Abstract
The use of echocardiography, a straightforward and widely available technique, allows for a comprehensive assessment of the patient with hypertrophic cardiomyopathy (HCM) under both resting and stress conditions. The true prevalence of HCM has been redefined over time by this imaging approach, which has also made it feasible to pinpoint parameters that clinicians may use to stratify patients at risk for adverse cardiovascular events. The current and emerging prognostic predictors in HCM, assessed with transthoracic echocardiography at rest and during provocation, are discussed in this review.
Keywords
Prognosis, hypertrophic cardiomyopathy, resting and exercise echocardiographyIntroduction
Hypertrophic cardiomyopathy (HCM) is a common condition, characterized by a heterogeneous spectrum of phenotypes and clinical trajectories, which can significantly vary even in the same family. While the disease may have a favorable course, heart failure (HF)-related complications are common, and potentially lethal arrhythmias may occur [1]. The identification of high-risk subsets remains an important challenge, which goes hand-in-hand with the daily efforts to provide answers to the patients on their state of health and risk profile. These clinical needs have inspired a bulk of studies based on imaging techniques, first and foremost echocardiography, which provides diagnostic and prognostic information, and allows the examination of HCM patients both at rest and during provocation. Based on over 3 decades of intense research, this review aims to provide an overview of well-established as well as innovative echocardiographic predictors of outcome in patients with HCM (Table 1).
Rest and stress echocardiographic predictors of outcomes in patients with HCM
| Outcomes | Resting exam | Stress exam |
|---|---|---|
| Cardiovascular death | - LA size - LV diastolic and systolic dysfunction - Resting LVOT obstruction - Progression of MR - RV dysfunction | Exercise: - MR ≥ moderate - Increased E/e’ - WMAs - PHT Vasodilator: CFVR |
| SCD | - Degree of hypertrophy - “Mixed” apical HCM - LA size and function - LV diastolic and systolic dysfunction - Resting LVOT obstruction - Apical aneurysms | Exercise: PHT |
| HF with/without hospitalization | - “Mixed” apical HCM - LA size - LV diastolic and systolic dysfunction - Progression of MR - RV dysfunction | Exercise: - MR ≥ moderate - Increased E/e’ - WMAs - PHT Vasodilator: CFVR |
| Ventricular arrhythmias | - “Mixed” apical HCM - LA size and function - LV diastolic and systolic dysfunction - Resting LVOT obstruction - Apical aneurysms - RV dysfunction | Exercise: - MR ≥ moderate - Increased E/e’ - WMAs Vasodilator: CFVR |
| AF | LA size and function | Vasodilator: CFVR |
| Stroke | Resting LVOT obstruction | Exercise: - MR ≥ moderate - Increased E/e’ - WMAs - PHT Vasodilator: CFVR |
AF: atrial fibrillation; CFVR: coronary flow velocity reserve; E/e’: early LV inflow velocity to early tissue Doppler annulus velocity; LA: left atrial; LV: left ventricular; LVOT: LV outflow tract; MR: mitral regurgitation; PHT: pulmonary hypertension; RV: right ventricular; SCD: sudden cardiac death; WMAs: wall motion abnormalities
Resting echocardiography
By allowing the evaluation of chamber dimension and function, presence of LVOT obstruction or valvular disease, transthoracic echocardiography provides crucial data in the assessment of risk for death or SCD, HF, AF, and stroke. The degree of hypertrophy, LV diastolic and systolic function, LA size and function, and LVOT obstruction, all have been identified as major echocardiographic predictors.
