Abstract
Aim:
Transthoracic echocardiography is commonly used to assess coronary artery dilatation in Kawasaki disease (KD). However, existing criteria often miss early abnormalities. This study examines the utility of a new parameter, coronary external diameter index (CEDi), for early diagnosis and monitoring in KD.
Methods:
CEDi of left main (LM) and right coronary artery (RCA), calculated as the ratio of coronary artery external diameter (i.e., the distance between the outer coronary edges measured in the proximal segment of the artery) and the diameter of the aortic annulus, was evaluated in 34 patients (age 23 mouths ± 13 months) with KD at the hospital admission and after 2 weeks and 8 weeks of treatment. The control group consisted of 210 healthy children aged 20 months ± 13.4 months. Z-score charts for LM and RCA coronary external diameter (CED) were obtained.
Results:
Compared with controls, KD patients had a markedly higher mean value of LM CEDi (0.53 ± 0.06 vs. 0.33 ± 0.04; P < 0.0001) and RCA CEDi (0.48 ± 0.05 vs. 0.31 ± 0.04; P < 0.0001) at hospital admission. By ROC analysis, LM CEDi of 0.41, and RCA coronary artery thickness index (CATi) of 0.39 were the best cut-offs to confirm the clinical diagnosis of KD, both exhibiting 100% sensitivity and specificity. Mean LM CEDi and RCA CEDi values decreased significantly (P < 0.0001) after 2 weeks of follow-up and were similar to controls (P = 0.53 and P = 0.12, respectively) 8 weeks after admission.
Conclusions:
In patients with KD, CEDi of LM and RCA is an accurate parameter to evaluate coronary artery involvement in the early phase of the illness and during follow-up.
Keywords
Kawasaki disease, echocardiography, Z-score, coronary artery dilatationIntroduction
Coronary artery aneurysm is the most serious complication of Kawasaki disease (KD), with prevalence ranging from 25% in untreated patients to 8% in those treated with a four low-dose immunoglobulin protocol to 5% in those treated with a single high-dose immunoglobulin protocol [1–5]. Transthoracic echocardiography is the first-line imaging technique to evaluate coronary lumen dilatation and aneurysm growth in KD [1–3, 6–8], allowing the classification of coronary damage [9, 10]. In particular, the coronary artery Z-score represents the widely used method to assess coronary artery dilatation in KD [11–17]. However, it shows a normal value in about 75% of cases at the time of hospital admission as the dilatation usually develops 7–10 days later [6–10]. Therefore, an early normal echocardiogram does not rule out the diagnosis of KD [6, 18], which can cause severe clinical relapses particularly in subjects with clinically incomplete clinical presentation [18, 19].
In this prospective study, we investigated the usefulness of a new echocardiographic parameter, such as the external coronary external diameter index (CEDi), for the early identification and follow-up of coronary artery involvement in patients with KD.
Materials and methods
Patients
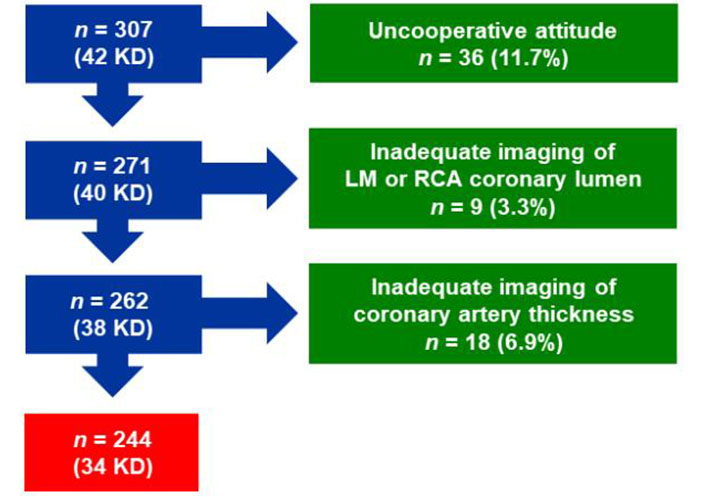
From January 2010 to September 2020, 42 patients with KD were admitted to the Pediatric Department of San Luca Hospital, Lucca, Italy, and underwent transthoracic echocardiography. Of these patients, 2 were admitted from March 2020 to September 2020 during the coronavirus disease (COVID) pandemic. Two patients were excluded due to an uncooperative attitude. Of the remaining 40 patients, 6 (15%) were excluded due to inadequate imaging of left main (LM; n = 1) or right coronary artery (RCA) lumen (n = 1) or coronary artery thickness (CAT; n = 4). Thus, 34 patients, including 17 males; mean [± SD (standard deviation)] age 23 months ± 13 months, formed the study group. Of them, 28 met the diagnostic criteria for KD [1, 6, 12], while 6 patients had an incomplete clinical presentation of KD, and the diagnosis was confirmed by additional abnormal laboratory criteria.
