Affiliation:
1Cardiology Unit, University Hospital of Parma, 43126 Parma, Italy
ORCID: https://orcid.org/0000-0002-1291-0306
Affiliation:
2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA
ORCID: https://orcid.org/0000-0003-2236-6970
Affiliation:
3Division of Artificial Intelligence in Medicine, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA
ORCID: https://orcid.org/0000-0002-6110-938X
Affiliation:
4Unit of Cardiology, Department of Experimental, Diagnostic and Specialty Medicine-DlMES, IRCCS AOU SOrsola-University of Bologna, 40126 Bologna, Italy
ORCID: https://orcid.org/0000-0002-4048-675X
Affiliation:
4Unit of Cardiology, Department of Experimental, Diagnostic and Specialty Medicine-DlMES, IRCCS AOU SOrsola-University of Bologna, 40126 Bologna, Italy
ORCID: https://orcid.org/0000-0001-8186-9717
Affiliation:
1Cardiology Unit, University Hospital of Parma, 43126 Parma, Italy
Email: ngaibazzi@gmail.com
ORCID: https://orcid.org/0000-0002-2207-3388
Explor Cardiol. 2024;2:31–39 DOI: https://doi.org/10.37349/ec.2024.00019
Received: October 23, 2023 Accepted: December 28, 2023 Published : February 29, 2024
Academic Editor: Andrea Borghini, Institute of Clinical Physiology - National Research Council (IFC-CNR), Italy
Myocardial bridging is a congenital defect characterized by the course of a segment of the coronary arteries within the heart muscle most frequently affecting the left anterior descending coronary artery (LAD). Patients with myocardial bridging may present with episodes of exertional anginal chest pain. The gold standard for non-invasive diagnosis of myocardial bridge is coronary computed tomography angiography (CCTA), which allows anatomical characterization. Coronary flow velocity reserve (CFVR) of the LAD on stress echocardiography (SE) can play an important role in the diagnosis of myocardial bridging of the LAD; a relationship between CVFR-LAD and coronary inflammation by estimating the attenuation of peri-coronary adipose tissue at CCTA has been demonstrated in patients without obstructive ischaemic heart disease. Therefore, coronary inflammation localized to the LAD has been assessed in patients diagnosed with myocardial bridging of the LAD and previous SE with CFVR-LAD in a case series.
Myocardial bridging is a congenital anomaly characterized by a coronary artery segment that takes a “tunnel-like” path within the heart muscle, most frequently involving the left anterior descending coronary artery (LAD). Patients with myocardial bridge often present with exertional chest pain; therefore, the same diagnostic tests indicated to confirm or exclude the presence of atherosclerotic obstructive ischemic heart disease are usually performed. In the diagnostic process of suspected chronic coronary syndrome, a predominant role is played by functional tests such as single-photon emission computed tomography (SPECT), stress echocardiography (SE) and anatomical tests, such as coronary computed tomography angiography (CCTA). As suggested by the European Society of Cardiology guidelines on chronic coronary syndrome, the choice of tests depends, among other clinical variables, on the center’s experience with a particular method and its availability [1–5]. In selected cases, provocative and anatomical examinations are sometimes performed in the same patient, in case of unclear results [6].
The role of coronary flow velocity reserve (CFVR) of the LAD during SE, according to the most recent scientific literature, is highly considered both in patients with suspected coronary artery disease (CAD) and in suspected microvascular angina [7–13].
The typical SE pattern in patients with a myocardial bridge is the absence of reversible wall motion abnormalities with reduced CFVR-LAD; however, to initially suspect the presence of a myocardial bridge, SE in particular if comprising LAD imaging can be helpful, as recently suggested [14].
In case of high suspicion for the presence of a myocardial bridge during SE, and in order to rule out obstructive CAD, in our center CCTA is often indicated, which in case of myocardial bridging is nowadays recognized as the diagnostic gold standard.