Degree of hypertrophy
Over the years, several studies have been conducted to assess the influence of hypertrophy on the disease course, with the earliest evidence focusing on the prediction of SCD. Two important studies [2, 3] conducted at the beginning of this millennium helped to pinpoint a maximum wall thickness (MWT) ≥ 30 mm as a major risk factor of SCD (Figure 1). Spirito and colleagues [4] first reported a proportional increase in SCD risk with the magnitude of myocardial wall thickness. Specifically, over a mean follow-up period of 6.5 years, the risk doubled from each subgroup to the next, starting from LV hypertrophy (LVH) ≥ 15 mm [4]. Young adults with MWT ≥ 30 mm appeared to be at high risk, even without outflow obstruction under basal conditions and with few or no symptoms. At the same time, this data reassured clinicians about the management of HCM population with mild hypertrophy (MWT < 19 mm), which represents about 40% of the total cases and for which the rate of sudden death was close to zero 10 years after the initial evaluation and less than 3% at 20 years [4]. In parallel, Elliot et al. [5] confirmed the positive association between increased MWT and higher rates of SCD or implantable cardioverter-defibrillator (ICD) discharge or death from any cause but emphasized the importance of focusing on a set of risk indicators rather than a single risk factor. This led to the development of two different multiparametric approaches endorsed by the European Society of Cardiology and the American Heart Association (AHA) to assess the risk of SCD [2, 3]. Beyond MWT, these include other echocardiographic indicators, such as LA size, resting LVOT gradient (LVOTG), LV ejection fraction (LVEF) < 50%, and LV apical aneurysm [3, 6]. Unlike MWT, there is no data regarding the relationship between LV mass or hypertrophy distribution and arrhythmic risk [4]. Noticeably, in children, an inverted U-shaped relationship was found between LVH and predicted SCD risk, indicating that MWT should not be used as a main criterion to guide ICD implantation [7]. While the direct association between LVH and SCD is well-demonstrated in adults, LVH does not seem to be directly associated with symptoms of worsening HF [8]. Moreover, distinguishing between hypertrophy patterns (asymmetric, concentric, and apical) has added little to prediction of outcomes [9], with one distinctive exception: the “mixed” apical HCM [10]. The latter—characterized by simultaneous apical and septal hypertrophy—shows a higher risk of arrhythmias, HF, and SCD [11], whether the apical aneurysm is present or not.
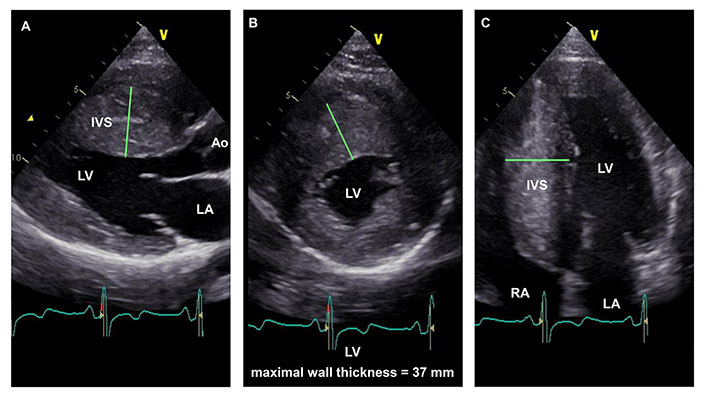
22-year-old HCM patient with extreme LVH. Parasternal long-axis (panel A), parasternal short-axis (panel B), and apical four-chamber (panel C) end-diastolic still-frame images demonstrate asymmetrical hypertrophy with a maximal LV wall thickness of 37 mm (green lines). The interventricular septum (IVS) shows a typical reversed curvature septal morphology (panels A and B). Ao: aorta; RA: right atrial chamber
Of note, conventional echocardiographic techniques do not allow accurate measurement of LV mass in HCM, a parameter shown to be a strong prognostic indicator in cardiac magnetic resonance (CMR) studies [12]. However, 3D echocardiography might hamper this limitation in the future [13, 14].
LA size and function
LA enlargement is a common finding in HCM and its aetiology is multifactorial, expressing a mix of primary atrial myopathy, elevated LV filling pressures, and MR (Figure 2). It has also been proposed that an intrinsic atrial myopathy may result from abnormalities in sarcomeric genes [15] or in genes involved in collagen formation and the renin-angiotensin-aldosterone pathway [16]. As an alternative, higher filling pressures derived from MR, LVOT obstruction, LV diastolic dysfunction, and myocardial fibrosis have been identified as causes of increased LA size [17]. In turn, patients with HCM and LA enlargement not only appear to show a 4 to 6-fold greater likelihood of developing AF compared to the general population but also higher rates of all-cause mortality and disabling symptoms [18].
