According to the definition established by biomaterial community, biomaterials are those materials of natural origin or synthetic, often made of multiple components that through their direct or indirect interactions with biological systems have the mission to augment, replace or/and modulate a natural body function [1]. Although the development of biomaterials is primarily for medical applications, they are also used in cells culture, processing cells and biomolecules in bioreactors, blood tests, gene and protein array-based diagnostic tools, cosmetics, food packaging, lab-on-a-chip devices, and most recently in addressing the challenges of COVID-19 through their use for the development of mRNA-based vaccines [2]. Yet, the advent of machine learning for data science-driven pathway such as ‘Biomaterialomics’ [3] and high-throughput methods for the discovery and investigation of biomaterials such as ‘Materiobiology’ [4] open also new and exciting horizons for the development of the new generation of biomaterials. This provides the biomaterials field with unique interdisciplinary and cross-disciplinary features at the edge of materials science, physics, chemistry, biochemistry, biology, engineering, and medicine. A google search indicated that in the last 20 years, over 50,000 articles, books, book chapters, and other types of communications have been dedicated to underline these unique features of biomaterial sciences and engineering (Figure 1A and 1B).
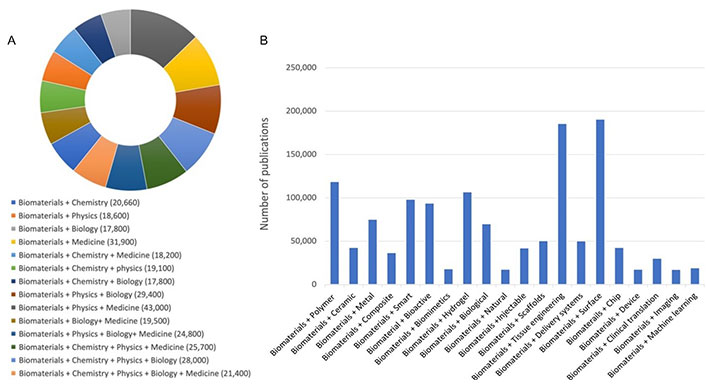
Google Scholar search (2003–2023). A. Showing the multidisciplinary and cross-disciplinary features of biomaterials; B. reporting on publications in main areas of biomat + X
Since the biomaterials have been established during the 1960s as a ‘discipline’ and as a ‘science’ standing on its own, there has been tremendous progress in the field [1, 5]. Particularly, a veritable boom in biomaterial research has occurred in the last 20 years thanks to progress in nano(bio)technology, pluripotent stem, and progenitor cell research along with 3-D and 4-D printing and micro and nanofabrication technologies (Figure 2A and 2B). However, we are still very far from the clinical translation of our research findings and data. For instance, among many tenths of thousands research articles in the field of nanoparticle-based drug delivery systems, only 5 types of nanoparticles based on iron oxide, hydroxyapatite, calcium phosphate, liposomes, pegylated proteins or enzymes have received the FDA approval [6, 7]. The future of the nanoparticle development is also hurdled by the demand for personalized and precision therapies and the need for tailored engineering design of nanoparticles for improved disease diagnosis and treatment specificity [8]. The same observation can be made for the development of biomaterials for tissue engineering. Although the field continues to evolve since the 1980s by creating complex organ tissues like heart, lung, and liver tissue in the lab, so far, the use of tissue engineering approaches plays a relatively small role in patient treatment. Artificial skin and cartilage, glycosaminoglycans, and polysiloxane, citrate-based polymers are a few examples of biomaterial-based engineered tissues that have been approved by the FDA [9].
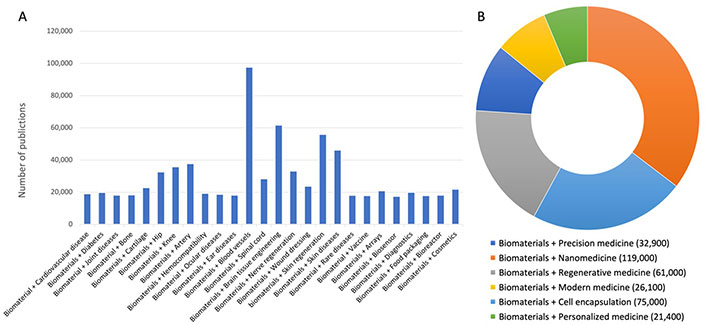
Google Scholar search (2003–2023). A. Presenting publications in the main field of applications of biomaterials; B. displaying the new challenges in applications of biomaterials
Multiple clinical trials are currently investigating acellular and cellular solutions for a variety of regenerative treatments [10]. However, the evidenced-based methodologies are urgently needed to translate such research data and results into validated scientific evidence [11].
The Exploration of BioMat-X has been launched in this context with the aim of covering the most cutting-edge research encompassing all these features in one place and in an accessible manner to the biomaterials’ community. We set our challenge to provide a new impetus to the breakthroughs in biomaterials science and engineering and implement the standards for the development of the next generation of medical devices and their clinical translation. The ‘X’ in the name of journal has purposely been chosen to indicate the endless possibilities arising from research opportunities in biomaterial fields. We thus welcome everyone interested in biomaterials to share their exciting scientific discoveries in all areas related to biomaterials, by submitting original research articles and reviews to Exploration of BioMat-X. Special attention will be paid to the research with translational components, as the challenge of the next decennium will be focused to respond to new requirements for biomaterials development and on the scalability of biomaterials’ community discoveries to commercially available therapies.
Declarations
Author contributions
The author contributed solely to the work.
Conflicts of interest
The author declares that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.