Affiliation:
1Department of Tissue Engineering, Hamidiye Institute of Health Sciences, University of Health Sciences Turkey, 34668 Istanbul, Turkey
ORCID: https://orcid.org/0000-0003-2612-3554
Affiliation:
1Department of Tissue Engineering, Hamidiye Institute of Health Sciences, University of Health Sciences Turkey, 34668 Istanbul, Turkey
ORCID: https://orcid.org/0000-0003-0437-7705
Affiliation:
1Department of Tissue Engineering, Hamidiye Institute of Health Sciences, University of Health Sciences Turkey, 34668 Istanbul, Turkey
2Experimental Medicine Research and Application Center, University of Health Sciences Turkey, 34668 Istanbul, Turkey
3Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, University of Health Sciences Turkey, 34668 Istanbul, Turkey
Email: erkanturker.baran@sbu.edu.tr
ORCID: https://orcid.org/0000-0002-0563-6943
Explor BioMat-X. 2025;2:101331 DOI: https://doi.org/10.37349/ebmx.2025.101331
Received: December 05, 2024 Accepted: February 10, 2025 Published: February 27, 2025
Academic Editor: Feng Chen, Zhejiang University of Technology, China
The article belongs to the special issue Trends in Biomaterials Research for Cardiovascular Applications
A potential solution for prosthetic heart valves is tissue-engineered heart valves. Tissue-engineered heart valves (TEHVs) are designed to replicate the complex properties found in natural tissues, such as stiffness, anisotropy, and composition and organization of cells and extracellular matrix (ECM). Electrospinning is regarded as a highly versatile and innovative approach for fabricating numerous fibrous designs. In this review, we discuss recent developments in electrospun heart valve scaffolds, including scaffold materials, cell types, and electrospinning setups used to prepare aligned nanofibers. Despite the fact that natural biomaterials provided excellent biocompatibility, nanofibers from synthetic materials provided the required mechanical compatibility. Accordingly, most studies highlighted the benefits of designing composite heart valves using biological and synthetic polymers. Various strategies, such as the application of motorized mandrel and micropatterned collector in electrospinning were effective in controlling nanofiber alignment. Studies also showed that aligned nanofiber’s mechanical strength and anisotropic structure promote cell proliferation, and differentiation, and promote attachment. Numerous studies have reported that multiple cell sources are suitable for producing heart valves. Successful results were obtained with human umbilical vein endothelial cells (HUVECs), since they provide a convenient cell source for cellularization of valve leaflets. A higher conductivity of scaffolds was achieved by using biomaterials that conduct electricity, such as polyaniline, polypyrrole, and carbon nanotubes, which resulted in better differentiation of precursor cells to cardiomyocytes and higher cell beating rates. In light of these attributes, nanofibrous scaffolds produced through electrospinning are expected to offer numerous advantages for tissue engineering and medical applications in the near future. However, multiple challenges were identified as cell infiltration and 2D nature of nanofiber mats necessitate further engineering approaches in electrospinning procedure leaflet production.
Calcification of the valve leaflet, endocarditis, and congenital valve anomalies are the main causes of heart valve dysfunction [1]. In pulmonary and systemic circulations, valves open in such a way that does not prevent flow to the ventricles under systolic pressure. Aortic valves (AVs) are exposed to shear stresses with high mechanical values due to the blood flowing through them in each cardiac cycle [2, 3]. All four types of heart valves are vulnerable to valve disease, but the most common are the AV and mitral valve (MV). Disorganization of the valve interstitial cells (VICs) and disruption of the ECM layer stratification are characteristics of diseased heart valves [4]. These structural defects have an impact on the function of heart valves. They become stenotic, restricting unilateral blood flow, affecting the function of the heart valves, and causing backflow of blood.
Anatomical, biomechanical, and structural properties of heart valves must be well understood in tissue engineering applications to mimic their function and physiology [5]. The heart is one of the most physiologically active organs in the human body. It nourishes every cell and removes waste products from metabolism by pumping blood that is rich in nutrients and oxygen in unidirectional throughout the body. There are four major chambers in the heart: The right and left atria are the upper chambers, and the right and left ventricles are the lower chambers [6]. The right ventricle receives blood from the atrium through the tricuspid valve. The tricuspid valve, also known as the pulmonary valve, regulates the flow of blood from the right ventricle into the pulmonary arteries and then into the lungs, where it is oxygenated again. Nutrient-rich blood enters the left atrium by the four pulmonary veins and exits the left ventricle via the left MV, also known as the bicuspid valve. The left ventricle pumps nutrient-rich blood through the AV (tricuspid valve) to the aorta and then to the whole body [7]. The heart valve opens and closes approximately 40 million times annually, and more than three billion times during a normal life span of 75 years [8]. The heart valve is an essential dynamic functional component that ensures unidirectional and unimpeded blood flow. The complexity of the macro- and micro-structure of heart valves plays a major role in their ability to fulfill their important properties [9]. The cusps, commissures, and supporting structures in the aortic and pulmonary roots are crucial components of the trileaflet semilunar valves, which include the aortic and pulmonary valves. The main components of atrioventricular valves (tricuspid and MVs) are the leaflets, commissures, annulus, chordae tendineae, papillary muscles, and arterial and ventricular myocardium [10, 11]. The heart valve leaflets are exposed to mechanical stress in the forms of shear, bending, and tension [12]. Each heart valve has unique anatomical features to promote optimal performance, even though valves and atrioventricular valves have comparable microstructures.
The AV consists of three half-moon leaflets enclosed in a connective tissue sheath [13]. Heart valve leaflet tissue is complex and multilayered, approximately 300 to 700 µm thick. The AV consists of three semilunar leaflets contained within a connective tissue sheath. In cross-section, the leaflet has three distinct layers: fibrosa (~45%), spongiosa (~35%) and ventricularis (~20%) [14]. In particular, the biomechanical function is met by fibrosa, spongiosa and ventricularis. The fibrosa, as the first layer, contains dense collagen fibers with endothelial cells lining the surface. Tensile strength is provided by collagen fibers directed toward the periphery when the valve is closed [15, 16]. In addition, the aligned collagen fibers make fibrous the main load-bearing layer. Collagen fibers in valve bend under non-stressful conditions and straighten under pressure. At the closure phase of the valves, the pressure is approximately 80 mmHg. During that time, the valves extend themselves to prevent backflow leakage. Collagen matrix receives the greatest stress in this phase [17]. Collagen shows high resistance to this elevated pressure and, therefore, is the essential component of the heart in mechanical performance.