Attenuation of peri-vascular adipose tissue (PVAT) on computed tomography (CT) is a relatively new method that allows non-invasive estimation of localized vascular inflammation; the measurement is performed in post-processing on CT scans that have previously been performed and therefore there is no additive risk in terms of ionizing radiations to the patient [15].
This PVAT measurement has already been applied in several vascular districts, such as the carotid arteries, the aorta and even around the left atrium in patients with atrial fibrillation [16–20]. In particular, an increased prevalence of fibrosis on tissue histological analysis has been shown in surgically excised aneurysms of the ascending thoracic aorta, following the finding of a high PVAT value on prior CCTA [21].
The major field of application of the PVAT method is probably in CCTA, in which peri-coronary adipose tissue attenuation (PCAT) has shown robust correlation with histologically documented peri-vascular inflammation and an independent prognostic stratification capability [22].
The PCAT measurement is able to predict the risk of both cardiac and all-cause mortality, when the analysis is performed in the LAD or right coronary artery, in this case excluding the most proximal 20 mm of the coronary artery [23].
Starting from the demonstrated independent association between CFVR-LAD and PCAT in the LAD, when tested in a group of patients suffering from coronary microcirculatory dysfunction in the absence of obstructive CAD [24], we therefore decided to evaluate PCAT in the LAD of patients with a CCTA diagnosis of myocardial bridge after a recent SE, to parallelly highlight PCAT behavior and SE findings at the same time.
Philips ie33 (Philips Medical Systems) was used with the standard S5 transducer (1–5 mHz). The protocol used for accelerated high-dose dipyridamole (0.84 mg/kg per 6 min) SE has been described elsewhere [12]. Briefly, it consists in serial assessments of wall motion abnormalities at rest and during pharmacological stress. Left ventricle ejection fraction was measured with Simpson’s biplane method. The left ventricle is divided in 17 segments according to the recommendations of the American Society of Echocardiography [25]. Segmental wall motion is graded as follows: normal = 1; hypokinetic = 2; akinetic = 3; and dyskinetic = 4. Reversible ischemia was defined as the occurrence of a stress-induced new dyssynergy or worsening of rest hypokinesia in ≥ 1 segment. Spectral Doppler CFVR-LAD was obtained as peak diastolic velocity stress/rest ratio, using a modified apical 3-chamber view, with small boluses of 0.3 mL microbubble ultrasound contrast (SonoVue, Bracco, Milan) if needed for signal enhancement, in conjunction with low mechanical index for color and pulse-wave Doppler imaging. Stress acquisition was performed 1 min after the end of the dipyridamole administration (0.84 mg/kg in 6 min).
Beta-blockers, non-dihydropyridine calcium-channel blockers or nitroderivatives were always suspended at least 48 h before SE.
All coronary CCTA examinations were performed using a Dual Source CT system (Somatom Definition FLASH, Siemens Healthcare, Forchheim, Germany). The data set was analyzed by two experienced readers using an off-line workstation software package (Leonardo, Siemens Medical Solutions, Forchheim, Germany). CCTA datasets of the reconstructed coronary vessels were created in the best phase of the cardiac cycle depending on the heart rate of the patient (end-diastolic cardiac phase usually set at 60% of the R-R interval or end-systolic phase set at 30% of the R-R interval). The presence of plaques was assessed using original axial images, multiplanar reconstruction, and cross-sectional reconstruction. The coronary segments were analyzed following the classification of the American Heart Association [26], and each segment was delimited by identifiable side-branches. For the final grading of stenosis severity, we used the following criteria for the LAD: 0 = no stenosis; 1 = mild stenosis (maximal diameter stenosis < 50%), 2 = intermediate stenosis (50–69%), 3 = severe stenosis (70–99%).
