Abstract
The increasing number of prosthetic hip replacement surgeries and their growing indication have led to a growing interest in understanding the factors that influence their long-term success. Total hip arthroplasty (THA) failure is mainly due to aseptic loosening. More rarely septic mobilization may occur. In the first case, many variables influence the bone-implant relationship and periprosthetic bone remodeling. Stress-shielding is the most evident but not fully explained manifestation of the bone implant interaction. Recently, three-dimensional (3D) printed titanium orthopedic implants have offered new perspectives in the field of hip prosthetics, enabling the customization and production of acetabular cups with enhanced biocompatibility. This review aims to evaluate the efficacy and reliability of 3D printed acetabular cups from the perspective of aseptic failure particularly related to the stress-shielding. The most recent clinical and preclinical studies will be reviewed, exploring the benefits and challenges associated with the use of these emerging technologies. Key factors, such as biocompatibility, mechanical stability, osseointegration, and wear resistance.
Keywords
3D printed, acetabular cup, hip arthroplasty, stress-shielding, aseptic looseningIntroduction
Hip replacements are a mainstay of modern orthopedic surgery, the purpose of which is to restore joint function and eliminate pain, thus contributing decisively to the improvement of the quality of life [1]. As shown in the latest SIOT (Società Italiana Ortopedia e Traumatologia) congress, in the last 15 years in Italy the number of prosthetic implants has increased by 141% for hip ones and 226% for knee ones [2]. In addition, the demand has increased especially in subjects with high functional demand in fact, the improvement in the quality of materials, surgical techniques, anesthesiologic techniques, and general medicine have widened the range of implants. However, the long-term success of hip replacements is often undermined by septic and aseptic complications [2]. In particular, cementless acetabular cups have been utilized more and more commonly in total hip arthroplasty (THA) in recent years [3], bringing excellent results from a clinical point of view but every year many cup failures, especially aseptic cup loosening, occur and lead to intractable revisions and heavy medical burdens.
Thus, surgeons and engineers have been working on prosthetic designs and materials for many years to improve the acetabular cups’ primary mechanical stability and secondary bone fixation.
In recent years, three-dimensional (3D) printing, also known as “additive manufacturing” (AM) [4], of titanium orthopaedical implants has attracted a great deal of interest in the field of orthopedics, offering the possibility of customizing hip prostheses according to the anatomical needs and the specific mechanical and biological characteristics of each patient [5]. However, the impact of 3D printed acetabular cups on reducing stress-shielding and preventing aseptic failure remains a subject of intense research and debate.
This review aims to explore in depth the relationship between the phenomenon of stress-shielding of hip replacements and the use of 3D printed acetabular cups. We started by discussing the main forms of aseptic failure with a priority towards the phenomenon of stress-shielding especially referring to retroacetabular one. We analysed, in general, the 3D metal-printing process with its strengths and critical points, evaluating techniques and materials available, through an overview of the available evidence we analysed the possible practical and clinical applications of this emerging technology.
Aseptic failure
Aseptic loosening means the absence of cohesion, without signs of infection, of the bone-implant system due to a lack of initial osseointegration (primary instability) or loss of stability at a later stage (secondary instability), due to changes in the peri-prosthetic bone or prosthesis or both. That is a significant challenge in orthopedic surgery, with various causes and clinical implications. According to epidemiologic projections by Kurtz et al. [6], the number of primary and revision hip replacements in the United States is expected to increase significantly through 2030, highlighting the growing importance of understanding and effectively managing this complication.
Since the causes contributing to the failure of a prosthesis are multiple, various damage scenarios can be defined. Huiskes [7], in one of his articles, identifies five such scenarios.
Accumulated fatigue damage
A high dynamic load generates mechanical microdamage in the materials or at the bone-implant and implant-cement interfaces that gradually accumulate over time [8]. In particular, these depend on the stresses generated by load transfer and mechanical factors such as material properties.
Microdamage generates micromovements at the interface, disconnection of the implant from the bone and subsequent bone resorption with the formation of fibrous tissue.
They are responsible for the failure of cemented prostheses due to the mechanical properties of the cement. In fact, the high stresses to which the hip joint is subjected induce the formation and propagation of micro-cracks in the cement mantle.
Wear
The surfaces of the prosthetic components and the joint wear due to their relative movements. In this case, the joint is no longer able to support loads and loses its mechanical integrity. However, this problem is largely solved thanks to the innumerable studies carried out on the design materials, with which it is now possible to obtain joint replacement devices that last at least 10 years [9].