Complete clinical recovery was achieved in all patients after treatment with aspirin and a single (n = 31) or double infusion of high-dose (2 g/kg) immunoglobulin (n = 3).
The control group consisted of consecutive children aged between 2 months and 48 months and with echocardiographic evidence of structurally and functionally normal heart who were referred from January 2010 to December 2013 to the outpatient echo lab of the San Luca Hospital for evaluation of heart murmur, musculoskeletal pain, electrocardiographic abnormalities, family history of congenital heart defects, minimal and hemodynamically insignificant findings such as patent foramen ovale, mild tricuspid or pulmonary valve regurgitation. Of the 265 initial children, 34 were excluded due to uncooperative attitude. Of the remaining 231 children, 21 (9%) were excluded due to inadequate imaging of the coronary artery lumen (3 LM, 3 RCA, 1 both arteries) or CAT (10 LM, 3 RCA, 1 both arteries). Consequently, the remaining 210 healthy children [125 males; mean (± SD) age 20 months ± 14 months] served as controls. The enrollment into the study is shown in Figure 1, reporting how many individuals were excluded at each exclusion step.
Coronary artery measurements
Echocardiographic studies were performed using a Philips iE33 Ultrasound System equipped with a high-frequency phased-array sector scanning probe (S8-3 ultrasound probe by Philips Healthcare) and second harmonic technology. The aortic annulus diameter, coronary lumen diameter, coronary external diameter (CED), CED Z-score, CEDi, and CAT of LM and RCA were evaluated in controls and KD patients at hospital admission. In these last subjects the same parameters were assessed also after 2 weeks and 8 weeks of treatment.
The diameter of the aortic annulus was measured in the standard parasternal long axis view, at early systole, inner to inner edge from hinge point to hinge point of the aortic cusps (Figure 2) [16].
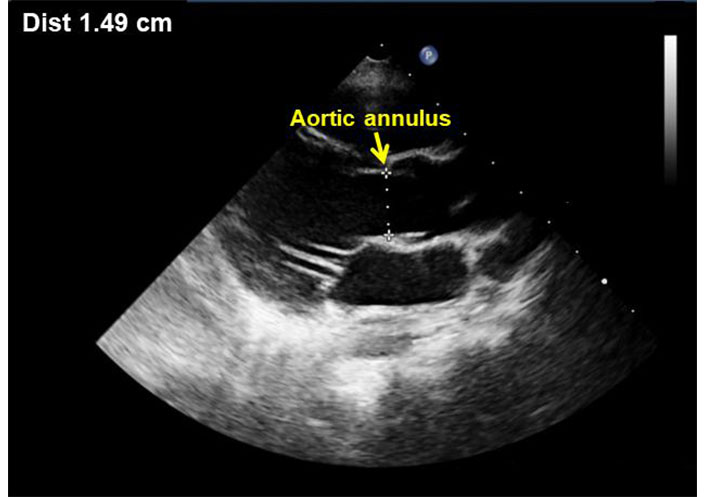
Parasternal long axis view showing measurement of the aortic annulus during end-systole
The short-axis parasternal view was used for LM and RCA measurements. In particular, the coronary lumen was obtained as the distance between the inner edges of the arteries [6, 13, 14], while CED as the distance between the outer edges of the arteries, thus including the lumen diameter and the thickness of the arterial wall (Figure 3). The measurements were made during early diastole and the optimal point was chosen 3–5 mm from the origin of the coronary artery. In the case of inadequate imaging of the coronary arteries from a short-axis parasternal view, a modified 5-chamber apical approach was adopted (Figure 4). At least 5 measurements were made in each subject and the results were averaged. All measurements were normalized by body surface area (BSA), calculated using the Haycock formula [BSA = 0.024265 × height (cm)0.3964 × weight (kg)0.5378], which is the best predictor of cardiovascular sizes [11, 12].