To measure PCAT one experienced reader used a dedicated image analysis software package (AutoPlaqueTM Version 2.5, Cedars-Sinai Medical Center). We traced proximal 40 mm segments of two major epicardial coronary vessels (right coronary artery 10 mm distal to the ostium, LAD starting right at the ostium) and defined peri-vascular fat as the adipose tissue within a radial distance from the outer vessel wall equal to the diameter of the vessel. We ascertained the PCAT by quantifying the weighted peri-vascular fat attenuation after adjustment for technical parameters based on the attenuation histogram of peri-vascular fat within the range −190 Hounsfield unit (HU) to −30 HU, as described previously [24].
In this study, we present several illustrative cases of myocardial bridging, along with the assessment of rest LAD imaging and CFVR-LAD on SE and PCAT-LAD using dedicated software (Autoplaque, Cedars Sinai), applied to the CCTA datasets. The method used for PCAT measurement and shows a curved planar reformation image of a deeply tunneled LAD coronary artery of a patient evaluated for chest pain: when fat attenuation quantification is applied to a vessel, fat tissue voxels are color-coded from green to red depending on their PCAT, measured in HU: green if closer to the –190 HU lower attenuation limit to define fat tissue, and red for the higher attenuation limit of fat tissue (–30 HU) is shown in Figure 1. According to this, the closer to –30 HU the more coronary inflammation is present, the closer to –190 HU the less inflammation is present. The concept of fat attenuation indirectly representing coronary inflammation is based on the changing adipocytes volume and water content as a paracrine response to coronary inflammation [22].
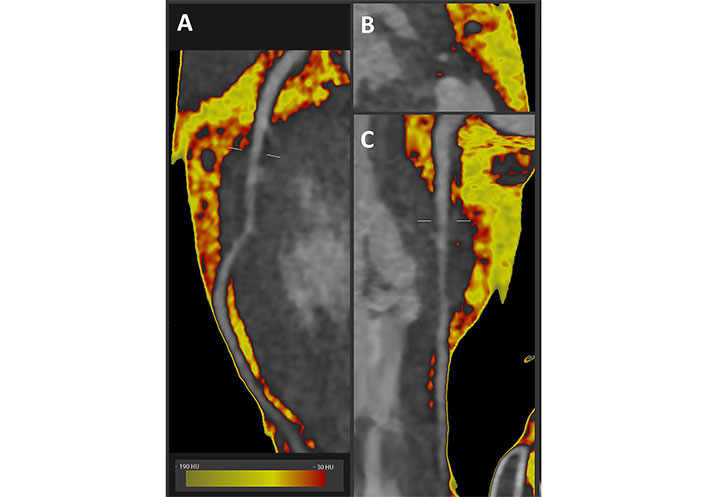
Fat attenuation CCTA imaging along the LAD in a deep myocardial bridge. A. Curved planar reformatted image; B. cross section image; C. straightened reformatted image
In brief, the rule of thumb is that a mean value of PCAT < –70 HU in the peri-coronary fat does not associate with inflammation, while > –70 HU (i.e. closer to 0) highlights the presence of inflammation. High values of PCAT (red pixels in Figure 1A, B and C respectively show curved, cross sectional or straightened planar reformation) are clearly seen here around the “tunneled” segment of the LAD artery.
The same case already demonstrated in Figure 1 is shown in Figure 2 together with echocardiography findings. The presence of the bridge was initially suspected on the contrast-enhanced color Doppler SE with vasodilators (Figure 2A) with LAD pulsed wave Doppler (PWD) sampling (Figure 2B), which shows the typical systolic flow reversal in proximity to the myocardial bridge segment. Also, in this case fat attenuation was performed and color-coded PCAT is shown as previously described (Figure 2C, D and E).