Particle reactions
These are caused by wear of the materials that make up the hip prosthesis. These include polyethylene particles that accumulate at the acetabular surface or debris that is abraded by the cement mantle at the implant-cement interface. This debris induces the activation of macrophages [10], which, however, are unable to eliminate the particles because they are too many or too large and, therefore, they fuse, giving rise to larger cells and generating inflammatory processes. These attract osteoclasts that cause, bone resorption, osteolysis, and implant mobilisation.
Failure of implant-bone bonding
The bone-prosthesis interface is not stable due to a lack of osseointegration and bone growth around the implant. When osseointegration is not effective, a layer of non-calcified fibrous tissue is interposed between the bone and the prosthesis, allowing elevated micro-movements at the bone-implant interface that can, in turn, cause aseptic dislocation or failure of the prosthesis. That scenario is typical of cementless THA in which the prosthesis fails to achieve its primary stability.
Stress-shielding
This is a phenomenon that determines bone resorption of the cortical bone around the stem of the prosthesis due to subnormal stresses.
More specifically, in any prosthetic replacement, the implant must be rigid enough to support the load but also to transfer it to the bone. Thus, the joint load, which before surgery was supported by the bone alone, is shared with the prosthesis.
The phenomenon of stress-shielding is dependent on purely mechanical factors such as the load acting on the joint, the bonding force at the interface, the mechanical characteristics of the bone, and the stiffness of the prosthesis.
In our review, we will focus on the correlation between this type of aseptic failure and the use of 3D printed prosthetic implants.
Stress-shielding
Stress-shielding occurs when natural mechanical stress is reduced around the implant of the prosthesis due to a nonphysiological load distribution, which can lead to a loss of bone density. This evidence was expressed by Julius Wolff in the 19th century: His law describes the capacity of healthy bone to adapt to variable loading conditions [11]. Currently, we have moved to the concept of “mechanosensation” introduced to indicate the ability of bone tissue to dynamically modify its morphology: New bone is added to withstand increased loads, whereas bone is resorbed when unloaded or disused [12].
Stress-shielding is mainly caused by the discrepancy between the mechanical properties of the prosthetic implant and those of the surrounding bone. Specifically, in hip prostheses, the metallic materials used such as titanium alloys or stainless steel have much greater stiffness than the surrounding bone. More specifically, cortical bone has an elastic modulus of 16 GPa, cancellous bone has an elastic modulus of 0.1 GPa, while Ti6Al4V alloy and CoCr alloy have an elastic modulus of 110 GPa and 230 GPa, respectively.
When the load is transmitted through the implant the underlying bone is no longer subjected to the same mechanical load to which it was accustomed before the surgery, and the result is a reduction of excessive stress on the bone, especially in cortical areas, which are particularly sensitive to mechanical stimulation. This leads to a decrease in bone density and a possible reduction in implant stability over time [13].
The consequences of stress-shielding are loss of bone density in the areas under less load leading to an increased risk of implant instability and possible periprosthetic fractures.
Retroacetabular stress-shielding
Previously, stress-shielding referred to bone mineral loss in the proximal femur with distally fixed stems. Now, it also considers the acetabulum exposed to press-fit device implantation. A decrease in bone mineral density (BMD) following press-fit fixation of acetabular implants has been described in a CT study. Wright and colleagues [14] propose that these changes represent a response to decreased regional bone stress due to the press-fit implant’s presence, termed retro-acetabular stress-shielding.
We can classify it according to various characteristics, namely:
Degree of retroacetabular bone density loss. The first level examinations for staging it are X-ray or CT. Currently, the most accurate examination is the dexa-scan (dual-energy X-ray absorptiometry). Wilkinson et al. [15] performed the first studies on acetabular periprosthetic BMD measurements. They concluded that a model that divided the retroacetabular space into four regions of interest (ROI), called “the Wilkinson regions”, as you can see in Figure 1 which was also described in the article by Wilkinson et al. [15], the regions of the pelvis and femur that correspond to the affected areas, offered the highest precision in BMD measurements.
Impact on the stability of the prosthetic implant by classifying it according to the presence or absence of signs of prosthetic instability, such as dislocation of the prosthesis, localised pain, laxity sensation, clonks, or abnormal noises during physical activity [16].