The middle layer of spongiosa, consisting of a fiberless gel-like layer, present in the middle of the leaflet. Also, proteoglycans, hydrated glycosaminoglycans (GAGs), VICs, and valvular endothelial cells (VECs) [18, 19] are primary components [20] of spongiosa. These properties produce heterogeneous mechanical forces upon the cells, while providing strength and flexibility in the leaflets upon tension. The last layer is ventricularis, which contains a dense layer of collagen and elastic fibers, lined with endothelial cells [21, 22]. The radially aligned elastin and collagen fibers provide flexibility in leaflet formation. Therefore, it can be deduced that the composition and alignment of collagen and elastin fibers play an important role in the strength and biomechanical behavior of heart valves [23].
The primary design objective for heart valve tissue engineering scaffolds is to mimic the structure and material characteristics of the natural AV, especially its anisotropy (Figure 1). It is crucial for developing tissue engineering constructs that replicate the structure of a natural leaflet in order to develop a heart valve. To enhance the anistropic feature of native mimicking structure, the electric conductive carbon fibers were previously used for cardiac tissue engineering [24]. Numerous biomedical fields, such as tissue engineering [25], drug delivery [26], wound healing [27], and regenerative medicine [28], have advanced significantly as a result of the versatility of electrospun polymer nanofibers. Recent advancements in electrospun heart valve scaffolds, in particular, scaffold types, cell types, electrospinning settings of aligned nanofibers, and tissue engineering applications are covered in this study.
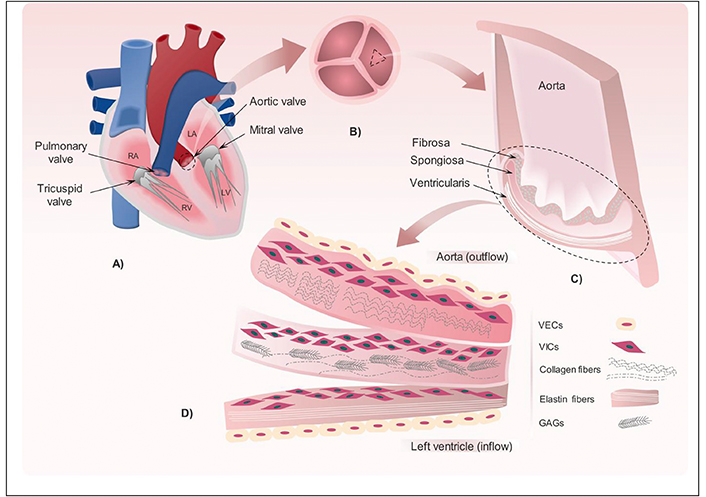
Representations of the various human body valves and the valve leaflet’s construction. (A) Four valves that present in the heart. (B) Overview of the three leaflets that compose the aortic valve (AV). (C) Cross section in one of the AV leaflets showing the three layers: fibrosa, spongiosa and ventricularis that compose the valve leaflet. (D) Detailed illustration for the key elements presents in fibrosa, spongiosa and ventricularis including valvular endothelial cells (VECs), valve interstitial cells (VICs), glycosaminoglycans (GAGs), radially aligned elastin fibers and circumferentially aligned collagen fibers. Reprinted from [29] with permission from Elsevier License number 5912680507723. © 2017 Elsevier Ltd.
The development of a scaffold that offers physiological support for cell adhesion, proliferation, and growth is necessary for heart valve tissue engineering. A heart valve’s architecture consists of two outer laminar anisotropic fiber layers layered on top of a spongy inner layer [30]. In an attempt to replicate the structure of the natural heart valve, several scaffold designs have been tested and proposed. For this objective, two primary scaffold types have been developed: (i) native heart valve scaffolds that have been decellularized (acellular) from allogeneic/xenogenic sources, and (ii) completely artificial scaffolds composed of natural (biological) and synthetic polymers [31].
Heart valves sourced from animals (xenografts) or obtained from human donors (allografts) represent some of the most apparent choices for scaffold materials [32]. The mechanical characteristics of decellularized allograft and xenograft heart valves are comparable to those of native tissue, and they include bioactive ligands that can encourage cell adhesion, making them preferred scaffolds [33]. Decellularization aims to remove donor cells and antigenic material while maintaining the integrity of the ECM. Several enzymatic or detergent-based techniques can be used to perform this procedure [34]. Once the valve is decellularized, cells can be reseeded onto the scaffold and recellularized in vitro in a bioreactor. The bioreactor system allows for sufficient movement of waste and nutrients and exerts dynamic stresses on the tissue [35, 36]. Due to the fact that dynamically cultivated valves have better mechanical qualities and deeper cell penetration than statically cultured valves, mechanical stimuli are necessary for appropriate tissue maturation [37].
In order to produce nanofibers, scientists have recently begun to employ natural biopolymers. These biopolymers include gelatin, collagen, silk fibroin, fibrinogen, chitosan, and chitin [38–43]. The use of biological polymers as starting matrices for scaffold materials in tissue engineering has been demonstrated as a useful strategy [44–46]. Some natural biopolymers used in tissue engineering are usually cell-synthesized ECM components, giving them better biocompatibility and micro-environment for tissue regeneration. As a result, these nanofibers have better cell adhesion and proliferation [47]. The ECM of the human body is mainly composed of fibrillar polymers such as collagen, GAGs, and other types of polysaccharides. In heart valve tissue engineering, biopolymers often used in the production of artificial ECM are collagen, gelatin, hyaluronate, GAGs, chitosan, alginate, silk fibroin, fibrin, dextran, and tumor ECM extracts (e.g., Matrigel®) [48]. Although pure biopolymers create challenges in electrospinning processing due to the insolubility of hydrophilic macromolecules in polar solvents, it would be useful to apply biopolymers as pure materials, blends, or co-spinning to increase biocompatibility.