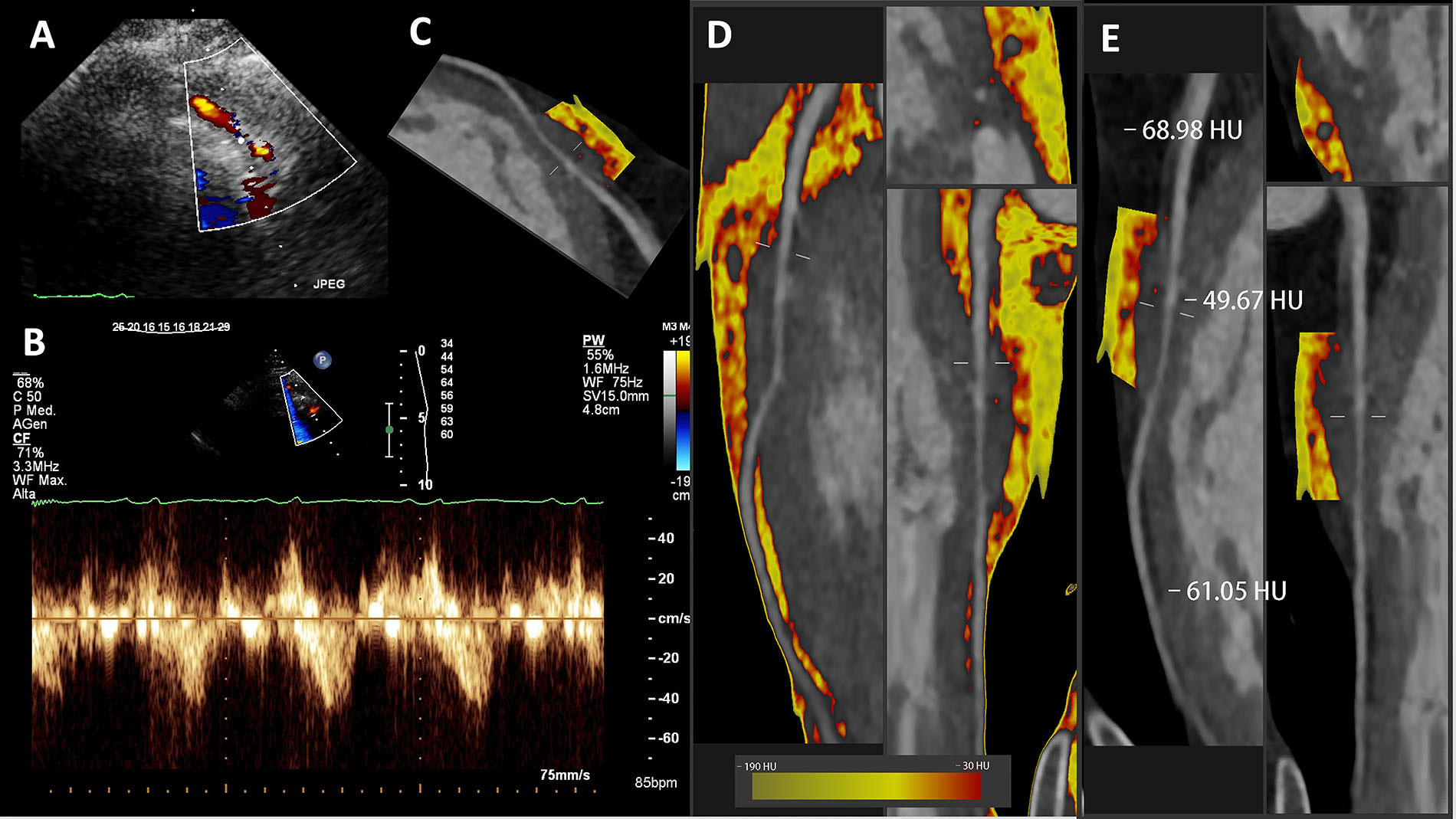
SE findings and fat attenuation imaging along the LAD in the same deep myocardial bridge showed in Figure 1. A. Color Doppler; B. Pulsed-wave Doppler; C–E. CCTA curved or straightened planar reconstructed images with color-coded PCAT
All the peri-coronary fat detected is shown in Figure 2D to demonstrate the paucity of fat in the distal part, while the volume is high in the proximal and mid segments. The red-coded (inflamed) component is also present to some extent in the proximal segment, but predominant in the mid part, in the context of the myocardial bridge segment.