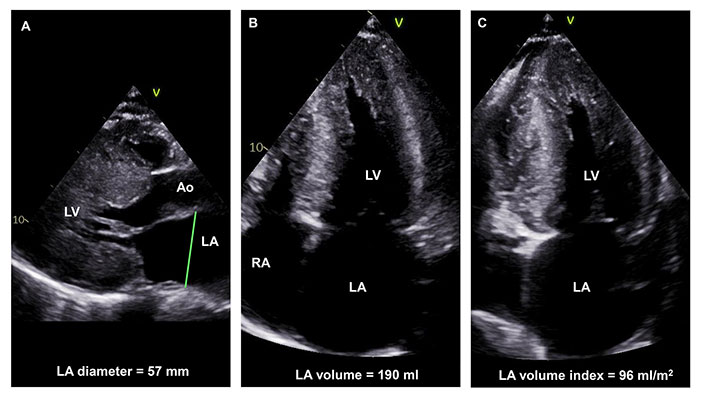
Severe LA dilation in a 29-year-old HCM patient with a pathogenic intronic variant in the cardiac myosin binding protein C gene. LA anteroposterior diameter in the parasternal short-axis view is 57 mm (panel A), indexed LA volume after biplane measurement in 4-chamber (panel B) and 2-chamber (panel C) views is 96 mL/cm2
Notably, LA diameter, volume, and strain, are all independently associated with new-onset AF [19]. Thus, even in individuals with LA diameter < 45 mm, LA volume and strain enhances prediction of new-onset AF, and both measures show higher sensitivity, negative predictive value, and positive predictive value than LA diameter [19].
The prediction of outcomes was tested in a nationwide Italian registry on 1,491 HCM patients, showing that each 5 mm increase in LA diameter was associated with a 20% increase in all-cause mortality, and identifying a cut-off of > 48 mm for LA diameter as an independent predictor of all-cause mortality, cardiovascular and HF death [20]. LA volume has also proven to be an independent predictor of adverse cardiovascular outcomes in general [20–22].
Finally, a correlation exists between LA and arrhythmic events. In fact, both LA size and function were associated with SCD, ventricular arrhythmias, and appropriate ICD discharge in independent studies [6, 23, 24]. In particular, the reduction of LA function as a reservoir (LA reservoir strain < 17%) displayed a significant additive predictive value on top of the HCM SCD risk score [24].
LV systolic and diastolic function
In the absence of LVOT obstruction, drug-resistant HF symptoms are usually caused by diastolic dysfunction which may be challenging to treat pharmacologically and may occasionally require heart transplantation. Subtended by a variety of mechanisms including delayed LV relaxation, diffuse myocardial fibrosis, and abnormal calcium homeostasis [25], severe diastolic dysfunction is a predictor of adverse outcome in HCM [21, 26–28]. Tissue Doppler imaging (TDI) has allowed identifying patients with higher risk not only of developing HF [28] but also of experiencing death, cardiac arrest, sustained ventricular tachycardia (VT), or ICD discharge [26]. Moreover, TDI is useful in the prediction of adverse cardiovascular events even in patients who are asymptomatic or mildly symptomatic [27]. Of note, TDI has also been suggested to have a role in detecting subtle alterations in genotype-positive, and phenotype-negative individuals [29].
Systolic dysfunction with LVEF below 50% identifies the so-called end-stage phase of HCM, characterized by severe and diffuse LV fibrosis [30]. However, abnormalities in regional systolic function can be identified at earlier stages, in which LVEF is preserved but fibrosis starts to accumulate, if alternative imaging modalities are used. Reduced systolic strain assessed by speckle tracking echocardiography identifies patients with more severe disease manifestation [31, 32] and an increased risk for major cardiac events [23, 33]. Specifically, in a study by Haland et al. [32], mechanical dispersion (calculated as the standard deviation of time from the beginning of the QRS complex on electrocardiogram to peak longitudinal strain in 16 LV segments) was a strong independent risk predictor of ventricular arrhythmias and related to the extent of fibrosis at CMR, improving risk stratification when added to the conventional SCD score.
Resting LVOT obstruction
Resting LVOT obstruction is present in about one-third of HCM patients [34] and is an independent predictor of all-cause or cardiovascular death [34, 35], HF hospitalization, stroke [35], and SCD or appropriate ICD discharge (Figure 3) [6, 35]. However, the risk does not increase with the augmentation of the gradient above the threshold of 30 mmHg [35]. In addition to the obstruction, the presence and progression of MR during follow-up (more than mild), together with mitral annular calcification, are significant contributors to poor outcomes in patients with HCM [36]. In children, however, recent evidence shows an opposite pattern, as patients with higher gradients seem to be at lower risk of SCD [37, 38].
