both a complete resumption of the sensation of smell and clinical remission according to Severe Asthma Network Italy (SANI) criteria [no exacerbations, no use of OCS, stability in forced expiratory volume in one second (FEV1), and Asthma Control Test (ACT) > 20] [3].
Unfortunately, in 2023, the patient complained of a reduction of nasal symptoms control and, after a multidisciplinary discussion with the otorhinolaryngologists, we decided to switch to dupilumab, aiming at a more complete coverage of nasal comorbidities. Before the administration of dupilumab, we performed a new lung CT scan to assess the status of bronchiectasis and the associated mucus plugs.
Just after the first administration, he reported a rapid improvement of nasal symptoms, associated with a complete control of asthma. After six months, we repeated lung functional tests and CT, which demonstrated an improvement in FEV1 and a contextual reduction of mucus plugs. Finally, at 12 months respiratory tests and CT revealed a stabilization of lung function and a further reduction of mucus plugs present at baseline according to Dunican et al. [8]’s criteria (Table 1 and Figure 1).
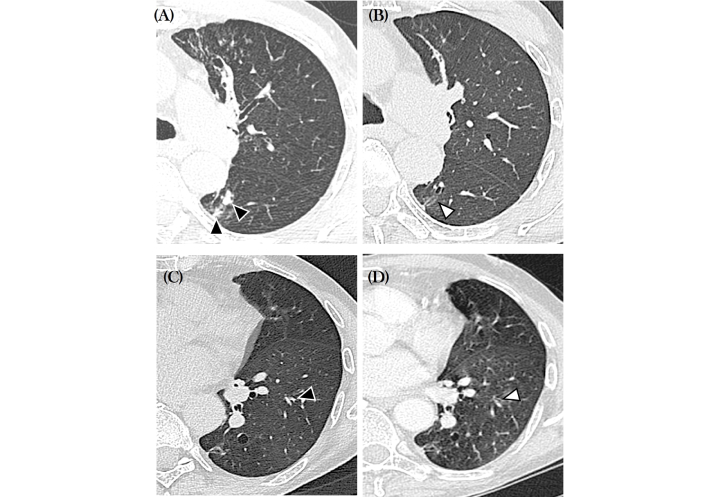
Axial computed tomography (CT) scans obtained at baseline (A and C) and six months (B and D). In A and C note mucus plugs in the bronchial divisions for the right upper lateral and left lower apical segments (black arrowheads). In B and D the mucus plugs have resolved (white arrowheads)
Currently, the patient is perfectly controlled both in nasal and asthma symptoms by its therapy, maintaining the remission criteria, as above described.
Discussion
More than 50% of patients with severe asthma have mucus plugs on chest CT scans, which are linked to airflow limitation [9]. Previous studies indicate that 82% of asthma patients with mucus plugs exhibit persistent mucus obstruction for over three years, and airway limitation may persist despite ICS and systemic steroid therapy. According to these studies, mucus plugs are one of the key therapy targets for severe asthma and have a detrimental long-term effect on severe asthma patients [9]. Findings from a prior investigation revealed that type 2 cytokines have a substantial correlation with mucus plug development. The crucial type 2 cytokine for the development of mucus is interleukin (IL)-13, which affects airway inflammation in severe asthma also in different ways. IL-13 increases the movement of thiocyanate into the airway lumen and stimulates the airway epithelium to release cysteine-rich MUC5AC mucin [9, 10]. Furthermore, the mucociliary transport in asthma is limited by the epithelial tethering of MUC5AC-rich mucus. In association with mucus plugs, patients also suffer from bronchiectasis, one of the comorbidities of severe asthma, able to make it more difficult to be treated [11–13]. Bronchiectasis themselves can be the cause of mucus plugs, regardless of whether asthma is present or not, facilitating bacterial proliferation, inflammation, and the development of new bronchiectasis [14–16]. In this case, bronchiectasis is a comorbidity of severe asthma and not a pathology in itself, but in the case of severe asthma is crucial to investigate them.
The case we present portrays, both from a functional and imaging point of view, the contemporary effect of dupilumab-mediated IL-4R and IL-13 inhibition on mucus plug production and respiratory function (Figure 2).