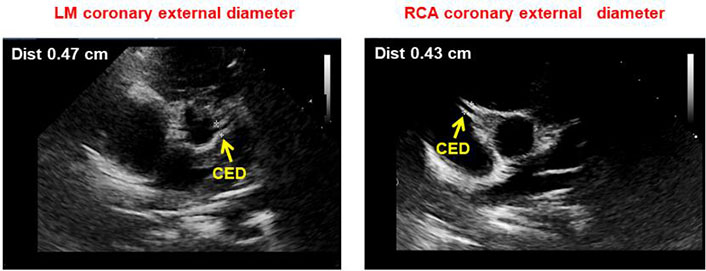
Parasternal short axis view showing LM (left) and right (right) CED measurement as the distance between the outer edges of the arteries
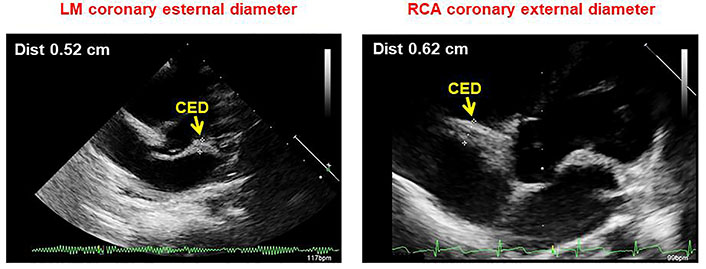
Apical modified 5-chamber view showing LM (left) and right (right) CED measurement as the distance between the outer edges of the arteries
LM and RCA CEDi were obtained from the ratio of the external diameter of the coronary artery to the diameter of the aortic annulus. Examples of the calculation of LM and RCA CEDi in a normal subject and a patient with KD are shown in Figure 5.
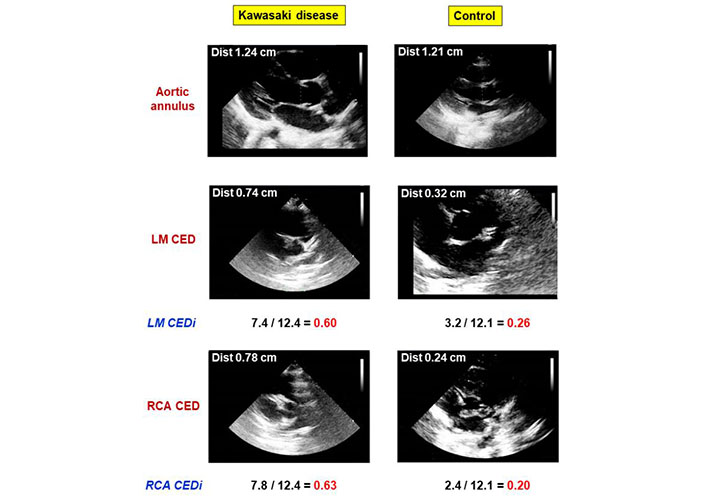
Comparative analysis of LM and RCA CEDi calculations in a patient with KD and a healthy subject. The left panels show the calculation of LM and RCA CEDi in a patient with KD. The aortic annulus (top), LM CED (middle), and RCA CED (bottom) were 12.4 mm, 7.4 mm, and 7.9 mm, respectively. Thus, LM CEDi of 0.60 and RCA CEDi of 0.63 were derived. The right panels show the calculation of LM and RCA CEDi in a normal subject. The aortic annulus (top), LM CED (middle), and RCA CED (bottom) were 12.1 mm, 3.2 mm, and 2.4 mm, respectively. Thus, LM CEDi of 0.26 and RCA CEDi of 0.20 were derived
CAT was obtained as the difference between CED and internal coronary lumen. All the studies were performed by the same senior sonographer (Andrea Azzarelli).