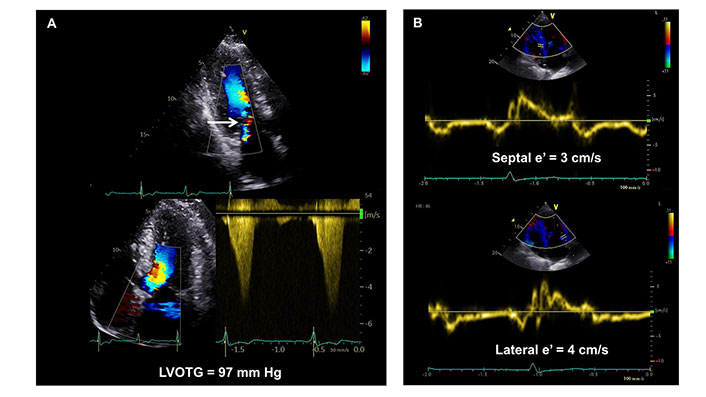
66-year-old man with symptomatic obstructive hypertrophic cardiomyo and a pathogenic variant in the cardiac Troponin I gene. End-systolic frame in apical 3 chamber view showing LVOT turbulence and functional MR (Panel A, upper row, white arrow). Continuous-wave Doppler recording of the LVOT in apical 5-chamber view revealing late-peaking and concave-to-the-left Doppler spectrum with midsystolic flow acceleration peaking at 4.9 m/s, yielding a peak dynamic LVOTG of 97 mmHg (panel A, lower row). Tissue velocity imaging shows reduced septal and lateral e’ velocities indicating severe diastolic dysfunction (panel B)
Specific features (apical aneurysm, RV involvement)
While current risk-scoring algorithms successfully identify high-risk individuals, especially in the late HCM stages, their accuracy is limited in patients without overt structural remodeling or advanced symptoms. This led to the investigation of other potential risk factors detectable during baseline echocardiography, such as apical aneurism (associated with risk of sustained VT) or RV involvement. The presence of apical aneurysms was incorporated in the stratification of SCD risk in the 2020 AHA /American College of Cardiology (ACC) guidelines on HCM. In the evaluation of this morphological alteration, contrast echocardiography has shown high sensitivity equal to CMR (Figure 4) [39]. Preliminary data, reported by a recent retrospective study of Chang et al. [40], recognized tricuspid annular plane systolic excursion and RV-free wall strain as independent predictors of hospitalization for HF, sustained VT, or all-cause death.
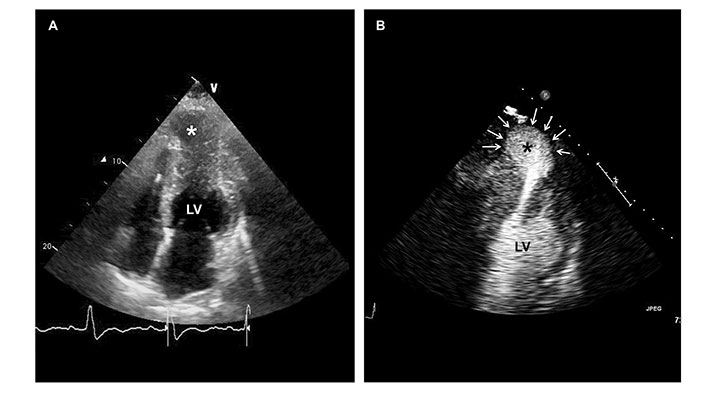
60-year-old woman with midventricular hypertrophy and apical aneurysm (asterisks). Baseline echocardiography was ineffective in defining the contours of the aneurysm (panel A). Echocardiography with a contrast agent provided better bedside visualization of the apical aneurysm (panel B, white arrows)
Stress echocardiographic predictors of outcomes in HCM
Stress echocardiography (SE) has gained an important role in the clinical evaluation of patients with HCM. Exercise is the most widely used modality for stress testing, albeit pharmacological testing provides invaluable information as well. The latest HCM guidelines recommend the execution of exercise SE for patients without LVOT obstruction at rest, with class 1 and 2 indications to rule out exercise-induced gradients [2, 3]. The recommendation is stronger (class 1) for symptomatic patients and weaker for asymptomatic individuals [class 2a (US) and 2b (Europe)]. These guidelines consider exercise SE mainly as a tool to uncover and quantify exercise LVOTG, which may be related to symptoms but has scarce and contradictory prognostic relevance. In contrast, emerging data demonstrate the precious functional and prognostic value of other stress echocardiographic parameters, such as regional WMAs, the ratio of E/e’, pulmonary pressures, or CFVR. Since exercise echo is operator-dependent, specific training for this technique, including expertise on HCM patients, is required to accrue meaningful data.