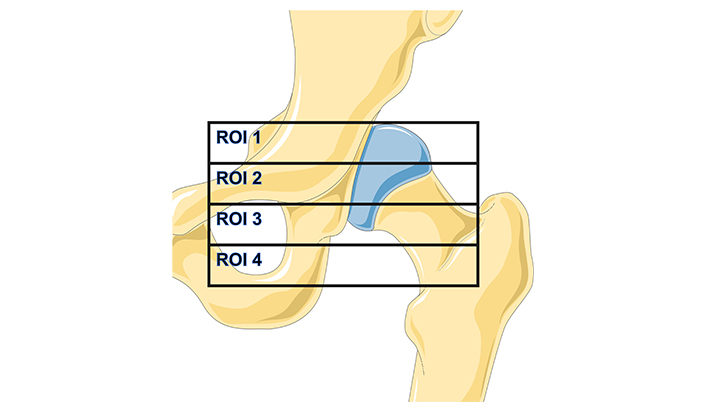
Representation of the modified Wilkinson regions of interest (ROI)
Note. The background image is adapted from Servier Medical Art, licensed under CC BY 4.0.
To reduce the risk of stress-shielding and its consequences, several approaches have been developed:
More bone-like materials: The use of prosthesis materials that have a more bone-like stiffness can help reduce stress-shielding. For example, prostheses made of ceramic or high-density polyethylene can provide better load distribution [17].
Implant design: Implant design can be optimized to reduce stress concentrated on specific areas of bone. This may include the design of more porous surfaces to promote bone engagement and even load distribution [18].
Tissue engineering treatments: Techniques such as electrical stimulation or the use of growth factors can be employed to promote bone regeneration and prevent loss of bone density around the implant.
As we will see below when analyzing the production procedure of 3D printed prosthetic components, it is precisely these first two characteristics that are sought after by this design and production technique.
3D printing manufacturing process, limitations, and risks
Manufacturing process
In recent years, advancements in technology have led to the adoption of 3D printing in the manufacturing of acetabular cups, offering several advantages over traditional manufacturing methods. Here are some key points about 3D printing acetabular cups:
Customization: One of the primary benefits of 3D printing is the ability to customize the design of acetabular cups according to patient-specific anatomical requirements. This customization can enhance the fit and stability of the implant, potentially leading to better patient outcomes and reduced risk of complications such as dislocation.
Complex geometries: 3D printing allows for the creation of complex geometries that may be challenging or impossible to achieve with traditional manufacturing techniques. This flexibility in design enables the optimization of structural integrity and biomechanical properties, leading to improved implant performance.
Material selection: 3D printing enables the use of a wide range of materials suitable for acetabular cup fabrication, including various metals, polymers, and ceramic composites. Material selection can be tailored to meet specific biomechanical requirements, such as strength, durability, and biocompatibility.
Porosity control: AM techniques offer precise control over the porosity of acetabular cups, allowing for the incorporation of porous structures that promote osseointegration, the process by which bone tissue grows into the implant surface. Enhanced osseointegration can improve implant stability and longevity.
Reduced lead times: 3D printing can streamline the manufacturing process, reducing lead times compared to traditional methods such as casting or machining. This rapid production capability can be particularly beneficial in cases where urgent or customized implants are required.
Research and development: 3D printing facilitates iterative design iterations and rapid prototyping, enabling researchers and manufacturers to quickly develop and evaluate new acetabular cup designs. This iterative process can lead to continuous improvement and innovation in implant technology.
Overall, 3D printing offers significant potential for enhancing the design, manufacturing, and performance of acetabular cups in THA. As the technology continues to evolve, it is expected to play an increasingly prominent role in the field of orthopedic implants [19].
Although 3D printing of metals, as AM is familiarly called, is still often seen by the general public as science fiction, it already started to reach an industrial scale.
Among many different technologies of AM, powder-bed technologies are the most widespread as they provide the highest resolution and so the widest options in the production of complex parts [20].
However, there are limitations and risks of defects that are unique to the 3D printing process, some of these can affect the orthopedic implants.
Specifically, in the manufacturing of orthopedic implants, the 3D printing, or AM, methods are classified as powder bed fusion (PBF) technologies, and two different processes fall under this category, depending on whether an energy source laser (SLM) or electron beam melting (EBM) selectively melts specific regions of a powder bed.