Making the main organic component of the hard and soft tissues, collagen fibrils, and their networks of ECM. Collagen exhibits varying morphologies in many tissues and is need to maintain the biological and structural integrity of the ECM architecture. In addition, it undergoes continuous remodeling to ensure physiological functions and is highly dynamic [49–51]. In addition to being naturally present in the body, collagen is an ideal biomaterial for tissue engineering processes, with properties such as low antigenicity, low inflammatory and cytotoxic responses, high water affinity, good cytocompatibility, availability of various isolation methods from various sources, and biodegradability [52]. Collagen type 1 is the main component of the fibrous layer of native AVs [53]. Hydrogels of collagen with the cell and matrix fiber alignment ability have potential in tissue-engineered heart valve (TEHV). Shi and colleagues [54] produced highly aligned and compacted collagenous structures. MVs were developed using collagen-based matrix, which was produced by combining solubilized collagen with neonatal rat aortic smooth muscle cells (SMCs). When mechanical constraint was applied, the collagen fibrils aligned in the direction of the constraint. This increased cell content and stimulated their metabolism, which ultimately resulted in stronger structures. In another research, Brougham et al. [55] developed a tri-leaflet TEHV scaffold using collagen-GAG-fibrin to evaluate the contraction of the CG-fibrin scaffold (CGF) when seeded with human vascular SMCs. They argue that the CGF scaffold’s capacity to maintain structural integrity and sustain the contractile pressures of embedded cells provides it with a promising candidate for use as an HV scaffold.
Gelatin is a natural biopolymer that is most similar to collagen and has cellular compatibility, low toxicity, and low inflammatory potential [56]. As it is a solubilized and denatured form of collagen, gelatin contains an arginine-glycine-aspartic acid (RGD) peptide sequence that promotes certain cell behaviors such as adhesion, proliferation, and differentiation, and a matrix metalloproteinase (MMP) degradation sequence that promotes cell enzymatic degradation [57]. Gelatin obtained from collagen type 1 by hydrolytic processes has been successfully used as a substitute for collagen because it exhibits lower antigenicity, ease of processing, and availability at lower cost while retaining functional moieties recognized by cells [54]. However, because of its poor mechanical qualities, it is frequently blended with synthetic polymers to get around this issue [58]. A thin and strong tri-layered structure was achieved by using a chitosan complex with gelatin and polyurethane (PU) to form heart valve leaflets [59]. Compared to poly(glycolic acid) (PGA)/poly(lactic) acid (PLA) and collagen-coated pericardium, the gelatin–chitosan complex added to PU provided an environment that was more favorable for the proliferation of endothelial cells. As a result, combining the gelatin–chitosan complex is expected to enhance cellular connections. The outcomes demonstrated further the possibility and appeal of using the electrospinning technology to produce complex structures like the tri-layered heart valve leaflets as well as thin, strong layered scaffolds. Methacrylic anhydride and a gelatin solution are the most frequent ways to create methacrylated gelatin (GelMA), which can then be crosslinked by UV light to create hydrogen. GelMA hydrogels can be micropatterned with different geometric characteristics (ranging from around 50 to 150 μm in height) to direct the production of 3D endothelial cords [60]. In one study, GelMA was successfully utilized as a platform for VICs, demonstrating the potential of this material for the development of heart valve-like culture models [61]. In another study, similarly, porous hydrogels formed by photo-crosslinking of GelMA were used to investigate VICs function in vitro. Relevant features of the native morphology of VICs cultivated in these hydrogels were visible within two weeks of cultivation [62]. Table 1 summarizes the use of biopolymer-based electrospun nanofibers in heart valve construction.
The use of biopolymer-based electrospun nanofibers for heart valve construction
| Material | Solution | Fiber diameter (nm) | Voltage (kV) | Flow rate | Needle-mandrel distances (cm) | Cell | Outcomes |
|---|---|---|---|---|---|---|---|
| Porcine decellularized valve: poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB) | Dichloromethane | - | 20 | - | 20 | Mesenchymal stromal cells (MSCs) | Hong et al., 2009 [63]
|
| Gelatin–chitosan polyurethane (PU) | N,N-dimethylformamide (DMF)/tetrahydrofuran (THF) | 720 ± 130 to 970 ± 160 | 20 | - | - | Endothelial cells | Wong et al., 2010 [59]
|
| Methacrylated hyaluronic acid and methacrylated gelatinPoly(glycerol sebacate) (PGS)–poly(ε-caprolactone) (PCL) | Chloroform and ethanol (9:1) | - | 12.5 | 2 mL/h | 18 | Mitral valve interstitial cells (MVICs) | Eslami et al., 2014 [64]
|
| Decellularized bovine pericardium: polycaprolactone-chitosan | Trifluoroacetic acid (TFA) | 128.78 ± 17.9 | 15 | 0.5 mL/h | 15 | Heart valve interstitial cells (hVICs) | Jahnavi et al., 2017 [65]
|
| Cellulose acetate | Acetone and dimethylacetamide (DMAc) (2:1) | 900 | 25 | 10–15 μL/min | 20 | Mouse fibroblasts L929 | Chainoglou et al., 2016 [46]
|
| Silk fibroin (SF) and poly(ester-urethane) urea (LDI-PEUU) | 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) | 465 ± 165 | 10 | 1 mL/h | 15 | Human umbilical vein endothelial cells (HUVECs) | Du et al., 2018 [66]
|
TEHVs: tissue-engineered heart valves
GAGs are polysaccharides that present in the pericellular space, inside cells, and in the ECM. These polysaccharide subunits consist of a hexuronic acid and an amino sugar linked by glycosidic bonds, and these variations in disaccharide composition are used to distinguish classes of GAGs, mainly hyaluronic acid (HA), chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), and heparan sulfate (HS) [67, 68]. Through their extensive structural variety and varied location with particular connections with hundreds of binding proteins, GAGs control biological processes like cell-renewal, differentiation, growth, inhibition, microbial invasion, and defense [69]. The multiple biological functions of GAGs indicate immense potential for tissue engineering as biomaterials. Therefore, studies have shown that electrospinning of gelatin with GAGs is a good candidate for the production of nanofibrous scaffold to be used as an artificial ECM in tissue engineering [70]. Previous research done by Flanagan et al. [71] developed collagen-GAG matrices as ECM analogs. Type I collagen-GAG hydrogels were examined as biomaterials for the production of MV tissue. In vitro, tissue-like structures were formed by the contraction of collagen and collagen-CS MV constructions. A biochemical test showed the collagen-CS constructions preserved more than 75% of the CS. Also, when comparing collagen-CS constructs to collagen constructs, morphological analysis revealed increased VECs covering the surface in the former. This study showed that a collagen-GAG matrix repopulated with both pig mitral VECs and VICs may produce viable MV tissue.