The mean PCAT is higher than the –70 HU in all the three segments, but clearly much higher over the myocardial bridge segment (Figure 2E).
Another case of myocardial bridge suspected at dipyridamole contrast SE is shown in Figure 3A and B in a patient with chest pain, where systolic turbulent flow is clearly shown on Color Doppler “aliasing”.
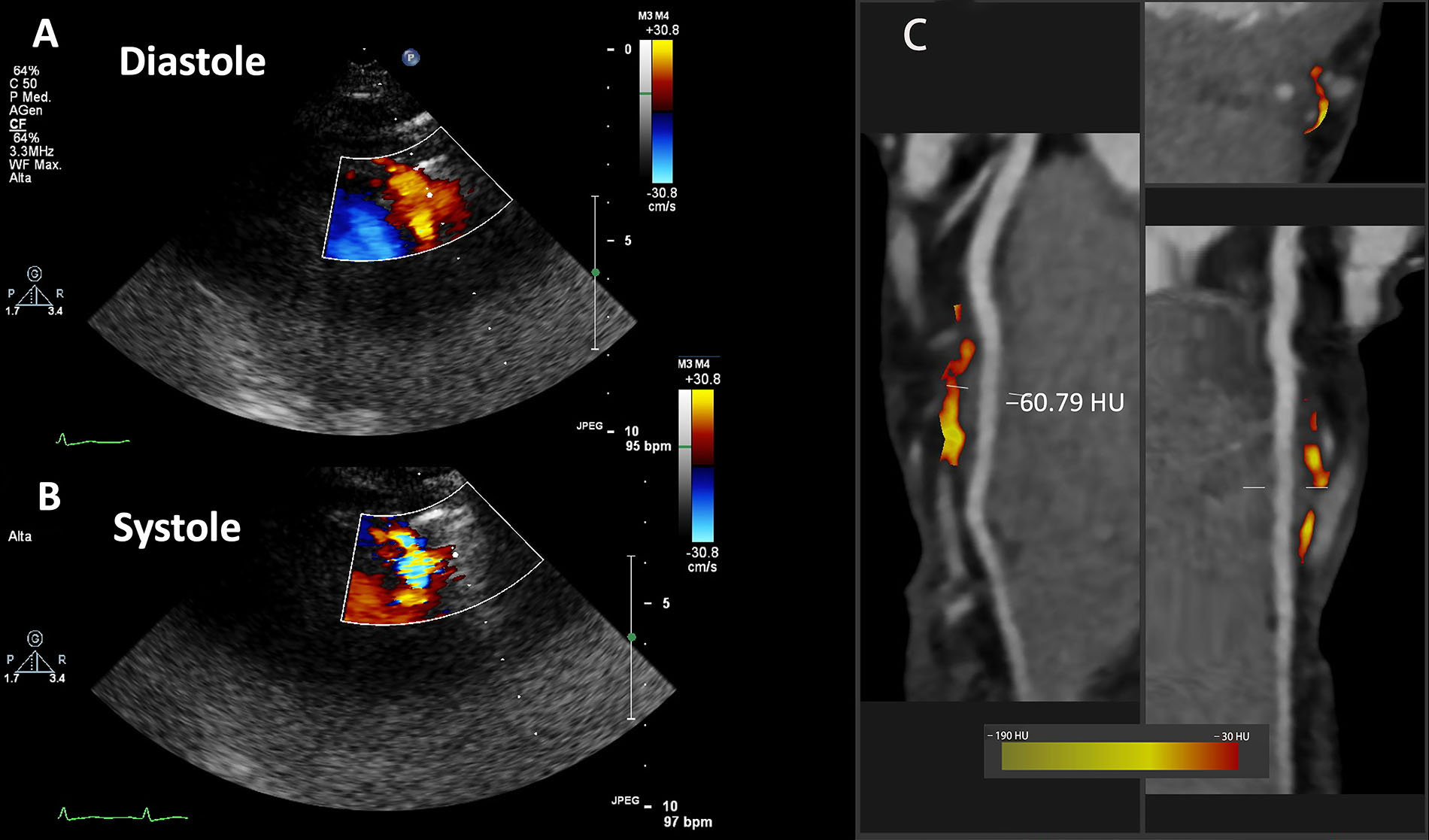
SE findings and fat attenuation imaging along the LAD in another case of bridge in the LAD. A. Color Doppler in diastole; B. Color Doppler in systole; C. CCTA curved, cross sectional or straightened planar reformation images
For further clinical evaluation this patient underwent CCTA, which confirmed the diagnosis of myocardial bridge in the mid LAD (less deep than the one shown in Figures 1 and 2). Moreover, the PCAT analysis limited to the myocardial bridge segment showed an inflammatory pattern with a mean value of –60.79 HU (Figure 3C).