To better melt metals, SLM technology needs to use laser beams with high absorptivity of metals. Therefore, laser beams with shorter wavelengths such as Nd:YAG laser (1.064 μm) and fiber laser (1.09 μm) are generally used. The advantage is that SLM technology uses pure metal powder, and the density of formed metal parts can reach nearly 100%; mechanical properties such as tensile strength are better than castings, and even reach the level of forgings. SLM is able to print a wide range of metals, including most iron, aluminum, nickel, cobalt, and copper-based alloys.
On the other side EBM, is similar to SLM technology, except that EBM uses the kinetic energy of high-speed electron beam to convert into thermal energy as a heat source for metal melting, and the working environment is vacuum. Using electron beam as heat source can achieve higher melting temperature than laser, and the furnace power and heating speed can be adjusted. It can melt refractory metals and fuse different metals. EBM is suitable for a smaller group of raw materials, including Ti6AL4V (a titanium alloy), Inconel® 718 (a nickel alloy), or CoCrMo (a cobalt alloy).
The process of fabricating 3D printing metal implants can be divided into four parts:
Personalized acquisition of patient imaging data, such as CT or MRI.
The orthopedic surgeon and engineer use CAD software to create a data model of the implant based on the patient’s needs, which is converted into a series of 2D layer slices and saved as stereolithography (STL) data.
Computer-controlled fabrication of the appropriate 3D printing technology melts the metal material layer by layer to print the molded model.
Final finishing of the implant, such as grinding, coating, and surface oxidation techniques [5].
Limitations and potential risks of 3D printing
We can mention some of the limitations which can affect the mechanical properties of the final construct:
The presence of voids or pores: These defects can be manifested by the entrapment of gas pores or by the spherical voids that compromise the final object [21]. Voids with elongated shapes can also be generated if a lack of fusion between subsequent layers occurs (lack of fusion defects) [22].
Too low/high energy source: In the first case, the presence of solid powder particles partially attached to the surface of the built part doesn’t allow the completely molten of the powder, in the second one the molten pool can form small “islands” that generate particles (balling phenomenon) and a phenomenon of loss of alloying elements can also occur. This depends on the metal alloy and can cause modification in the alloy composition [23].
Cracking and delamination: These can occur due to solidification shrinkage (thermal contraction) of the structure, which subsequently generates tensile stresses that form cracks at the grain boundaries if the strength of the material is exceeded. When the residual stresses overcome the strength of the metal at the interface of subsequent layers, then a delamination phenomenon of these consecutive layers can occur.
In the final steps of the manufacturing process, cleaning all parts of the new object from the residual molten power is challenging, especially for medical implants such as complex porous structures [24].
Rationale for 3D printing in orthopedics
In recent years, AM has gained significant traction within the medical field, particularly in orthopedics, with rapidly evolving applications. Traditionally, metallic orthopedic implants are fabricated through subtractive machining, which involves the gradual removal of material from a solid metal block until the desired form is achieved. Another conventional method involves formative shaping, where mechanical forces are employed to mold raw material into the desired shape, akin to casting or forging. Currently, the predominant commercial activity in orthopedics revolves around standard-sized implants produced through these traditional methods.
Implants created via AM techniques, characterized by internally engineered porous architectures, not only enhance osseointegration but also reduce the stiffness mismatch between natural bone and the implant. This reduction in stiffness disparity may alleviate stress-shielding-induced bone resorption, thereby increasing the longevity of orthopedic implants. While porous materials demonstrated clinical efficacy prior to the advent of AM, this technological advancement allows for the integration of porous structures with diverse geometric complexities, including fine surface textures, interconnected internal porosities, and nonporous components, all within a single manufacturing process.
AM implants can be designed to match the specific geometry of bone and optimize the microscopic 3D structure with desired porosity and stiffness at surgical sites, facilitating bone ingrowth and long-term implant fixation. Notably, AM implants are typically employed as a one-time solution when standard off-the-shelf implants fail to meet surgical requirements, which can limit flexibility during the procedure.
