Abstract
Pulmonary embolism (PE) is the third leading cause of cardiovascular mortality worldwide. Percutaneous mechanical thrombectomy is indicated in patients with contraindications to thrombolysis. Complications include atrial or ventricular perforation causing tamponade. We describe one case of pericardial tamponade in an elderly woman who underwent thrombectomy for acute PE. An 88-year-old woman presented with acute shortness of breath. She was tachycardic, oxygen saturation of 80% and blood pressure of 95/57 mmHg. Bedside ultrasound showed a dilated right ventricle. Electrocardiogram showed large S wave in lead I, Q wave and inverted T wave in lead III. Computed tomography (CT) angiogram of the chest revealed an extensive saddle PE. Tissue plasminogen activator was deferred given patient’s age. Full dose anticoagulation was started and she underwent a successful percutaneous thrombectomy with FlowTriever device. Two hours later, she developed severe back pain and hypotension to 88/63 mmHg. Hemoglobin dropped from 13.7 g/dL to 8.8 g/dL. CT chest angiogram showed dense pericardial effusion, likely hemopericardium, with mass effect on the heart. Bedside pericardiocentesis was attempted and converted to pericardial window given sustained hypotension. She went into cardiac arrest. Emergent thoracotomy revealed significant hemothorax. The pericardium was opened yielding a blue, globally ischemic, and non-contracting heart. Cardiac massage and intra-cardiac epinephrine attempted unsuccessfully. Percutaneous thrombectomy using the large bore design FlowTriever system has shown promising results in reducing clot burden and improving hemodynamics. Attention must be paid to life threatening complications such as cardiac tamponade which can be precipitated by using these devices.
Keywords
Cardiac tamponade, percutaneous thrombectomy, pulmonary embolism, mechanical thrombectomy, FlowTriever deviceIntroduction
Patients with life-threatening pulmonary embolism (PE) may require additional treatment beyond anticoagulation, including thrombolysis and thrombectomy. Thrombectomy is indicated in patients with hemodynamically unstable PE in whom thrombolytic therapy is contraindicated which is both diagnostic and therapeutic. Percutaneous mechanical thrombectomy has a success rate of over 80% and is indicated in patients with contraindications to thrombolysis [1]. Treatment of pulmonary emboli with mechanical thrombectomy was shown to reduce intensive care unit stay and immediately improve hemodynamic parameters, including pulmonary artery systolic pressure as well as cardiac index. However, there are potential complications such as the risk of bleeding, atrial, or ventricular perforation causing tamponade. Although rare, there is a risk of pulmonary artery perforation which can lead to life-threatening pericardial tamponade. We attempt to describe one such case of pericardial tamponade leading to the death of an 88-year-old woman who underwent thrombectomy after being diagnosed with acute saddle PE. We report this case in order to bring light to a potential complication which can be avoided. Due to the novelty of some devices used for mechanical thrombectomy, there is a need for standardization of procedure and awareness of adverse events in the treatment of life threatening PE, especially in a vulnerable population of patients in whom thrombolysis is not indicated.
Case report
An 88-year-old-woman with a past medical history of hypertension and obesity presented with shortness of breath and generalized weakness for two weeks. Her vitals were significant for tachycardia to 108 bpm, blood pressure of 113/50 mmHg, and oxygen saturation of 80% on room air, which improved to 98% with 4 L on nasal cannula oxygen. Bedside ultrasound showed dilated right ventricle (RV) with “D” sign [“D” shaped left ventricle (LV)]. Blood work was significant for troponin of 0.135 ng/mL and pro-BNP (brain natriuretic peptide) of 59,256 ng/mL. Electrocardiogram (EKG) showed sinus tachycardia with S1, Q3, and T3 changes. Computed tomography (CT) angiogram of the chest showed massive saddle PE extending into the bilateral main pulmonary arteries with evidence of right heart strain and right lower lobe infarction (Figures 1 and 2).