Statistical analysis
Continuous variables are expressed as mean ± SD. Differences between groups were compared using Student’s t and chi-square test, as appropriate. Correlations of CED and CEDi with age, weight, height, and BSA in the control group were estimated with Pearson’s coefficients. A receiver operating characteristic analysis was adopted to determine the best value of LM CEDi and RCA CEDi to confirm the clinical diagnosis of KD. Statistical significance was set at P < 0.05. Inter-observer and intra-observer agreement was calculated based on overall agreement (observed percent exact agreement) and tested using linear regression analysis.
By analyzing the data of the 210 subjects of the control group, the Z-score tables for LM and RCA CAT were obtained. Since the assumption of logistic regression is that the residuals should be normally distributed, the Shapiro-Wilk and Kolmogorov-Smirnov tests have been used to evaluate the normality of the residuals [11]. The regression equation with the highest R2 (goodness-of-fit) was selected for LM and RCA CAT. The heteroskedasticity used to describe the behavior of the variance of the residuals was then verified using the White test and the Breusch-Pagan test. The statistical package for the social sciences (IBM SPSS Statistics V22.0) was used for the analysis.
Results
Out of the total population included in the study (KD patients and controls), the number of subjects who were able to undergo an adequate examination of both coronary arteries was 244/307 (79%).
The following Table 1 shows the best-fit model, its R2 value, and the regression equation that allows deriving the Z-score plots for LM and RCA CED (Figure 6) in the group of 210 normal subjects. In these individuals, LM and RCA CED correlated significantly with age, height, weight, and BSA, whereas LM and RCA CEDi failed to do it (Table 2).
Coefficients for regression equations relating echocardiographic measurements and BSA
| Measurement | Intercept | B | SEE (√MSE) | R2 | SW | KS | BP | W |
|---|---|---|---|---|---|---|---|---|
| RCA CED | 1.506 | 0.448 | 0.128 | 0.484 | 0.255 | 0.087 | 0.471 | 0.600 |
| LM CED | 1.605 | 0.471 | 0.134 | 0.485 | 0.385 | 0.200 | 0.114 | 0.187 |
| BSA Haycock. (ln[y] = a + b*ln[x]); Z value = exp((ln[Measurement] – (Intercept + B*ln[BSA]))/√MSE) | ||||||||
B: regression coefficient; SEE (√MSE): standard error of the estimate; R2: coefficient of determination; SW: Shapiro-Wilk test; KS: Kolmogorov-Smirnov test; BP: Breusch-Pagan test; W: Wald test
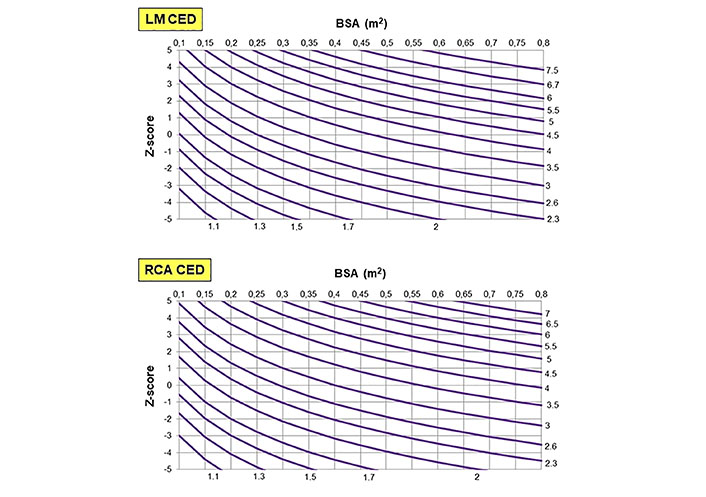
Percentile charts for LM CED (top), and RCA CED (bottom) according to BSA calculated using the Haycock formula
Relation between age, height, weight, and BSA with CED and CEDi of LM and RCA in controls
| CED and index | Age | Height | Weight | BSA | ||||
|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | r | P-value | |
| LM CED | 0.63 | < 0.001 | 0.66 | < 0.001 | 0.68 | < 0.001 | 0.68 | < 0.001 |
| RCA CED | 0.38 | < 0.001 | 0.43 | < 0.001 | 0.46 | < 0.001 | 0.46 | < 0.001 |
| LM CEDi | 0.08 | 0.22 | 0.04 | 0.51 | 0.06 | 0.35 | 0.06 | 0.39 |
| RCA CEDi | 0.02 | 0.74 | 0.13 | 0.90 | 0.01 | 0.84 | 0.01 | 0.90 |
The main clinical and echocardiographic parameters in KD patients on admission and in controls are shown in Table 3. No difference between groups was observed for age, height, weight, and BSA, while aortic annulus diameter was greater (P = 0.001) in subjects with KD.