Currently, clinical outcomes for AM implants are primarily evaluated through short-term, small-scale case series. Perticarini et al. [25] were among the pioneers in documenting the use of trabecular titanium acetabular cups in European patients with primary hip osteoarthritis (OA), avascular necrosis (AVN), and developmental dysplasia of the hip (DDH), reporting significant improvements in functional recovery and pain relief over a minimum five-year follow-up period for 133 patients. Castagnini et al. [26] compared the survival rates and reasons for revision between a specific off-the-shelf 3D printed design (Fixa Ti-Por; Adler Ortho, Milan, Italy) and other cementless cups used from July 2007 to December 2015, finding a higher survival rate (98.7% vs. 97.9%) and a lower incidence of aseptic loosening with the 3D printed socket. Geng et al. [27] reported a 99.1% cup survival rate and a 91.3% patient satisfaction rate, with excellent surgical safety and efficiency, no instances of cup loosening, and excellent bone ingrowth.
Beyond technical performance, the economic impact of AM implants is notable. Though comprehensive data on production costs are lacking, studies indicate that patients planned with 3D printed models experienced reduced operative times and shorter hospital stays compared to those planned with standard methods, leading to significant cost savings. In advanced procedures like revision arthroplasty for acetabular defects, the use of aMace implants demonstrated a cost-saving advantage.
Classification and analysis of 3D printed acetabular cups
Although most THA procedures still utilize conventionally manufactured cups, the use of 3D printed acetabular components is increasingly prevalent in both primary and revision hip surgeries. Presently, there are over fifteen different commercially available designs of 3D printed acetabular cups (Table 1), with a consistent increase since their initial release in 2007. By 2017, it was estimated that more than 60,000 acetabular cups produced via EBM technology had been implanted globally, and this number is expected to continue rising.
3D printed acetabular cup currently commercially available
| Company | Cup brand |
|---|---|
| Adler Ortho (Milan, IT) | Agilis Ti-Por®CustomFixa Ti-Por®Omnia Ti-Por®PolyMax Ti-Por® |
| Corin (Cirencester, UK) | TrinityTM Plus |
| Implantcast (Buxtehude, DE) | C-Fit 3D® (custom) |
| Lima Corporate (Udine, IT) | Delta TTDelta ONE TTDelta Revision TTPromade (custom) |
| Materialise (Leuven, BE) | aMace® (custom) |
| Medacta (Castel San Pietro, CH) | Mpact® |
| Smith&Nephew (Memphis, USA) | Redapt |
| Stryker (Mahwah, USA) | Trident® II |
| Zimmer Biomet (Warsaw, USA) | G7 |
Note. Adapted from “3D Printed Acetabular Cups for Total Hip Arthroplasty: A Review Article” by Dall’Ava L, Hothi H, Di Laura A, Henckel J, Hart A. Metals. 2019;9:729 (https://www.mdpi.com/2075-4701/9/7/729). CC BY.
Conclusions
Retroacetabular resorption following implantation of a total hip prosthesis remains a major cause of aseptic failure in hip prosthetics. The advent of 3D printing has transformed the production of customized orthopedic implants, with potential implications for standardized implants as well. The aim of our study was to evaluate the advantage of using 3D-printed cups for hip prosthetics in preventing stress-shielding. We have mentioned various studies in which results emerge in relation to reduced rates of loosening, stress-shielding, and the improvement of rating scales such as the Harris Hip Score (HHS) and Western Ontario and McMaster Universities Arthritis Index (WOMAC), both in absolute terms for 3D-printed cups and in comparison, with cups produced using the standard technique. Furthermore, we have shown how a reduction in hospitalisation costs can be achieved, and another key point is that their use also finds a very valid rationale in hip revision prosthetics.
However, it is important to note some limitations of these innovative techniques: All these reports were based on short- to mid-term follow-up. Long-term follow-up studies have not been published yet [28], the sample size and the diversity among the implants analyzed and compared are not adequate, and the available number of studies is not adequate to ensure absolute certainty regarding biological complications such as periprosthetic infections and inflammation of soft tissues.
In conclusion, we can say that hip replacement cups produced with AM technology are a highly valuable tool for the orthopedic surgeon, which will easily find more use although there is a need to carry on research to fully know their advantages and limitations and to be able to standardize their use in surgical practice.
Abbreviations
| 3D: | three dimensional |
| AM: | additive manufacturing |
| BMD: | bone mineral density |
| EBM: | electron beam melting |
| SLM: | source laser |
| THA: | total hip arthroplasty |
Declarations
Author contributions
AB: Conceptualization, Investigation, Writing—original draft. GTG: Investigation, Writing—original draft, Writing—review & editing. AZ: Conceptualization, Validation, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.