A wide variety of synthetic polymers are employed in scaffolding [72]. Electrospun scaffolds can be made using either one polymer or a variety of combinations with natural polymers. The main advantages of synthetic polymers are that they are mostly electro-winnable and cost-effective. Often, modification of the surfaces of polymers is required for augmenting cell adhesion. In TEHV applications, hydrolytically biodegradable polymers such PGA, PLA, polycaprolactone (PCL), poly(glycerol sebacate) (PGS), and PU have demonstrated potential [73]. These polymers are biocompatible and have good mechanical qualities that make them resistant to the strains and stresses that valves encounter in a hemodynamic environment. Fiber scaffolds resembling the natural valve ECM structure can be produced from these polymers using methods like electrospinning [74].
PCL is the most widely used polymer type in the polylactone family. PCL is considered a non-toxic and tissue-compatible material. As a semi-crystalline polymer that is highly soluble in organic solvents and readily combined with other polymers, PCL is considered as a non-toxic and tissue-compatible substance [75]. PCL polymer is usually synthesized by ring-opening polymerization. Its tensile strength is around 23 MPa, and it is elastic. These properties make PCL suitable for the production of tissue-engineered valves, such as electrospinning or 3D printing. The mechanical properties and biodegradability of PCL scaffolds can be modified by adjusting the molecular weight of PCL [76]. The degradation rate of PCL is quite slow. However, because the scaffold cannot be reabsorbed when new tissue grows, the slow breakdown rate might have both benefits and drawbacks [77]. Together with PCL, Jahnavi et al. [65] established aligned and randomly electrospun chitosan fibers. They coated nanofibres with a decellularized bovine pericardium polycaprolactone-chitosan (DBPDBP-PCL-CH) to produce biohybrid scaffolds that had advantageous structural and biomechanical characteristics for TEHV. In their study, scanning electron microscope (SEM) and fluorescence micrographs demonstrated that the biohybrid scaffolds have superior cellular adhesion to the decellularized bovine pericardium and cells are highly aligned along the polymeric fibers in comparison to the randomly electrospun samples. Furthermore, aligned biohybrid scaffolds showed ideal pore and fiber diameters, which ultimately reduced the rate of degradation and greatly enhanced uniaxial mechanical characteristics. In another study, by electrospinning a PCL, Ravishankar et al. [78] developed a composite scaffold by embedding the mat in GelMA hydrogels. These hydrogels comprised of aligned fibers and gelatin GAG exhibited valve-mimetic mechanical properties, making a highly compatible microenvironment for tissue engineering of heart valves. The hydrogels based on gelatin GAG provided an environment that encourages the growth of VICs, and the fibrous component improved the mechanical properties to produce stronger materials, providing the advantages of both hydrogel and fibers. According to this study, VIC cells showed increased alpha-smooth muscle actin (α-SMA) expression during a 14-day period. Collectively, these findings provide an environment that triggers cell activation, which may be essential for the synthesis and release of ECM.
PGS, which is produced by condensation polymerization of glycerol and sebacic acid, has quickly found application as biomaterials due to its advantages [79]. PGS polymer shows high biocompatibility and degradation within 4–6 weeks [80]. In a study by Masoumi et al. [81], PGS shows good biodegradability, stiffness, and cell adhesion when compared to PGA in TEHVs. Tensile Young’s modulus of PGS ranges from 0.025 MPa to 1.2 MPa, with a maximum tensile strength above 0.5 MPa and a reported strain of 330%. In contrast, natural human heart tissue has a tensile Young’s modulus and strain at 0.5 MPa, and 260%, respectively. The mechanical properties of PGS largely depend on three factors: (1) curing time, (2) curing temperature, and (3) molecular ratio of glycerol and sebacic acid. Chen et al. [82] presented the elastic modulus of PGS as 0.056 MPa, 0.22 MPa, and 1.2 MPa at 110°C, 120°C, and 130°C, respectively. Therefore, it was concluded that there is a direct relationship between temperature and Young’s modulus. Eslami et al. [64] combined PGS-PCL electrospun microfibers into methacrylated HA and GelMA (Me-HA/Me-Gel) hybrid hydrogels. The objective was to develop a composite biomaterial that mimics the mechanical characteristics of PGS-PCL elastomeric microfibers and the beneficial ECM-mimicking capabilities of hydrogels to produce a cellular environment and mechanical characteristics that are comparable to those of native heart valve tissue. The migration of cells into the hydrogel component leads MVICs to be more adherent to the fibrous component and present at varying depths. The preservation of mechanical qualities combined with all these benefits of microfibrous hybrid scaffolds give the composite hydrogel/PGS-PCL scaffolds a better 3D structure to develop scaffolds for HVTE. In order to create fibrous scaffolds with aligned fibers that approximated native heart valve leaflet ECM networks, Masoumi et al. [83] utilized a directional electrospinning technique using PGS-PCL polymer blend. According to the study’s findings, cells cultivated on aligned scaffolds were more than 50% aligned in the direction of the deposited fibers, while cells cultivated on randomly deposited PGS:PCL combinations were less than 20% aligned. These demonstrate that the anisotropic properties of the native heart valve could be mimicked by PGS-PCL scaffolds.
Polysulfones (PSfs) are part of a broad biomaterial family. One of the most often utilized materials in the production of hemodialysis membranes is PSf and its derivatives thanks to its negative charge [84]. PSf membranes are hydrophobic and biologically stable. They exhibit excellent mechanical and thermal properties and can remove large molecular-weight toxins [85]. As it is known, electrostatic repulsion occurs between the negatively charged components of the blood (such as platelets, leukocytes, and erythrocytes) and the membrane surface when the membrane surface has negative charges. Therefore, low protein adsorption and low platelet adhesion occur on the membrane surface, which increases blood compatibility on the surface [86]. PSf membranes are often mixed with hydrophilic polyvinylpyrrolidone (PVP) to improve hemocompatibility [87]. PSf polymer was utilized for the first time in tissue engineering of the heart valve by Gürbüz et al. [88]. In this study, researchers constructed radial aligned nanofibrous scaffold of PCL, PGS, and PSf polymers by combining 3D printing and electrospinning techniques. It was provided that HUVECs on PCL/PGS/PSf scaffolds radially aligned with a rate of 72% while that was only 65.3% on PCL. This investigation demonstrated that PCL/PGS/PSf could generate an anisotropic and biomimetic endothelium. Table 2 summarizes the use of polymer-based electrospun nanofibers in heart valve tissue engineering.