Clinical and echocardiographic characteristics of patients with KD at admission and controls
| Clinical and echocardiographic characteristics | KD patients (n = 34) | Control subjects (n = 210) | P-value |
|---|---|---|---|
| Age (months) | 23 ± 13 | 20 ± 14 | 0.15 |
| Height (cm) | 84 ± 14 | 81 ± 16 | 0.28 |
| Weight (kg) | 11 ± 3 | 11 ± 4 | 0.63 |
| BSA | 0.51 ± 0.10 | 0.49 ± 0.13 | 0.44 |
| Aortic annulus diameter (mm) | 11.8 ± 1.5 | 10.7 ± 1.7 | 0.001 |
| LM lumen diameter (mm) | 2.3 ± 0.7 | 1.6 ± 0.3 | < 0.0001 |
| LM lumen Z-score | 0.5 ± 0.3 | 0.2 ± 0.1 | < 0.0001 |
| LM lumen Z-score ≥ 2 | 4 (12%) | 0 | < 0.0001 |
| LM CED (mm) | 6.3 ± 1.1 | 3.6 ± 0.6 | < 0.0001 |
| LM CED Z-score | 4.1 ± 1.2 | 0.01 ± 1 | < 0.0001 |
| LM CAT (mm) | 3.9 ± 0.9 | 1.9 ± 0.5 | < 0.0001 |
| LM CEDi | 0.5 ± 0.1 | 0.3 ± 0.1 | < 0.0001 |
| RCA lumen diameter (mm) | 2.0 ± 0.5 | 1.6 ± 0.3 | < 0.0001 |
| RCA lumen Z-score | 0.4 ± 0.2 | 0.2 ± 0.1 | < 0.0001 |
| RCA lumen Z-score ≥ 2 | 3 (9%) | 0 | < 0.0001 |
| RCA CED (mm) | 5.7 ± 1.1 | 3.3 ± 0.6 | < 0.0001 |
| RCA CED Z-score | 4.1 ± 1.2 | 0.01 ± 0.9 | < 0.0001 |
| RCA CAT (mm) | 3.7 ± 1.0 | 1.7 ± 0.4 | < 0.0001 |
| RCA CEDi | 0.4 ± 0.1 | 0.3 ± 0.1 | < 0.0001 |
CED, CEDi and CAT of LM and RCA were markedly higher (P < 0.0001) in KD patients than in controls (Table 3). The former had also a larger lumen diameter (P < 0.0001) of either LM or RCA. However, an abnormal Z-score (≥ 2) for LM and RCA at admission was found in only 4 (12%) and 3 (9%) KD patients, respectively (Table 3).
With a ROC analysis, LM CEDi of 0.41, and RCA CEDi of 0.39 at hospital admission were the best cut-offs to confirm the clinical diagnosis of KD, both showing 100% sensitivity and specificity.
In KD patients, CEDi of LM and RCA decreased progressively at 2 weeks and 8 weeks of follow-up (Figure 7). In particular, at 8 weeks the mean value of LM and RCA CEDi were comparable to those of controls (P = 0.53 and P = 0.12, respectively), and 33 of 34 patients had LM CEDi < 0.41 and RCA CEDi <0.39. The mean values of CED, CED Z-score, CEDi, and CAT of LM and RCA in KD patients at admission, and 2 weeks and 8 weeks after hospitalization, as shown in Table 4.
