Affiliation:
1Department of Cardiology, City Hospital, Sandwell and West Birmingham Hospital NHS Trust, B18 7QH Birmingham, UK
Email: Zafraan.zathar2@nhs.net
ORCID: https://orcid.org/0000-0003-2495-9884
Affiliation:
2Department of Nuclear Medicine, Birmingham City Hospital, Sandwell and West Birmingham Hospital NHS Trust, B18 7Q Birmingham, UK
Affiliation:
2Department of Nuclear Medicine, Birmingham City Hospital, Sandwell and West Birmingham Hospital NHS Trust, B18 7Q Birmingham, UK
Affiliation:
1Department of Cardiology, City Hospital, Sandwell and West Birmingham Hospital NHS Trust, B18 7QH Birmingham, UK
4Institute of Cardiovascular Sciences, University of Birmingham, B15 2SQ Birmingham, UK
Explor Cardiol. 2024;2:67–78 DOI: https://doi.org/10.37349/ec.2024.00022
Received: November 28, 2023 Accepted: March 01, 2024 Published: April 15, 2024
Academic Editor: Antonello D’Andrea, University of Campania Luigi Vanvitelli, Italy
Aim: The pattern and severity of coronary artery calcification (CAC) can influence prognosis and outcome in percutaneous coronary intervention. An objective assessment of CAC during invasive angiography may provide additional prognostic information. This study aimed to assess the correlation between the angiographic Birmingham calcium score (BCS) and the Agatston coronary calcium score (CCS) performed as part of single-photon emission computed tomography myocardial perfusion imaging (SPECT-MPI).
Methods: In this retrospective observational study, patients undergoing SPECT-MPI and invasive coronary angiography as part of their routine management were included. BCS was calculated by reviewing angiography images in retrospect by an observer blinded to the SPECT-MPI calcium score. Spearman’s correlation was used to analyze the correlation between BCS and SPECT-MPI. Receiver operating characteristic curve was used to detect cut-off for BCS that would detect clinically significant CAC [> 400 Agatston units (AU)]. Kaplan-Meier was used to report on outcomes at 5 years follow-up.
Results: In this cohort of 151 patients, there was a positive correlation between BCS and CCS [Spearman correlation coefficient (r) = 0.558, P < 0.001]. Cumulative BCS of 1 was able to identify clinically significant CAC [area under the curve 0.788, 95% confidence interval (CI) 0.714–0.863]. Cumulative BCS ≥ 3 was associated with major adverse outcomes at 5 years follow-up (log rank P = 0.013).
Conclusion: BCS correlates well with established higher CCS. Application of BCS during invasive coronary angiography will aid risk stratification, management, and follow-up with no extra patient involvement, radiation, or costs.
Coronary artery calcification (CAC) can lead to poor cardiovascular outcomes [1, 2]. The two recognised types of coronary calcification are intimal and medial calcification. Renal dysfunction, hypercalcemia, hyperphosphatemia, parathyroid hormone abnormalities, and duration of dialysis are associated with medial calcification [3]. Intimal calcification is seen in advanced age, diabetes mellitus, dyslipidemia, hypertension, male gender, cigarette smoking, and renal disease.
Invasive coronary angiography has a restricted ability to detect and localise coronary calcification [4, 5]. Yet CAC is both a marker for significant coronary artery disease (CAD) and a major determinant of the success of percutaneous coronary intervention (PCI) [6, 7]. It is an independent predictor of mortality and future cardiac events [8–11]. An appreciation of calcium burden during angiography can allow calcium modification tools to be used improving the success of PCI. Indeed, in a prospective multicentre global investigational device exemption study (Disrupt CAD III), authors relied on invasive coronary angiography to identify severely calcified lesions to enable the use of intravascular lithotripsy to improve procedure success and post-procedure complications [12]. There is no recognized classification system for grading calcium burden in a coronary lesion during invasive angiography and it is unclear if this will correlate well with established and validated tools for coronary calcium scoring.