An overview of key electrospun synthetic nanofibers used for heart valve tissue engineering applications
| Material | Solution | Fiber diameter (nm) | Voltage (kV) | Flow rate(mL/h) | Needle-mandrel distances (cm) | Cell | Outcomes |
|---|---|---|---|---|---|---|---|
| Poly(ethylene glycol) diacrylate/polycaprolactone (PEGDA-PCL) | Tetrahydrofuran and N,N-dimethylformamide (1:1) | ~700 | 12 | 1 | 20 | Valve interstitial cells (VICs) | Tseng et al., 2014 [89]
|
| Poly(ether ester urethane) urea (BPUR) | 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) | 500 ± 70 | 7.5 | 0.3 | 20 | VICs | Puperi et al., 2016 [90]
|
| Polyurethane (PU) | Chloroform and methanol (50:50) | 153 ± 4 | 20 | 1 | 100 | Human umbilical vein endothelial cells (HUVECs) | Firoozi et al., 2017 [91]
|
| Poly(p-dioxanone) (PDO) and PEUU | HFIP | 1,068 | 11 | 1 | 15 | HUVECs | Du et al., 2019 [92]
|
| Poly(1,3-diamino-2-hydroxypropane-co-glycerol sebacate)-co-poly (ethylene glycol) (APS-co-PEG)/PCL | Ethanol and chloroform (1:9) | 1,246 ± 81 | 17 | 1 | 12 | Human valve interstitial cells (hVICs) | Xue et al., 2020 [93]
|
| Polylactic acid | Dimethyl sulfoxide (DMSO) and dichloromethane (DCM) 9:1 | 1,600 | 18 | 1.5 | 15 | HUVECs | Wang et al., 2024 [94]
|
| PCL | Chloroform | 2,000–6,000 | 10–14 | 0.7–1.2 | 10–12 | Porcine valve interstitial cells (PVICs) | Synder and Jana, 2023 [95]
|
VICs within the valves represent a diverse group of cell types located throughout the valve structure. A variety of sub-phenotypes of these cells can resemble SMCs, fibroblasts, and mesenchymal stem cells (MSCs) [96]. The ability of VICs to differentiate into osteoblasts, adipocytes, and chondrocytes makes them act similar as MSCs. Their capacity to produce ECM proteins including collagen, GAG, and elastin also gives them fibroblast-like characteristics. However, VICs differ from other fibroblasts such as pericardial or dermal fibroblasts [97]. According to earlier research, VICs also exhibit positive production of contractile proteins and α-SMA, which are characteristics of SMCs and “activated” fibroblasts. In developing valves, VICs are in an activated state with expression of α-SMA and vimentin. In contrast, vimentin is the only protein expressed by healthy valve cells, indicating that the cells are fibroblast-like and quiescent. VICs may contract in the disease state as a result of increased expression of α-SMA. They also produce more ECM and secrete more growth factors, proteases, and cytokines [98]. The use of specific cell types may be crucial for heart valve regeneration in addition to the production of appropriate TEHV scaffolds. Because VICs are thought to be able to synthesis the right ECM, they have been utilized to construct heart valves [99].
The valve leaflet’s blood contacting surfaces are lined by a layer of cells called VECs. These monolayer cells are responsible for the non-thrombogenicity of the leaflets. Furthermore, through paracrine signaling, VECs control inflammatory and immunological responses and contribute to the regulation of the VIC phenotype [100]. It has long been assumed that VECs behave similarly to vascular endothelial cells. The primary distinction between VECs and vascular endothelial cells lies in their orientation; VECs are arranged either circumferentially or at right angles to blood flow, while vascular endothelial cells are positioned in alignment with the flow direction. In comparison to vascular endothelial cells, VECs have also been demonstrated to proliferate more quickly. Additionally, VECs play a key role in paracrine signaling that modulates VIC function [101]. As previously mentioned, nitric oxide production by VECs is protective in terms of keeping VICs in a quiescent phenotype. VICs have been demonstrated to display a more active phenotype with enhanced α-SMA expression when VECs function dysfunctional [102]. Furthermore, the position of VECs on the AV leaflets seems to affect their phenotypic and genetic profiles. This could be as a result of the ventricular side of the leaflets experiencing constant pulsatile flow, which promotes a more protective phenotype, whereas the aortic side encounters disrupted flow when the valve closes during valve diastole [103].
The primary constituents of the human heart valves are two types of cells: endothelial cells and interstitial cells. These cell types are thought to generate and preserve ECM, which provides the required flexibility and physical strength to the heart valve [104]. Endothelial cells make up the cellular layer that covers the matrix and interstitial cells. They serve a variety of purposes, such as preventing thrombus formation and serving as a selective barrier [105]. One of the primary goals of tissue engineering is the reconstruction of these biological structures. Studies have proven that many cell sources are suitable for the production of heart valves. Nowadays, autologous cells extracted from umbilical cord, saphenous veins, or umbilical cord blood are typically used in research to produce TEHVs [106]. Since 1973, HUVEC has been the most widely employed endothelial cell type in in vitro studies of vasculature and angiogenesis because of its easy, quick, and affordable isolation procedure [107, 108]. It is evident that vascular cells from the human umbilical cord mimic the normal cellular structures and functions observed in heart valves because endothelial cells isolated from HUVECs resemble pulmonary heart valve endothelial cells [109]. In a study, the original endothelial cells of heart valves were replaced with HUVECs and endothelialized on the scaffold surface. HUVECs completely covered the valve surface and were observed to be oriented toward the flow direction. In another study, HUVECs were utilized to replace the existing endothelial cells of heart valves and were subsequently endothelialized on the scaffold’s surface [110]. In this regard, human endothelial cell activity is better represented by HUVEC than by cell lines.
Fibroblasts, the primary cell type of connective tissue, are spindle-shaped and are thought to form the ECM that keeps the tissue structurally intact. In addition, fibroblasts represent a diverse group of cells that are present in various tissues and originate from mesenchymal cells. Fibroblasts from different anatomical regions all have similar morphology [111]. Fibroblasts are the main cell type responsible for the development and repair of connective tissue in a healthy heart. Fibroblasts provide a highly ordered, collagen-rich meshwork that supports force transmission, stabilizes the heart wall, and permits intricate tissue deformation patterns [112]. Dermal fibroblasts perform a variety of tasks, including autocrine and paracrine interactions, proliferation and migration in response to chemotactic, mitogenic, and modulatory cytokines, and the synthesis and deposition of ECM [113]. In the study by Syedain et al. [114], dermal fibroblasts were seeded into the decellularized heart valve using a bioreactor system, and TEHV that was analyzed in a pulse duplicator system showed similar results to the native structure. By employing dermal fibroblasts to produce a structure appropriate for recellularization by host cell infiltration, this study offered a viable substitute for current tissue valve replacements [114].