All echocardiographic findings must be considered in conjunction with the other pieces of information that can be derived by exercise testing, such as blood pressure response, heart rate response, and exercise capacity [41–44]. Although technically challenging to perform in the same session, the addition of cardiopulmonary testing may also add prognostically important information [45–47].
Inducible LVOT obstruction
Increased LVOTG during exercise may be attributable to effort-related symptoms in patients with HCM, whereas the relation between provoked LVOTG and clinical outcomes remains uncertain. Inducible LVOT obstruction was associated with worse outcomes in 5 studies, [48–52] whereas it showed no association with prognosis in other 6 [41, 42, 53–56]. In the most recent one, Lu and coworkers [55] examined 705 HCM patients (91% with treadmill SE) and, surprisingly, found that patients with obstruction only on provocation had the lowest event rates and the best event-free survival for composite cardiovascular outcome compared to nonobstructive and rest obstructive patients. The same group revealed also that there is a bi-modal distribution of events and exercise LVOTG. In detail, latent obstructive patients can be subdivided into a benign latent subgroup (rest LVOTG < 30 mmHg & provoked gradient 30–89 mmHg) and an adverse latent sub-group (rest LVOTG < 30 mmHg & provoked gradient ≥ 90 mmHg). They revealed that the benign latent HCM subgroup had the best event-free survival among the overall four categories, followed by the adverse latent, non-obstructive, and rest obstructive groups [57]. This pattern is reminiscent of that observed in children based on resting gradients only, and suggests that, in specific scenarios, the capacity to generate gradients with effort is a reflection of a preserved contractile reserve, rather than a marker of risk [38].
Evidence of ischemia and CFVR
Regional LV WMAs during stress are relatively infrequent findings in patients with HCM, occuring in about 6–13% of patients. They have a complex and multifactorial pathophysiology and are usually not related to epicardial coronary artery disease [53, 54, 56, 58, 59]. It is more frequently detected during physical than pharmacological stress and carries a substantial risk of future adverse events [53, 56, 60, 61]. Patients with peak exercise WMAs are characterized by poorer exercise capacity, lower rest and peak stress ejection fraction, lower rest and post-exercise LVOTG, and more extensive myocardial fibrosis on cardiac resonance imaging [53, 58]. In 2015, Peteiro et al. [53] demonstrated that exercise WMAs have an independent prognostic value in predicting follow-up events and that their prognostic value is additive to the presence of extensive myocardial fibrosis.
Another ischemia-related stress parameter of the utmost importance is CFVR of the left anterior descending (LAD) coronary artery during vasodilator SE. CFVR during dipyridamole or adenosine SE is thought to be a marker of microvascular function in HCM, a complex and multifactorial phenomenon [62]. CFVR ≤ 2 proved to be a powerful predictor of unfavorable outcomes in several studies [61, 63, 64]. Cortigiani and his colleagues [61] prospectively evaluated 68 HCM patients undergoing dipyridamole SE with CFVR evaluation. At a median follow-up of 22 months, decreased CFVR was an independent predictor of future adverse events including death, non-fatal myocardial infarction, ICD implantation, hospitalization for HF or unstable angina, syncope, and AF. In their study, reduced CFVR was related to symptoms, greater LV wall thickness, and LVOT obstruction. However, its prognostic value was independent of all these parameters. Furthermore, asymptomatic individuals with decreased CFVR had a 10-fold greater risk for events compared to asymptomatic patients with preserved CFVR. Subsequent studies on larger sample sizes and longer follow-ups confirmed the prognostic value of vasodilator CFVR in HCM [54, 64]. Tesic et al. [64] subjected 150 HCM patients to CFVR evaluation with adenosine. During a median follow-up of > 7 years, 52% of patients with reduced CFRV experienced cardiac events compared to 9% of patients with preserved CFVR (P < 0.001). Patients with impaired CFVR were more often females, with more advanced functional class, had a greater need for diuretic therapy, greater LV wall thickness, larger LA, lower septal e’, higher E/e’, higher RV systolic pressures, higher baseline heart rate, and higher resting coronary flow velocity when compared to patients with preserved CFVR. At multivariable Cox analysis, CFVR ≤ 2 was the only parameter that independently predicted poor cardiac outcomes, with an odds ratio of 6.5 (confidence interval 2.8–16.3).