Myocardial perfusion scintigraphy (MPS) single-photon emission computed tomography (SPECT) is a well-recognized non-invasive technique in detecting myocardial ischemia and evaluating prognosis. This imaging modality shows great ability in the detection of lesions with flow restriction [13, 14]. MPS is helpful in identifying low and high-risk groups among heterogeneous populations of both symptomatic and asymptomatic patients [15, 16]. With the widely available nuclear medicine hybrid cameras, the SPECT myocardial perfusion imaging (SPECT-MPI) can be combined with low-dose calcium score computed tomography (CT) for a more complete evaluation of coronary arteries.
To date, no study has reported on the direct comparison between objective assessment of invasive angiographic calcification and coronary calcification on SPECT-MPI. Appreciation of calcium burden during invasive angiography can aid in risk stratification, management, and future follow-up. Therefore, the Birmingham calcium score (BCS) was used to assess angiographic calcification in an objective manner (Figure 1) [17, 18].

BCS
Note. Adapted with permission from “37 Coronary calcification on coronary angiography versus agatston score - does it matter?” by Zathar Z, Pandit M, Notghi A, Sharma V, Karunatilleke A. Heart. 2023;109:A42–3 (https://heart.bmj.com/content/109/Suppl_3/A42). © 2023 BMJ Publishing Group Ltd & British Cardiovascular Society.
The aim of this study was to evaluate the correlation of coronary calcium score (CCS, performed during MPS study) with coronary angiographic calcium by BCS.
This is a single-center retrospective analysis of patients who underwent invasive coronary angiography, where indicated, as part of their standard care, after a diagnostic SPECT-MPI between 2015 and 2016. All patients ≥ 18 years who underwent SPECT-MPI with CCS and invasive angiography were included. This study was approved by the local audit committee and conducted as a local service improvement project (approval number 2040). This study adhered to the principles of the Declaration of Helsinki and “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies” [19, 20].
During MPS patients underwent a gated SPECT (17 min duration) followed by attenuation correction CT (no breath-hold, non-diagnostic images with extremely low radiation) and then a breath-hold gated CT for calcium scoring (diagnostic quality with ultra-low dose radiation lasting 20 s in duration). The MPS, attenuation correction CT, and calcium score CT were performed in quick succession which minimized additional procedure time with relatively small additional radiation, with mean additional estimated radiation dose (ED) of 0.92 mSv (dose length product = 54 mGy*cm) for calcium scoring. Discovery NM/CT 670 GE hybrid camera with BrightSpeed Elite 16 slices CT scanner (GE healthcare, Milwaukee, Wisconsin, USA) was used for myocardial perfusion imaging (MPI)/CT. Total coronary artery calcium score [Agatston units (AU)] was measured using corridor-4DM (Michigan University) software.
Angiographic scoring of calcium was calculated by retrospective review of coronary angiograms and classified according to the BCS (Figure 1), score 0: vessel wall calcification only; score 1: spiculated calcium within the lesion; score 2: focal calcium within the lesion, < 50% of lumen diameter; score 3: focal calcium within the lumen, > 50% of lumen diameter.
All coronary angiography was reviewed by an observer who was blinded to the CT calcium score along with all demographic data. In case of doubt or discrepancy, a second observer (senior cardiologist) reviewed the angiographic images and a consensus was reached. Interobserver variability for BCS in a proportion of the coronary angiograms was assessed by Cohen’s kappa to ensure the reproducibility of the BCS [21]. Each of the major coronary vessels was given a BCS which was then aggregated to obtain the cumulative BCS for all three coronary arteries (Figure 2). In cases of calcification in multiple segments, the highest BCS was used. The various calcium scores were analyzed with regard to their demographic and clinical characteristics. The presence or absence of cardiovascular risk factors such as diabetes, hypertension, hypercholesterolemia, smoking status, family history, and prior cardiovascular events were recorded and compared between 3 groups: cumulative BCS 0–1, BCS 2–3, and BCS > 3.