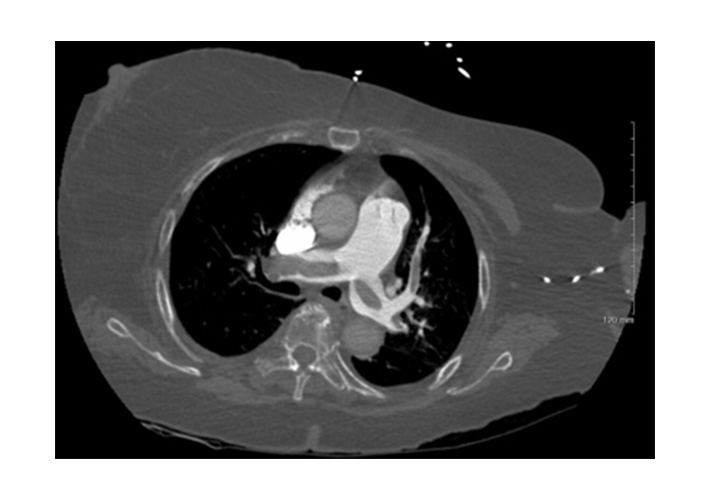
Initial PE protocol chest CTA. Axial CT image shows large embolic burden with partial saddle embolus and clot extending into bilateral main, lobar, and segmental pulmonary arteries. CTA: computed tomography angiography; CT: computed tomography
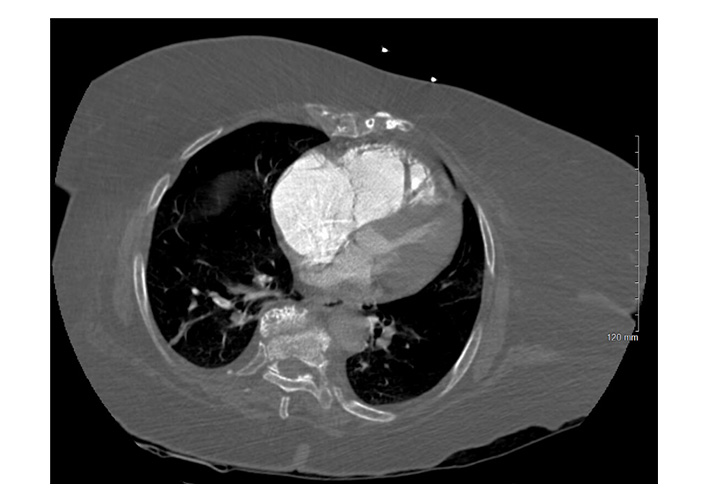
Same initial PE protocol chest CTA. Axial CT image shows CT findings suggestive of right heart strain, including massive dilation of the RV and atrium with an RV:LV ratio > 1, leftward bowing of the interventricular septum and significant narrowing of the left ventricular chamber. PE: pulmonary embolism; CTA: computed tomography angiography; CT: computed tomography; RV: right ventricle; LV: left ventricle
CT of the abdomen and pelvis demonstrated large bowel obstruction and a calcified extraluminal mass within the mesentery measuring 4 cm × 2 cm × 2 cm. Doppler ultrasound showed an acute deep venous thrombosis of the left lower extremity from the left common femoral to the popliteal vein. General surgery recommended no acute surgical intervention. Patient was then started on a heparin infusion. Thrombolysis with alteplase was deferred given the patient’s age, possible colonic malignancy, and current state of hemodynamic stability. Interventional radiology was consulted to perform a mechanical thrombectomy. She underwent a pulmonary arteriogram with successful bilateral pulmonary artery mechanical thrombectomy (Figure 3).