Polymer solutions have been electrospun effectively, notably synthetic ones that dissolve readily in organic solvents, to produce random and aligned fiber mats to promote heart valve cell proliferation (Table 3). Electrospinning is a highly adaptable and effective method for producing materials that can be scaled up with ease. Continuous production of fibers with micron or nanometer dimensions from a variety of polymeric materials is now feasible thanks to electrospinning [115, 116]. Through the optimization of the solvent system and manufacturing conditions, a wide variety of synthetic and natural biopolymers are employed in electrospinning [117]. Biopolymers such as collagen, chitosan, gelatin, fibrinogen, chitin, HA, and silk are examples of biopolymers that are electrospun. Despite their potential, only a limited number of these polymers have been utilized in producing heart valves. Mechanical anisotropy can be achieved by manipulating the alignment of electrospun microfibres [118]. Electrospinning is an adaptable and promising method for producing scaffolds in tissue engineering. In electrospinning, a high-voltage power supply, a collector, and a syringe pump are the basic requirements of the electrospinning apparatus (Figure 2) [119]. A force transfers the polymeric solution in a syringe to the needle, causing the conical protrusion to produce fibers (Figure 2a). 3D configuration of scaffolds is crucial in the manufacturing of heart valves, which may require the development of specialized tools like innovative collectors for electrospinning machinery [120]. For example, the motorized mandrels, which rotate at high speed could align fibers during deposition (Figure 2b). Furthermore, 3D printing of molds and subsequent metalization could offer a novel way of alignment as recently studied in research group (Figure 2c) [121]. The fiber is accelerated and collected on a grounded target [122]. A variety of microstructures can be produced through electrospinning, depending on the parameters selected. These include polymer type, solvent, flow rate, physical distance between the target and collector, voltage difference, and rotational speed of the mandrel [123]. The 3D configuration of scaffolds is crucial in the manufacturing of heart valves, which may require the development of specialized tools like innovative collectors for electrospinning machinery [124].
A summary of electrospinning methods used for sythetic biomaterials for heart valve tissue engineering
| Polymer | Collector type | Orientation of nanofiber | Cell type | References |
|---|---|---|---|---|
| PGS-PCL | Conventional collector (aluminum plate) | Non-aligned | Human umbilical vein endothelial cells (HUVECs) | Sant et al., 2011 [125] |
| PGS-PCL | Conventional collector (aluminum plate) | Non-aligned | Valve interstitial cells (VICs) | Sant et al., 2013 [126] |
| PGS-PCL | Teflon sheet collector | Aligned | VICs | Masoumi et al., 2014 [83] |
| PCL | Spoke-in-ring collector | Aligned | VICs | Jana and Lerman, 2016 [127] |
| PCL-PLLA | Conventional collector (aluminum plate) | Non-aligned | Porcine valve interstitial cells (PVICs) and C57/B1 mice cardiac stem cells (CSCs) | Hasan et al., 2018 [128] |
| PCL | Ring-with-dot collector | Aligned | PVICs | Jana and Lerman, 2019 [129] |
| PCL | Conventional collector (metal plate) | Non-aligned | PVICs | Jana et al., 2019 [130] |
| PCL-gelatin | Centrifugal jet spinner setup-reservoir surface | Aligned | PVICs | Ravishankar et al., 2021 [78] |
| PCL | Conventional collector (aluminum plate) | Non-aligned | Human endothelial colony-forming cells (ECFCs) and human induced-pluripotent stem cells-derived MSCs (iMSCs) | Lutter et al., 2022 [131] |
| PCL/PGS/PSf | Centered pillar collector | Aligned | HUVECs | Gürbüz et al., 2024 [88] |
PGS: poly(glycerol) sebacate; PCL: polycaprolactone; PLLA: poly L-lactic acid; PSf: polysulfone
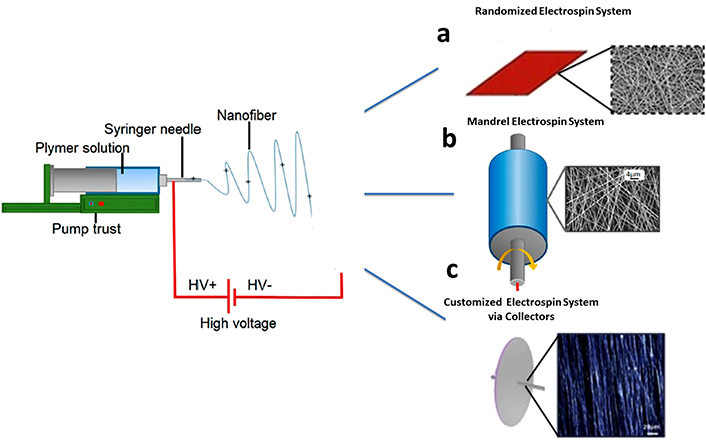
Illustration of electrospinning process. (a) Randomized electrospin system with aluminum plate. Adapted from [132] with permission from Elsevier License number 5966110342256. © 2020 Elsevier B.V. (b) Mandrel electrospinning process with motorization. Adapted from [133], © 2023 by the authors. CC BY 4.0. (c) Customized electrospinning process with collectors. Adapted from [121] with permission from Elsevier License number 5966110728207. © 2020 Elsevier Inc.
Anisotropy describes the way physical properties depend on direction and is a crucial feature of specific tissues such as cartilage, muscle, tendon, and ligament [134]. ECM can optimize its function along the direction of anisotropy. For instance, these special structures can offer efficient contractility and force transfer to support the regeneration of healthy muscle fibers. Structurally and mechanically anisotropic scaffolds are necessary to mimic the properties of the target tissue and provide a distinct microenvironment for cell growth. By controlling the alignment and arrangement of the electrospun nanofibers, many 3D electrospun nanofibers with the essential anisotropy features have been established [135–137] (Table 3). For instance, the myocardium, which is the primary tissue involved in systole and diastole, has a hierarchical design with aligned cells established in an anisotropic and micropatterned ECM [138]. Heart valve tissue engineering is a promising approach for the repair of damaged or diseased heart tissue. In a study by Wu et al. [139], an electrospun fiber network with heterogeneous and anisotropic properties was formed by textile methods to replicate the natural properties of heart tissue. When combined with a hydrogel made from methacrylated HA and GelMA that contains cells, this composite scaffold demonstrated superior mechanical properties, improved cell growth, and consistent ECM remodeling, showing resistance to the degradation and shrinkage in comparison to individual materials [139]. The arrangement of cells in 3D nanofibre/cell constructs, achieved through layering enabled by nanofibres, can be hindered by the small inter-fibre pore sizes in electrospun nanofibre scaffolds, which restricts cell movement between layers and slows down the development of a cohesive tissue structure [140]. To overcome this challenge, efforts are being undertaken to enhance cell penetration through nanofibre scaffolds by changing the density distribution and spatial arrangement of the fibers during electrospinning.