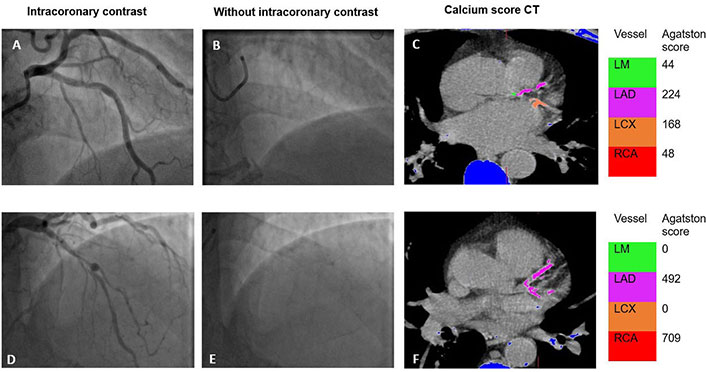
BCS and CCS on CT. Example of (A–C) BCS 2 and (D–F) BCS 3 depicting coronary calcification on invasive angiography along with Agatston score on CT. Blue areas in figures C and F represent calcification outside the coronary arteries. LM: left mainstem; LAD: left anterior descending; LCX: left circumflex; RCA: right coronary artery
All data was analyzed using Statistical Package for Social Sciences for Windows (SPSS version 26). Continuous variables are presented as mean (± standard deviation) and categorical variables are presented as percentages. Comparison of categorical variables was done using Fisher’s or χ2 test. Spearman’s correlation was used to assess the correlation between BCS and CT CCS. Receiver operating characteristic (ROC) curve analysis was performed to detect cut-off BCS that would predict significant CAC (defined as coronary calcium > 400 AU [22]). Kaplan–Meier estimator was used to report on 5 years major adverse outcomes. Interobserver variability was assessed by Cohen’s kappa as discussed above. Confidence interval (CI) for significance was set at 95% with P < 0.05.
The primary outcome measure was to find the correlation between the BCS and CT CCS. The other outcome measures were to correlate BCS with traditional cardiovascular risk factors and report on the correlation between BCS and major adverse endpoints (readmission, revascularisation, myocardial infarction, and all-cause mortality).
Of the 151 patients included in the study, 90 (60%) were male and 61 (40%) were female (Table 1). Mean age of the population was 76 years (± 9 years). Hypertension was the most common risk factor. Aside from peripheral vascular disease (PVD), all patient demographics were similar in the three BCS groups (Table 2).
Baseline characteristic
| Variables | Total (n) | Percentage (%) |
|---|---|---|
| Age in years | ||
| ≤ 5051–6061–70≥ 70 | 0928114 | 061975 |
| Gender | ||
| MaleFemale | 9061 | 6040 |
| Diabetes mellitus present | 65 | 43 |
| Hypertension present | 94 | 62 |
| Hypercholesterolemia present | 62 | 41 |
| Smoking status | ||
| Current smokerEx-smokerNon-smoker | 1725109 | 111772 |