Ejection fraction, MR, E/e’, and pulmonary systolic arterial pressure during exercise
During the last few years, many studies have brought attention to other SE imaging metrics that may help to better risk stratify patients with HCM. Peteiro and coworkers [56] examined the feasibility and prognostic value of comprehensive echocardiography at peak and early post-exercise treadmill SE. They found that several variables offered incremental prognostic value, namely peak exercise LVEF, peak stress WMAs, and post-exercise E/e’ ≥ 14. Their results showed that the worst outcome corresponded to patients with WMAs at peak exercise (fixed or new) and raised post-exercise E/e’. The annualized hard event rate was 5.9% for patients with elevated post-exercise E/e’ and peak WMAs compared to the 4.0% event rate for patients with elevated post-exercise E/e’ and normal wall motion. Notably, they also showed that patients with exercise WMAs and elevated E/e’ (≥ 14) during treadmill exercise have almost 5-fold higher annual hard event rates when compared to patients with exercise WMAs but low E/e’ (5.9% vs. 1.2%). This is an important message since, even in the presence of WMAs during stress, the operator should not refrain from searching for additional predictors such as E/e’ (Figure 5). In the same study, blunted ejection fraction (≤ 65%) and MR (moderate or severe) on exertion also identified HCM patients at higher risk [56]. It is important to evaluate exercise-induced MR which is a dynamic phenomenon and is frequently associated with LVOT obstruction and systolic anterior motion in HCM patients [52]. Data on its prognostic value is limited since only two small studies were available before Peteiro’s work in 2021 [56]. Exercise-induced MR was associated with adverse cardiovascular events in both studies [50, 52], although in one of them [52] the authors questioned its real independent prognostic value. They stated that it may define a group of patients with either a severe LVOT obstruction or morphologic abnormalities who have a higher risk of cardiovascular events. In addition, similarly to LVOT obstruction, the different methods and definitions used for the evaluation of exercise MR limits our understanding of its functional and prognostic role in HCM.
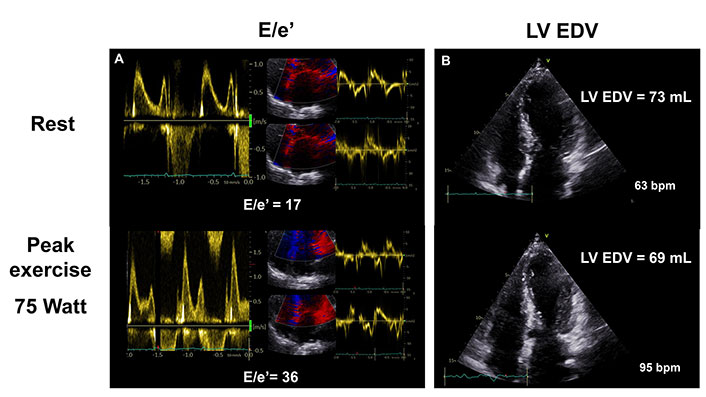
A 66-year-old nonobstructive female HCM patient with dyspnea and chest paint on effort and negative coronary angiography. Exercise SE revealed worsening diastolic dysfunction indicated by increased E/e’ ratio (panel A) and falling LV end-diastolic volume (EDV) at peak exercise (panel B). bpm: heart beats per minute
During exercise, approximately 1 out of 5 HCM patients with normal resting systolic pulmonary artery pressure (SPAP) develop PHT which, according to recent studies, may play a role in risk stratification (Figure 6) [65, 66]. Hamatani et al. [65] found that HCM patients with PHT during semi-supine exercise (stress SPAP ≥ 60 mmHg) have a higher cumulative incidence of HCM-related events. In a recent study, Re et al. [66] used a lower cut-off (stress SPAP > 40 mmHg) and showed that exercise PHT is linked to higher risk for a composite endpoint comprising death, heart transplantation, aborted SCD, nonfatal myocardial infarction, stroke or HF hospitalization.