A grounded conductive surface is typically utilized to collect the electrospun nanofibres during the electrospinning process. By adjusting the electric field distribution during the fiber collection process, a special spatial arrangement of the fibers can be formed to mimic the anisotropy of the native ECM. For instance, the orientation of fibers accumulated on a revolving mandrel is parallel to the rotational direction. Additionally, fiber deposition can be influenced appropriately to give distinctive fiber organization within the electrospun scaffolds by interfering with the electric field distribution utilizing a patterned collection surface [141, 142]. Research has demonstrated that these patterned fiber constructions can improve mechanical strength and cause noticeable biological reactions on their surface [143]. However, it is still uncertain if the electrical field’s influence on fiber configuration can actually increase the distance between fibers enough to allow for cell infiltration. Therefore, the main goal of these type of research is to find whether periodic changes to the organization and distribution of fibers within electrospun nanofiber constructs can result in high cell penetration and, as a result, make it easier to create incorporated tissue constructs using nanofiber-enabled cell layering technology [144]. In one study, a combination of PCL and collagen was utilized to enhance cell adhesion and proliferation. The mesh-patterned nanofiber scaffolds’ shape and pore size were assessed under a microscope, and the number of cells that passed through them was used to count the degree of cell infiltration. The results showed that the random, aligned, and patterned nanofiber scaffolds had respective values of 2.2 µm, 4.1 µm, and 35.4 µm. The highest cell penetration is observed in the patterned nanofiber structure [145]. It was thought that the control of proper cell phenotype for the development of functional tissue depended significantly on the restoration of the fundamental morphological characteristics of the native ECM. The arrangement of the collector determines whether the electrospun fibers in the tissue scaffold are anisotropic or isotropic, depending considerably on how the electric field is distributed between the grounded surface and the syringe tip [146, 147]. Cell morphology provides knowledge about the condition and behavior of cells. According to reports, nanofibers can alter the cytoskeleton’s organization and cell shape. In a study, normal human oral keratinocytes were used to electrospin silk fiber nanofibres and examine the impact of cell spreading. SEM pictures revealed that the cells formed a 3D network of the nanofibrous structure by interacting and integrating well with the surrounding fibroin nanofibers, and indicated growth in the direction of fiber orientation [148]. In an another study, fibroblasts grown on polyamide nanofibres were shown to have a longer shape than those grown on a glass surface, which led to a significant difference in actin organization and cell spreading [149]. The arrangement of cells is a biological process in various tissues across the body, and the structural organization of the ECM significantly influences cellular behavior and functionality in organisms.
Despite advancements, production of a tissue substitute that mimics the molecular structure and architecture of the ECM to guide cell orientation remains a challenge. It has been widely reported that aligned nanofibres can guide cell morphology and direct cell orientation. Similarly, scaffolds serve as a structural template that facilitates the 3D arrangement of cells. One study showed that both aligned PCL and PCL/gelatin nanofibres were able to direct the orientation of rabbit cardiomyocytes on scaffolds [150]. However, compared to aligned PCL fibers, aligned PCL/gelatin nanofibers generated higher cell alignment, suggesting that scaffold topography, chemical and biological signals, and scaffold components all affect cell morphology.
In order to produce tissues that are structurally and functionally similar to their natural counterparts, they need also provide cells with the appropriate signals. It is generally acknowledged that the cellular architecture and ECM of natural tissues are important factors in defining their mechanical and biological function [151]. Moreover, most studies focused on the impact of nanofibers on cell behavior have utilized 2D cultures on nanofiber mesh surfaces, which significantly differs from the natural conditions where cells are integrated into a complex and fluid 3D fibrous network of the ECM. However, little work has been done to explicitly examine how the configuration of ECM fibers affects the behavior of cells that produce heart valve tissue because there is a lack of suitable model systems.
There are few studies on the electrical conductivity of scaffolds in the field of heart valve tissue engineering. Electrical conductivity was identified as a critical factor influencing cell regeneration. The electrical conductivity and mechanical contractility support cell adhesion, migration, differentiation, and proliferation [152]. For cardiac tissue engineering, a scaffold should be electrically conductive, mechanically stable, and have elasticity comparable to that of the native myocardium [153]. The electrical conductivity of the scaffold can be increased by functionalizing various materials that are frequently used for scaffold construction, such as collagen, silk, alginate, and chitosan, with nanostructures like graphene or carbon nanotubes [154]. Martinelli et al. [155] studied the behavior of newborn rat cardiomyocytes by depositing pure carbon nanotubes on glass surfaces. They found that there was increased proliferation and tight contact formation among the cardiomyocytes [155]. In another work, scientists developed an electrically conductive nanoscale scaffold by adding carbon nanotubes to electrospun PCL. They successfully differentiated human mesenchymal stem cells into cardiomyocytes by using electrical stimulation. In the presence of carbon nanotubes, cardiac markers such as Nkx-2.5, Cx43, GATA-4, and cardiac troponin T expressions were upregulated and the cell shape became elongated [156]. In a study by Mawad and colleagues [157], the conductive scaffold immobilized by PLA. Experiments have shown that PLA/polyaniline (PANI) nanofibrous sheets exhibit similar cell viability and proliferation to PLA nanofibrous sheets. However, they showed that the synchronized beating rate of cardiomyocytes produced on PLA/PANI nanofibrous sheets was significantly higher than that of PLA nanofibrous sheets [157].