| Family history of CAD | 21 | 14 |
| Previous myocardial infarction | 20 | 13 |
| Previous PCI | 10 | 7 |
| Previous cerebral vascular accident | 16 | 11 |
| PVD | 8 | 5 |
Cumulative BCS and risk factors
| Variables | BCS 0–1 n (%) | BCS 2–3 n (%) | BCS > 3 n (%) | P–value |
|---|---|---|---|---|
| Age in years | 0.874a | |||
| ≤ 5051–6061–70≥ 70 | 0 (0)6 (4)21 (14)78 (52) | 0 (0)2 (1)5 (3)28 (19) | 0 (0)1 (1)2 (1)8 (5) | |
| Gender | 0.109b | |||
| MaleFemale | 57 (38)48 (32) | 26 (17)9 (6) | 7 (5)4 (3) | |
| Diabetes mellitus present | 41 (27) | 20 (13) | 4 (3) | 0.156b |
| Hypertension present | 65 (43) | 24 (16) | 5 (3) | 0.383b |
| Hypercholesterolemia present | 47 (31) | 9 (6) | 6 (4) | 0.090b |
| Smoking history | 29 (19) | 6 (4) | 7 (5) | 0.011b |
| Family history | 14 (9) | 5 (3) | 2 (1) | 0.855a |
| Previous myocardial infarction | 14 (9) | 5 (3) | 1 (1) | 0.905b |
| Previous PCI | 10 (7) | 0 (0) | 0 (0) | 0.100a |
| Previous cerebral vascular accident | 12 (8) | 2 (1) | 2 (1) | 0.396a |
| PVD | 1 (1) | 4 (3) | 3 (2) | < 0.001a |
a Fisher’s exact test was used; b χ2 test was used
A large proportion of participants had clinically significant high calcium, defined ≥ 400 AU [22], as noted on CT (Table 3). Majority of the patients had ischemia on reversibility testing, however, those without reversible ischemia (28.5%, n = 43) on the SPECT-MPS who underwent invasive angiography had ongoing symptoms and/or very high calcium score (1,159 AU ± 1,535 AU) to warrant invasive testing. There was a positive correlation between cumulative BCS and total Agatston score [Spearman correlation coefficient (r) = 0.558, P < 0.001]. Using cumulative BCS ≥ 1 as cut off, it was possible to detect clinically significant calcium burden (> 400 AU) (Figure 3). Cumulative BCS ≥ 1 had a statistically significant correlation with > 400 AU (r = 0.408, P = 0.002). BCS < 1 also had a statistically significant correlation with < 400 AU (r = 0.555, P < 0.001). ROC curve identified BCS of 1 as cut off for identifying significant coronary calcification (> 400 AU) with sensitivity of 60% and specificity of 77% (area under the curve 0.788, 95% CI 0.714–0.863). Cohen’s kappa for the cumulative BCS was 0.412 (Cohen’s kappa 0.41–0.60 = moderate agreement). No differences in BCS scores were observed between the sexes (P = 0.27).