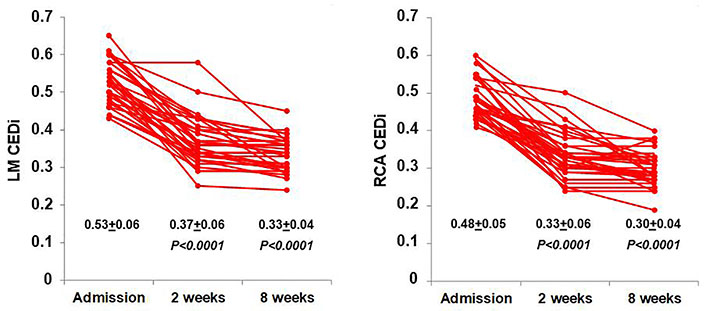
Behavior of LM (left) and RCA CEDi (right) values evaluated at hospital admission, after 2 weeks and 8 weeks in the 34 patients with KD
Values of CED, CED Z-score, CEDi, and CAT of LM and RCA in KD patients at admission, and 2 weeks and 8 weeks after hospitalization
| Echo Stages | LM CED | LM CED Z-score | LM CEDi | LM CAT | RCA CED | RCA CED Z-score | RCA CEDi | RCA CAD |
|---|---|---|---|---|---|---|---|---|
| Admission | 6.3 ± 1.1 | 4.1 ± 1.2 | 0.5 ± 0.1 | 3.9 ± 0.9 | 5.7 ± 1.1 | 4.1 ± 1.2 | 0.4 ± 0.1 | 3.7 ± 1.0 |
| 2 weeks | 4.2 ± 0.8 | 0.8 ± 1.2 | 0.4 ± 0.1 | 2.6 ± 0.7 | 3.9 ± 0.1 | 0.8 ± 1.1 | 0.3 ± 0.1 | 2.3 ± 0.8 |
| 8 weeks | 4.0 ± 0.5 | 0.5 ± 0.7 | 0.3 ± 0.1 | 2.1 ± 0.7 | 3.5 ± 1.1 | 0.3 ± 0.9 | 0.3 ± 0.1 | 1.9 ± 0.7 |
In the assessment of inter-observer variability by 2 independent observers (Andrea Azzarelli, Lauro Cortigiani Cuono Cucco) in a set of 40 individuals (20 with KD and 20 controls), a significant correlation was found for LM CED (r = 0.97), RCA CED (r = 0.96), LM CEDi (r = 0.96), and RCA CEDi (r = 0.95), and the agreement for LM CEDi > or < 0.41 and RCA CEDi > or < 0.39 was evidenced in all cases. In the same way, the intra-observer variability was very high (r = 0.99) for both CED and CEDi.
Discussion
In this prospective study on KD patients with a relatively low level of coronary dilatation, we found that CEDi of LM and RCA is a feasible and accurate tool for assessing and monitoring coronary artery involvement. In particular, LM CEDi ≥ 0.41 and RCA CEDi ≥ 0.39 at hospital admission provided a diagnostic sensitivity and specificity of 100%, allowing confirmation of the diagnosis of KD even in the case of incomplete clinical presentation. Of interest, the parameter progressively decreased to normalize in 97% of cases at 8 weeks of follow-up.
CEDi shows a stable value in the whole age group considered as it is not affected by BSA. However, the aortic annulus diameter is the determinant for its calculation, and CEDi cannot be used in children with aortic disease accordingly. For this reason, we provided Z-score nomogram charts describing the correlation of LM and RCA with BSA. Our charts are practical and easy to applicate clinicians. In fact, by measuring the external coronary artery diameter and using the Z-score normograms it is possible to determine the normal range regardless of the presence of aortic artery disease.