Studies on conductive scaffolds have generally indicated encouraging findings and opportunities regarding maturation of cardiomyocytes cells and production of coordinated beats. The potential of injectable hydrogel scaffolds for myocardial repair following myocardial infarction was highlighted by Mihardja et al. [158] using polypyrrole (PPy) and alginate. The findings demonstrate that PPy and alginate together improve cell proliferation and adherence while facilitating myofibroblast penetration into the infarct region [158, 159]. Saghebasl et al. [160] developed four distinct electrospun scaffold types based on poly(glycerol sebacate)-polyurethane (PGU), PGU-soybean oil (Soy), gelatin (G) Soy/G, and simvastatin-loaded PGU-Soy/G scaffold as an effective in vitro scaffold to provide the ideal scaffold for cardiac regeneration. Their findings demonstrated that the electrical conductivity of nanofibrous scaffolds was enhanced by the addition of soy oil, a semiconducting substance. Furthermore, research showed that the simvastatin-loaded polymeric system improved the proliferation and attachment of cardiomyoblasts and could potentially be used as a drug release carrier in cardiac tissue engineering. In another study, an electrically conductive PPy-chitosan hydrogel was developed by Mihic et al. [161] conjugating the conductive PPy onto chitosan side chains. When injected into rat hearts following myocardial infarction, the conductive hydrogel greatly improved heart function and was able to coordinate myocyte function ex vivo.
A conductive polymer that was designed for cardiac tissue repair is a hydrogel made of gelatin, aniline pentamer (AP), and glutathione (gelatin/AP-GSH) composite as shown by Li et al. [162]. Due to the antioxidant properties of glutathione (GSH), this construct may also lower local reactive oxygen species concentrations, which may improve the results of heart tissue healing. Based on the findings, the gelatin/AP-GSH scaffold’s conductivity was comparable to that of the native myocardium, ranging from 3.4 × 10−5 S/cm to 1 × 10−4 S/cm. According to these findings, gelatin/AP-GSH composite scaffolds showed potential in cardiac tissue engineering [162, 163]. The mechanical and electrical properties of PU/chitosan/CNT electrospun scaffolds were compared by Ahmadi et al. [164] by electrospraying of carbon nanotubes by (PU/Chitosan/CNT.sp) or blending (PU/chitosan/CNT.bl). The produced PU/chitosan/CNT composite nanofibrous scaffolds were shown to be electroconductive, and aligned nanofibers may be regarded as potential scaffolds with nanoscale properties for infarcted myocardial regeneration [164]. By mixing the conductive polymer PANI with poly(ethylene glycol) diacrylate (PEGDA), Haq et al. [165] produced hydrogels and a conductive ink for 3D printing. Their major objective was to use 3D printing projection micro-stereolithography to generate electrically conductive scaffolds that, from the perspective of technology, resemble the hierarchical orientation of heart myofibers. The resulting scaffolds had an average pore size of 300 nm and showed semi-conducting characteristics (~10–6 S/m). Scaffolds with conductive properties exhibit adjustable conductivity and create an ideal setting for the growth of mouse cardiac progenitor cells.
The crucial concerns in the field of cardiac tissue engineering require further research on the application of electrically conductive nanostructured materials. All things considered, research on conductive scaffolds has produced encouraging findings and opportunities for applying these materials to produce mature cardiomyocyte cells and coordinated beats. In spite of significant efforts in cardiac tissue engineering, there is still a lack of conclusive materials, methodologies, and cellular resources, leading to ongoing research, particularly because in vivo studies have been limited.
Electrospinning is regarded as a beneficial technique for developing scaffolds in the field of tissue engineering, as it is simple, low-cost, adaptable, and capacity to form structures that mimic the ECM. A significant number of studies confirmed electrospun scaffolds were more similar to the ECM nanostructure. It has been applied in a variety of techniques to incorporate different morphological features with material properties for tissue engineering such as aligned nanofibers. By far, the post-potent technique was to employ patterned collectors which could orient the electric field effectively. In construction of heart valves aligned nanofibers proved the best approach to mimic anisotropy of valve leaflets. By mimicking the native heart valve anisotropic structure, they influence cell orientation and enhance tissue function. It was also observed that aligned nanofiber’s mechanical strength and anisotropic structure promote cell development. However, it is important to consider the constraints that come with using aligned nanofibers. Among the difficulties that must be taken into account while producing aligned nanofiber-based heart valve applications are reproducibility and scalability. Furthermore, the biocompatibility, hemocompatibility, and degradation characteristics of the materials chosen for electrospinning-based heart valve construction should be carefully considered. It was also concluded that nanofibers itself may not perform physiological functions arising from the lack of soft tissue properties. Some of the studies therefore focused on using nanofiber mats within hydrogel matrix to increase cell infiltration and provide more vascular structure. Another significant challenge detected in using nanofiber scaffolds was cell permissiveness between leaflets. Therefore, it would be essential to apply novel engineered collectors to adjust the inter-fiber distances and hence increase the infiltration rate of heart valve cells.
This review sought to overview the up-to-date status of heart valve scaffold production, biomaterials, and cells used in the scaffolds, aligned electrospinning methods, and tissue engineering of heart valve scaffolds. This section on future directions reflects our thoughts on potential fixes and upcoming studies that could result in better heart valve scaffold. In designing nanofibrous scaffolds, it is often necessary to specify the application and to modify features depending on particular cell type and leaflet structure of valve. Nanofibrous heart valve constructs should investigate multifunctional scaffolds that transmit bioactive signals to aid in tissue regeneration in addition to physical cue-providing microarchitecture. It was detected that the majority of published research has been performed in vitro, and mostly in static conditions. The composition and architecture of polymeric nanofiber scaffolds require additional optimization for in vivo applications. Through expanded research investigation in the near future, it is highly probable that all of the challenges and limitations encountered in the production, research, and development, and processing of electrospun nanofibers will be clearly resolved.
AP: aniline pentamer
AVs: aortic valves
CGF: CG-fibrin scaffold
CS: chondroitin sulfate
ECM: extracellular matrix
GAGs: glycosaminoglycans
GelMA: methacrylated gelatin
GSH: glutathione
HA: hyaluronic acid
HUVECs: human umbilical vein endothelial cells
MSCs: mesenchymal stem cells
MV: mitral valve
PANI: polyaniline
PCL: polycaprolactone
PGA: poly(glycolic acid)
PGS: poly(glycerol) sebacate
PGU: poly(glycerol sebacate)-polyurethane
PLA: poly(lactic) acid
PPy: polypyrrole
PSfs: polysulfones
PU: polyurethane
SEM: scanning electron microscope
SMCs: vascular smooth muscle cells
TEHVs: tissue engineered heart valves
VECs: valvular endothelial cells
VICs: valve interstitial cells
α-SMA: alpha-smooth muscle actin
BG and EB: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. ETB: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This research was supported by Scientific Research Project of University of Health Sciences Turkey [2022/165] and TUBITAK 1002 ARDEB Project [122M795]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Nolan G. Schwarz ... Ivan T. Lima