Cumulative BCS vs. cumulative Agatston score
| AU | BCS < 1 n (%) | BCS ≥ 1 n (%) | P–value |
|---|---|---|---|
| 0–200 | 32 (38.6) | 7 (10.3) | < 0.0001b |
| 201–400 | 16 (19.3) | 7 (10.3) | |
| 401–999 | 16 (19.3) | 22 (32.4) | |
| ≥ 1,000 | 19 (22.9) | 32 (47.1) | |
| Total | 83 (100) | 68 (100) |
b χ2 test was used
Note. Adapted with permission from “37 Coronary calcification on coronary angiography versus agatston score - does it matter?” by Zathar Z, Pandit M, Notghi A, Sharma V, Karunatilleke A. Heart. 2023;109:A42–3 (https://heart.bmj.com/content/109/Suppl_3/A42). © 2023 BMJ Publishing Group Ltd & British Cardiovascular Society.
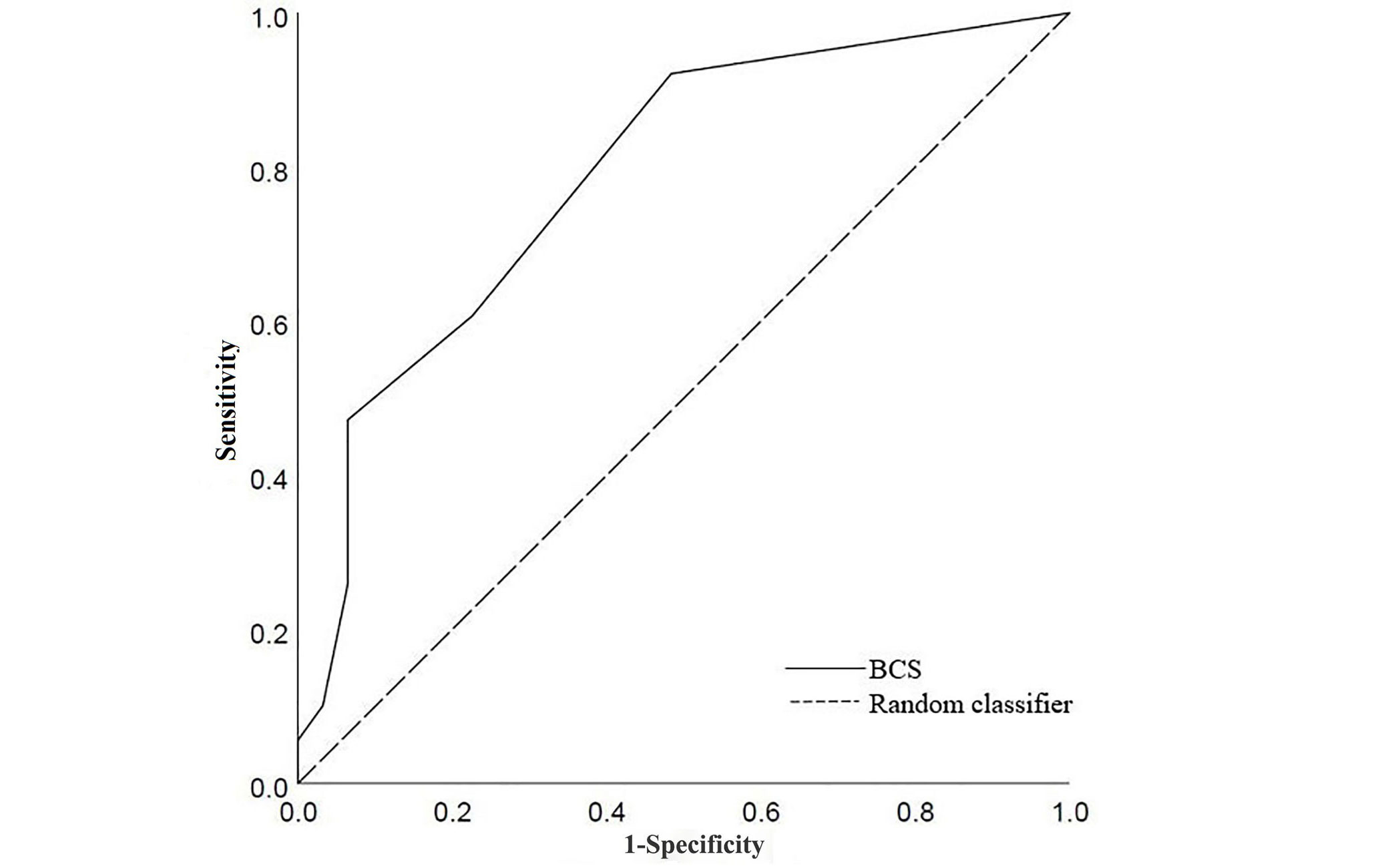
ROC curve for BSC to detect significant calcium (> 400 AU). Area under curve 0.788 (95% CI 0.71–0.863)
In this cohort, 70/151 (46%) patients undergoing invasive angiography had PCI performed. Majority of these patients had a successful procedure (n = 65, 93%). As expected, higher BCS scores were associated with the use of calcium modification tools during angiography (r = 0.285, P = 0.02). Patients with unsuccessful procedures (n = 5), on average, had higher cumulative BCS scores (mean BCS 3) compared to successful procedures (mean BCS 1). Of those with unsuccessful procedures, one represented with chest pain, and two patients died during the follow-up period. Issues that lead to procedural failure include chronic total occlusion (n = 3), coronary artery tortuosity (n = 1), and dissection (n = 1). Patients that had no coronary intervention performed 81/151 (54%) had either mild non-obstructive CAD (n = 65) or were referred to coronary artery bypass grafting due to their severe disease (n = 16).
With respect to long-term outcomes, cumulative BCS of ≥ 3 was associated with poor outcomes at 5 years follow-up. Kaplan–Meier analysis showed that the incidence of major adverse outcomes (re-admission, revascularisation, myocardial infarction, and all-cause mortality) differed between the cumulative BCS ≥ 3 and BCS < 3 cohorts (log rank P = 0.013) (Figure 4).