As reported, all these cases show that the bridges segments are more inflamed at the CT PCAT analysis. It has been showed that myocardial bridging is associated with coronary endothelial dysfunction and altered vasoreactivity. Hence, we interpreted these values of PCAT in the context of vascular inflammation related to dysfunction of the endothelium and impaired vasoreactivity.
The case of a patient with coexisting tortuosity in the distal part of the LAD course and a superficially bridged myocardial segment in the mid portion is shown in Figure 4. This was suspected again on vasodilator SE (Figure 4A), which showed the typical “reverse S” sign of tortuosity on contrast color-Doppler. Remarkably, also in this case there is a mildly more inflamed PCAT in the tortuous tract with a mean value of –67.90 HU. We interpreted this mild inflammation in the context of an alteration in vasoreactivity in this segment and they are in line with some data that demonstrated a correlation between coronary tortuosity and microvascular dysfunction [27].
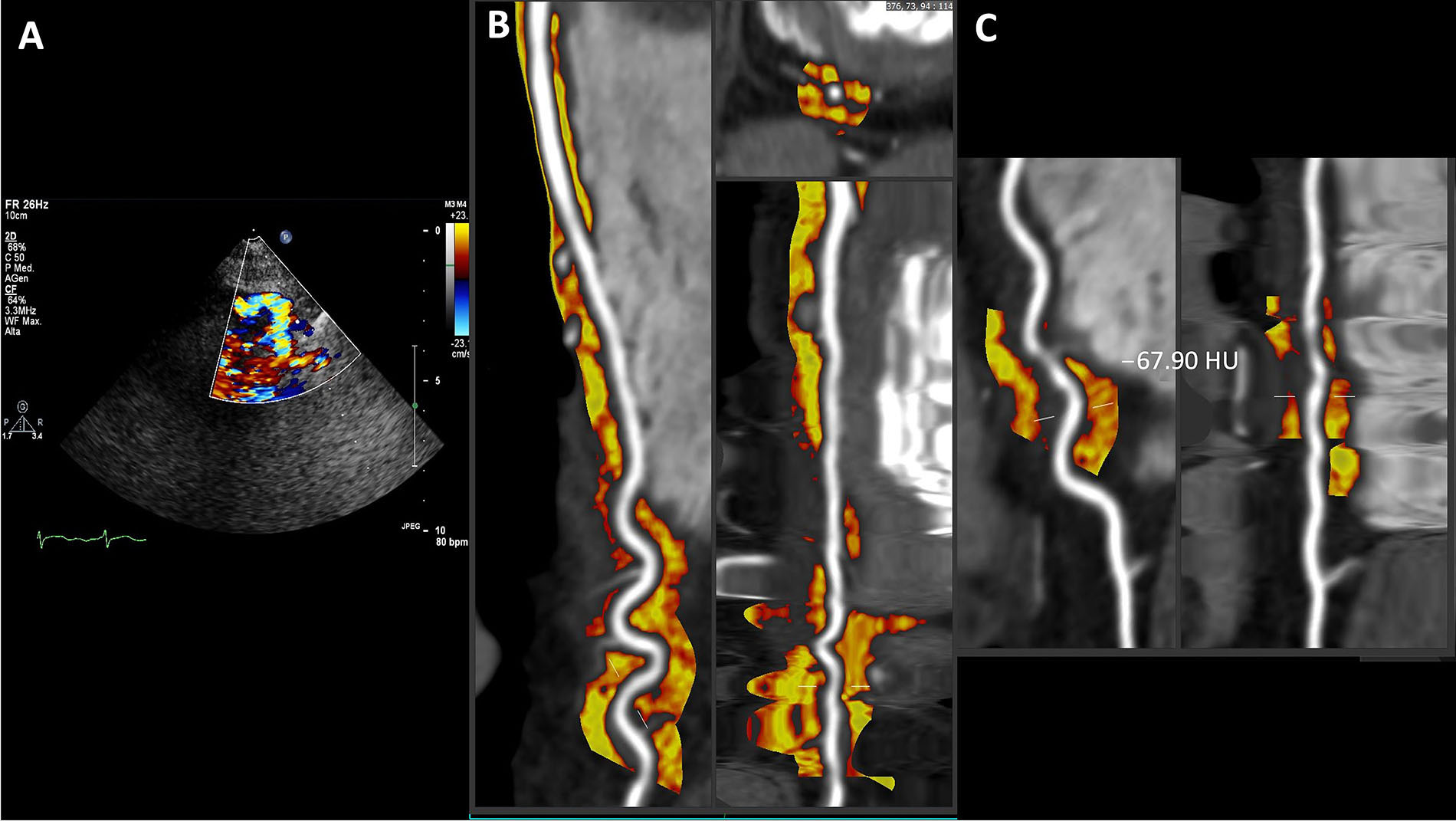
Coexistent tortuosity and bridging in LAD coronary artery. A. Color Doppler; B. curved, cross sectional or straightened planar reformation; C. focus on the tortuous tract with a mean PCAT value of –67.90 HU
As depicted in the images, our findings reveal significantly higher levels of inflammation (higher PCAT) along the bridged coronary segments when compared to the normal extra-myocardial segments of the LAD. The presence of coronary inflammation in myocardial bridges may be due to the previously documented endothelial dysfunction and altered vasoreactivity