Comparison with previous studies
KD is characterized by systemic vasculitis involving the medium-sized arteries of multiple organs including the heart [6, 20–22]. Coronary artery involvement can result in a coronary artery aneurysm, ranging from a slight dilatation to the formation of a giant aneurysm [1, 7, 23, 24], usually appearing eight days after the onset of fever. Although the incidence of coronary artery aneurysm has been reduced to approximately 5% by immunoglobulin treatment [1–4, 9, 10, 23], it remains the most serious complication of KD. Echocardiography is the first-line method for identifying and characterizing changes in the coronary arteries, due to its non-invasive nature and good accuracy. Of note, the coronary artery lumen can be evaluated in most segments of the coronary artery [1–3, 5, 25–27]. The first classification of coronary dilatation was based on the absolute or relative lumen diameter, regardless of patient size, weight, or BSA [24]. In recent years the Z-score method, which normalizes the lumen of the coronary artery to the BSA, representing the number of standard deviations from the mean, has been increasingly adopted to evaluate coronary artery dilatation [12, 17]. A classification of coronary dilatation based on Z-score and raw size has recently been recommended [6]. However, approximately 75% of KD patients have normal coronary arteries on admission echocardiogram, and 30% to 50% of those with a normal coronary lumen (Z-score < 2) show a progressive reduction in lumen size during follow-up, suggesting initial dilatation that resolves within 4–8 weeks [6–9]. Consequently, normal or mild dilatation of the coronary lumen has a low positive predictive value for the early diagnosis of KD, adversely affecting the accuracy of the echocardiogram at admission [28–30]. This lack of sensitivity of the echocardiographic technique is particularly relevant in individuals with clinically incomplete KD and in children less than 1 year of age who are at increased risk of coronary aneurysms [18, 19]. Our results confirm the lack of sensitivity of the LM and RCA lumen Z-score at hospital admission, demonstrating abnormal values in only 12% and 9% of cases, respectively. Conversely, LM CEDi ≥ 0.41 and RCA CEDi ≥ 0.39 were observed in all patients with KD at hospital admission and in no subjects of the control group.
Clinical implications
The increase in CEDi in KD is likely due to the involvement of the coronary wall and pericoronary tissue during the acute inflammatory phase, thus resulting not only in the slight dilatation of the coronary lumen but also in the increased thickness of the coronary artery wall. Previous reports with ultrasound and non-ultrasound (optical coherence tomography) techniques have shown the increased coronary wall thickness in the acute phase of KD, and its reversibility with treatment [20, 31, 32], corroborating the results of the current study reporting markedly higher mean values of CAT at admission in KD patients compared to controls.
Assessment of CEDi may have important clinical implications for the management of patients with KD. Since CEDi of LM and RCA was increased in all of our patients at hospital admission but in no control, it may represent a useful additional parameter to confirm the diagnosis of KD allowing for early starting medical therapy. Furthermore, the serial evaluation of CEDi during the follow-up can allow use to evaluate the evolution of the coronary involvement.
Study limitations
Our study is single center and all examinations were performed by the same expert pediatric echocardiographer. Furthermore, the number of patients is quite small despite the 10-year enrollment period, which is largely due to the relatively low incidence of KD in our country. Therefore, it would be desirable if our preliminary results were confirmed by a multicenter study conducted on a larger patient population. Sedation for uncooperative patients was not available in our echo lab and this fact did not allow use to investigate all consecutive patients hospitalized for KD thus reducing the feasibility of the study. Since the control group consisted of afebrile subjects without echocardiographic evidence of cardiac disease, it remains to be defined whether CEDi is a specific marker of KD. The size of the diameter of the aortic annulus was slightly greater in KD patients than in controls confirming the results of previous experiences [29, 30]. However, this fact seems to strengthen our findings by lowering the CEDi value in KD patients compared to controls.
Abbreviations
| BSA: |
body surface area |
| CAT: |
coronary artery thickness |
| CED: |
coronary external diameter |
| CEDi: |
coronary external diameter index |
| KD: |
Kawasaki disease |
| LM: |
left main |
| RCA: |
right coronary artery |
Declarations
Author contributions
AA, CC and LC: Conceptualization, Investigation, Writing—original draft. VA, FB, RD, AV and FV: Investigation, Date curation. MS and CC: Formal Analysis. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study complies with the Declaration of Helsinki and was approved by the institutional ethic committee (Azienda USL Toscana Nordovest, decreto N. 1954, June 19, 2018).
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.