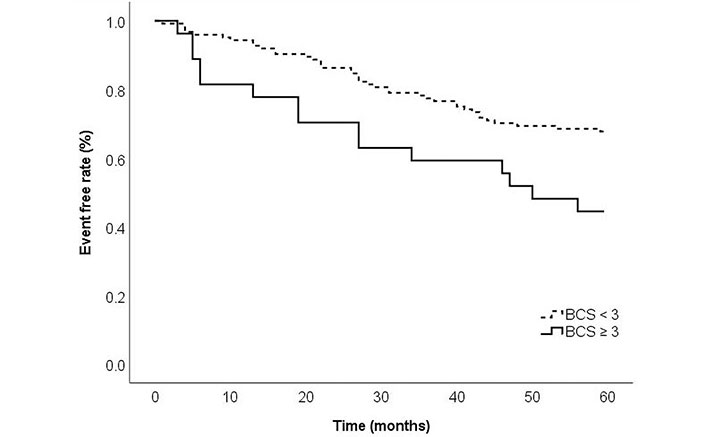
Kaplan–Meier curves comparing major adverse outcomes (re-admission, myocardial infarction, and all-cause mortality) among patients with BCS ≥ 3 vs. BCS < 3 at 5 years follow up. Log rank P = 0.013
Note. Reprinted with permission from “37 Coronary calcification on coronary angiography versus agatston score - does it matter?” by Zathar Z, Pandit M, Notghi A, Sharma V, Karunatilleke A. Heart. 2023;109:A42–3 (https://heart.bmj.com/content/109/Suppl_3/A42). © 2023 BMJ Publishing Group Ltd & British Cardiovascular Society.
This single-center, retrospective study, has demonstrated that BCS is a useful tool that correlates well with established calcium scoring in the form of CT. In patients whose cumulative BCS ≥ 1, the calcium burden is likely to be very high. Cumulative BCS score of 1 can be used as a useful cut-off to estimate the likelihood of clinically significant coronary calcium (> 400 AU). Furthermore, cumulative BCS ≥ 3 can identify patients most at risk from major adverse outcomes in the next 5 years.
The European Society of Cardiology (ESC) recognizes that CAC scoring can contribute to pre-test probability in the assessment of obstructive CAD [23]. The American Heart Association (AHA) advises that CAC scoring can be used to inform treatment decision-making if risk-based treatment decision is uncertain [24]. However, CAC score ≥ 400 AU can make CT imaging difficult, as such, the National Institute for Health and Care Excellence (NICE) recommends invasive coronary angiography in this population, if considered clinically appropriate.
As established elsewhere in the literature, CAC score closely correlates with coronary stenosis in invasive coronary angiography [25–27]. Rosen et al. [28] assessed CAC score and the severity of coronary stenosis in coronary angiography during follow-up- they noted that the CAC scores predicted the severity of coronary stenosis as well as the extent and distribution of CAD in patients who underwent clinically driven coronary angiography. Furthermore, they noted that 66% of patients with CAC score < 100 AU did not have significant coronary stenosis whilst 13% of patients with CAC score > 400 AU had no coronary stenosis. In this study, a CAC cut-off of > 400 AU was chosen as this was highly predictive of significant CAC and stenosis as reported elsewhere in the literature [28–32]. Ho et al. [31] specifically correlated CAC score with the degree of coronary artery obstruction and noted CAC score 1–400 AU was consistent with minimal to moderate atherosclerosis and < 10% rate of obstructive stenosis. However, CAC score of > 400 AU was consistent with severe arthrosclerosis and obstructive lesions > 2 times the frequency of those with CAC < 400 AU.
Although observational studies have demonstrated an increased risk of future cardiovascular events with higher calcium scores, a recent meta-analysis from Nerlekar et al. [33] would suggest that calcified plaque has a weak association with major adverse outcomes compared to non-calcified plaque. This would suggest that the pattern of coronary calcium and plaque morphology are important determinants of major adverse outcomes. In this study, 35/80 (39%) of patients with CAC > 400 AU received a BCS score < 1. In this subgroup, 28/35 (80%) received a BCS score of 0 suggesting vessel wall calcification as opposed to no calcium. The differences in major adverse outcomes observed at 5 years follow-up (Figure 4) further support the current evidence base that the pattern of calcium is more important than the total amount of calcium (AU). Spotty calcification is a commonly observed high-risk plaque feature that could double adverse event rates [33–35]. It can be a marker of plaque vulnerability which differs from the pattern of calcium seen in patients with stable CAD [36]. As BCS allows an objective assessment of calcified lesions, it can provide additional clinical information to aid aggressive preventative pharmacotherapy and regular follow-up.
In this study, cumulative BCS ≥ 1 had a statistically significant positive correlation with an Agatston score of ≥ 400 AU. This can provide an additional prognostic marker in patients who can then benefit from aggressive cardiovascular risk management. As CAC score of > 400 AU is considered high with 10-year coronary heart disease events exceeding 20%, prompt identification of these patients during invasive angiography may help clinicians risk stratify their patients [37].