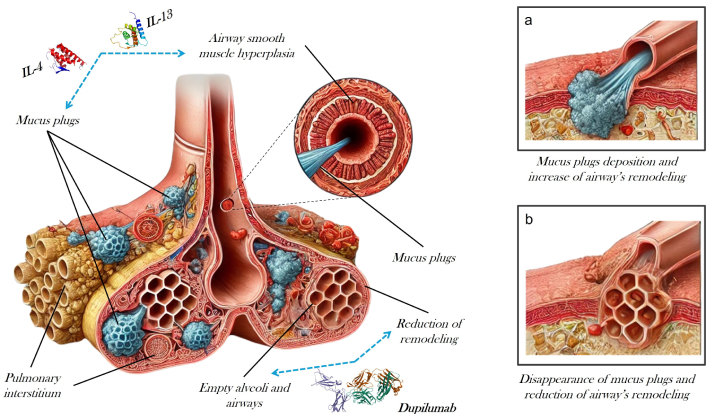
Bronchus before and after introduction in the therapy of anti interleukin (IL)-4R (dupilumab)
The formation of plugs, at the bronchial and bronchiolar level, has long been known and was extensively studied as a cause of acute and chronic disease in asthmatic patients [17]. The particularity of the case we presented concerns the efficacy of the drug on a patient chronically affected by the presence of plugs, which could not be removed, due to their density, during bronchoscopy, already undergoing treatment with another biologic drug, who at the switch managed to gain about 400 mL in FEV1 and to remove several of the plugs that had been obstructing the bronchi for long time. The effect of dupilumab on mucus plugs and lung function is well described in clinical trials, where the drug was administered to patients with moderate severe asthma [18]. Svenningsen et al. [18], demonstrating the drug’s efficacy, reported a 3-point reduction in CT mucus score after 16 weeks of treatment in patients receiving dupilumab 300 mg compared to a placebo-treated control group. Also, in “VESTIGE” trial, the efficacy of dupilumab in reducing mucus plugs, mucus volume, and airway inflammation in patients treated with the anti-IL-4R drug was demonstrated. Furthermore, as in our case report, in the above mentioned trial lung function improved, and this improvement was associated with a decrease in fractional exhaled nitric oxide (FeNO) levels [19], and was observed not only in FEV1 but also in resistance, reactance, indicating an effect in small airways [20]. It is well known that the improvement of FEV1 in asthmatic patients leads to a reduction in daily symptoms [21], exacerbations, and OCS use [22]. Given the current limitation of assessing airway remodeling in asthma through invasive biopsy, a non-routine procedure in patient management, changes in specific parameters, such as FEV1, are considered potential indicators of therapeutic effects on remodeling.
Dupilumab also, as in the case of our patient, demonstrated significant efficacy on nasal polyposis, both as a single disease and as a comorbidity of asthma. Indeed, the combined effect on IL-4 and IL-13 makes it possible to reduce the volume of polyps, demonstrating both in real life and in clinical trials that it acts effectively on this district [23–27].
In conclusion, our case report demonstrates in real life the efficacy of dupilumab treatment in the management of patients affected by severe asthma, low lung function, and mucus plugs at CT scan, suggesting that the drug must be considered in all patients but particularly in those with the described characteristics.
Abbreviations
| CT: | computed tomography |
| FEV1: | forced expiratory volume in one second |
| IL: | interleukin |
| OCS: | oral corticosteroids |
Declarations
Acknowledgments
During the preparation of this work, authors used ChatGPT for generating the bronchus picture of Figure 2, while the mucus plugs, muscle, and the remaining part of the picture were done by the authors themselves. After using the tool, authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Author contributions
DB, RP, and BB: Conceptualization, Investigation, Writing—original draft. VC, ET, and CM: Supervision, Validation. FB: Supervision, Validation, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
Diego Bagnasco who is the Editorial Board Member of Exploration of Asthma & Allergy, Fulvio Braido who is the Associate Editor of Exploration of Asthma & Allergy, had no involvement in the decision-making or the review process of this manuscript. The other authors declare that there are no conflicts of interest.
Ethical approval
The study was approved by the Genoa Ethics Committee (EC 2017, ID 3663) and complies with the Declaration of Helsinki.
Consent to participate
Informed consent to participate in the study was obtained from the patient.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
The data of this manuscript could be available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.