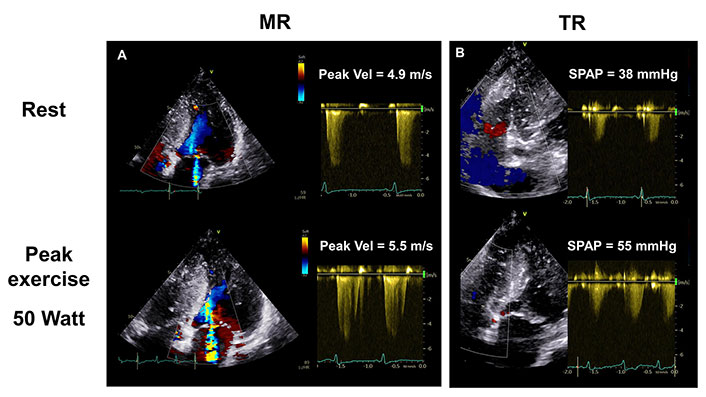
Increasing MR (panel A) is associated with worsening pulmonary hypertension in a 59-year-old woman with nonobstructive HCM during exercise SE. Estimated SPAP rose from resting 38 mmHg to 55 mmHg at low peak workload (panel B). TR: tricuspid regurgitation
Future perspectives
The ABCD protocol is a new and promising SE methodology that provides comprehensive information on the different vulnerabilities of HCM patients. This approach makes it possible to uncover concealed myocardial ischemia, pulmonary congestion due to diastolic dysfunction, preload reserve and contractile reserve impairment, coronary microcirculatory dysfunction, and cardiac autonomic dysfunction easily and systematically [67]. The same approach proved to be feasible and effective in refining phenotypes and risk classification in patients with chronic coronary syndromes [68]. Large-scale prognostic validation of the protocol and other additive steps in HCM has started recently as a specific subproject of the SE 2030 study endorsed by the Italian Society of Echocardiography [67].
Conclusion
The precise prognostic evaluation of HCM patients requires a detailed echocardiographic structural and functional assessment. Several echocardiographic variables contribute to patients’ risk stratification and have been incorporated in the clinical algorithms. Resting parameters with a well-established prognostic value are the degree of LVH, LV systolic and diastolic function, LA size and function, and LVOT obstruction. Some of these may worsen during exercise, such as diastolic dysfunction and MR, thus providing further information on patient outcomes. Other echocardiographic parameters such as WMAs or PHT have no prognostic relevance at rest but their presence on effort provide meaningful information on future adverse events. The impaired coronary microvascular function is a powerful prognostic indicator in patients with HCM and can only be assessed echocardiographically by CFVR measurement in the LAD during dipyridamole or adenosine stress.
Abbreviations
| AF: |
atrial fibrillation |
| CMR: |
cardiac magnetic resonance |
| E/e’: |
early LV inflow velocity to early tissue Doppler annulus velocity |
| EDV: |
end-diastolic volume |
| HCM: |
hypertrophic cardiomyopathy |
| HF: |
heart failure |
| ICD: |
implantable cardioverter-defibrillator |
| LA: |
left atrial |
| LV: |
left ventricular |
| LVEF: |
left ventricular ejection fraction |
| LVH: |
left ventricular hypertrophy |
| LVOT: |
left ventricular outflow tract |
| LVOTG: |
left ventricular outflow tract gradient |
| MR: |
mitral regurgitation |
| MWT: |
maximum wall thickness |
| PHT: |
pulmonary hypertension |
| RA: |
right atrial |
| RV: |
right ventricular |
| SCD: |
sudden cardiac death |
| SE: |
stress echocardiography |
| SPAP: |
systolic pulmonary artery pressure |
| TDI: |
tissue Doppler imaging |
| VT: |
ventricular tachycardia |
| WMAs: |
wall motion abnormalities |
Declarations
Acknowledgement
EDP was a recipient of the Erasmus+ grant for medical and doctorate students in Florence between September–December 2021 and January–December 2022.
Author contributions
ADF: Conceptualization, Investigation, Methodology, Data curation, Writing—original draft, Writing—review & editing. EDP: Investigation, Methodology, Data curation, Writing—original draft, Writing—review & editing, Visualization. GP: Investigation, Methodology, Data curation, Writing—original draft. IO: Conceptualization, Supervision, Writing—review & editing.
Ethical approval
Not applicable.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.