BCS can be readily calculated during invasive angiography or shortly after the procedure in retrospect to inform further decision-making. BCS was not affected by the majority of the patient’s cardiovascular risk factors (Table 2). A statistically significant difference was observed between the BCS outcomes for patients with PVD; a larger proportion of patients with BCS ≥ 2 had a history of PVD. Vascular calcification is commonly associated with PVD and it possible that this group of patients are also having higher coronary calcifications through the same pathophysiological mechanisms [38, 39]. Although it is well established that PVD is an indicator of underlying CAD, due to the small sample size we cannot draw any reliable conclusions between PVD and BCS.
Data from a large population observational study by Peng et al. [40] noted that patients with very high calcium (≥ 1,000 AU) had a higher risk for major adverse outcomes in the form of CAD and all-cause mortality. This study has established that a cumulative BCS of ≥ 3 was associated with adverse outcomes at 5 years follow-up relating to re-admission, revascularisation, myocardial infarction, and all-cause mortality (Kaplan–Meier log rank P = 0.013). Prompt identification of these high-risk patients during angiography can guide management strategy including aggressive preventative pharmacotherapy and regular follow-up.
CAC can pose a challenge to successful PCI due to suboptimal stent placement, higher stent failure rates, and longer procedure time [41, 42]. This has, to some extent, been overcome by intracoronary imaging and calcium modification techniques such as cutting balloons and intravascular lithotripsy. However, the severity of calcification in coronary targets may predict outcome and success [42]. As BCS can correlate well with coronary calcium burden, it may have utility in management and procedure planning.
This was a single-center study with a cohort of 151 patients that could be subject to selection bias. It is recognized that there could be a subjective element to the BCS classification based on experience; however, this tool is designed for use by the interventional cardiologist who will have prior experience in recognizing coronary calcification. The retrospective observational nature of the study is also a limitation. Although the angiography images were reviewed in retrospect, the application of the scoring system is unlikely to lengthen the overall procedure duration. This study did not look at the correlation between BCS and percutaneous intervention procedure duration or complications, this could be a focus of future work. More importantly, data regarding intracoronary imaging was unavailable which may have helped further quantify the correlation between MPS CAC, BCS, and intracoronary imaging.
In conclusion, this is the first study to report on the direct correlation between an objective assessment of calcium during invasive angiography (BCS) and an established tool for coronary calcium assessment (CT). This study assessed the correlation of CAC (performed during MPS study) with coronary angiographic calcium BCS in order to assess the potential added value of BSC during routine angiography. The proposed cut-off of cumulative BCS ≥ 1 can identify patients who are likely to have a significant calcium burden. Also, a cumulative BCS ≥ 3 is associated with significantly worse major adverse outcomes at 5 years. Therefore, it can identify high-risk patients who would benefit from aggressive lifestyle risk modification and pharmacological prophylaxis. A large prospective cohort study may allow the BCS to be validated for independent use.
AU: Agatston units
BCS: Birmingham calcium score
CAC: coronary artery calcification
CAD: coronary artery disease
CCS: coronary calcium score
CI: confidence interval
CT: computed tomography
MPS: myocardial perfusion scintigraphy
PCI: percutaneous coronary intervention
PVD: peripheral vascular disease
r: Spearman correlation coefficient
ROC: receiver operating characteristic
SPECT: single-photon emission computed tomography
SPECT-MPI: single-photon emission computed tomography myocardial perfusion imaging
ZZ: Conceptualization, Methodology, Data curation, Formal analysis, Writing—original draft. MP: Methodology, Resources, Writing—review & editing. AK: Data curation, Resources. AN: Methodology, Resources, Writing—review & editing. VS: Conceptualization, Methodology, Formal analysis, Writing—review & editing, Supervision. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
The authors declare that they have no conflicts of interest.
This study was approved by the local audit committee and conducted as a local service improvement project (approval number 2040, Sandwell General Hospital, West Bromwich). This study adhered to the principles of the Declaration of Helsinki and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.
Consent from participants is waived as the project is a retrospective study undertaken as an audit with local governance.
Not applicable.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3115
Download: 27
Times Cited: 0