associated with this congenital anomaly. However, prior to the introduction of the PCAT measurement method, coronary inflammation in this setting had never been reported nor semi-quantitatively assessed using non-invasive imaging tools. On resting transthoracic echocardiography (TTE) and vasodilator SE, myocardial bridging can be suspected by 3 features in the LAD flow pattern: a peculiar “reverse S” sign on contrast color-Doppler; a negative component (flow reversal) of systolic flow on PWD; a (less specific) reduction of CFVR-LAD. Decreased CFVR has been linked to inflammation measured with PCAT in a prior study. This provides support for the theory that inflammation, reduced coronary flow, and myocardial bridging are interconnected, with myocardial bridging likely being the trigger both for inflammation and a reduction in CFVR.
CAD: coronary artery disease
CCTA: coronary computed tomography angiography
CFVR: coronary flow velocity reserve
CT: computed tomography
HU: Hounsfield unit
LAD: left anterior descending coronary artery
PCAT: peri-coronary adipose tissue attenuation
PVAT: peri-vascular adipose tissue
SE: stress echocardiography
NG: Conceptualization, Writing—original draft, Writing—review & editing, Validation, Supervision. DT: Conceptualization, Writing—original draft. DD, PJS, CP, and LB: Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
According to the Comitato Etico Area Vasta Emilia Nord (AVEN) conditions, for this study the ethical approval is not required.
Informed consent to participate is obtained from all participants.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3301
Download: 30
Times Cited: 0