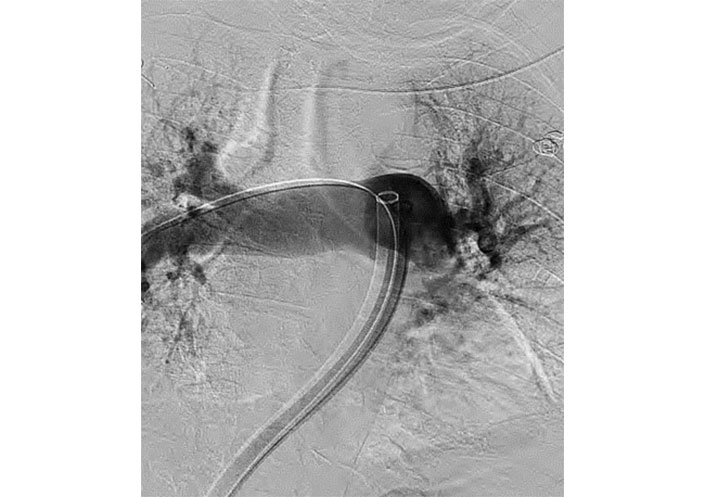
Pulmonary arteriogram with successful bilateral pulmonary artery mechanical thrombectomy
Two hours after the procedure, the patient began to complain of severe back pain and was hypotensive to 88/63 mmHg. Labs were significant for a reduction in hemoglobin level to hemoglobin (Hb) 8.8 g/dL (13.7 g/dL on admission). A bedside ultrasound was performed and was grossly non diagnostic due to shadowing from bowel loops. She was transported emergently for a CT angiogram of the chest, abdomen, and pelvis due to suspicion of retroperitoneal hematoma, which showed dense pericardial effusion with Hounsfield units of 80, likely to be hemopericardium, exerting mass effect on the heart with narrowing of cardiac chambers (Figure 4A and 4B).
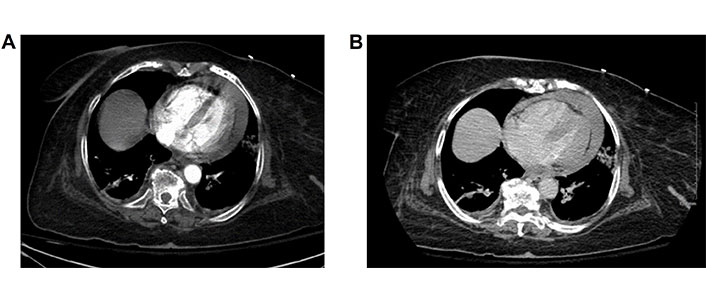
Arterial and 5 min delayed venous phases of follow-up chest CT showing new high density pericardial effusion consistent with hemopericardium. The heart is contracted in size when compared to prior study and there is straightening of the interventricular septum. Cardiac chambers grossly similar in size. No change in the size of the pericardial fluid between arterial and delayed phases to suggest rapid accumulation. CT: computed tomography
Cardiology, cardiothoracic surgery, and trauma surgery were urgently consulted given the imaging findings. Ultrasound guided bedside pericardiocentesis was attempted by the trauma surgery team as a temporizing maneuver before proceeding to the operating room, which was unsuccessful due to the patient’s body habitus as well as cephalad displacement of diaphragm due to bowel obstruction and presence of bowel loops, providing difficulty with visualization. Within a matter of minutes, patient was intubated, and pericardiocentesis was then converted to a pericardial window due to rapid hypotension. Massive transfusion protocol was initiated. Approximately 150–200 cc of non-clotted blood was aspirated, after which patient was bradycardic and lost her pulse. Advanced cardiac life support (ACLS) protocol was initiated and an emergent bedside thoracotomy through the left infra-mammary fold, with an incision placed anterior to the level of the sixth rib. The pericardium was opened yielding a blue, globally ischemic, and non-contracting heart. Open cardiac massage and intra-cardiac epinephrine was attempted, however, there was no return of cardiac activity.
Discussion
PE is a relatively common, potentially devastating clinical entity with high early mortality rates. If not treated acutely, RV failure develops, which is defined as a rapidly progressive syndrome with systemic widespread vascular congestion resulting from impaired RV filling with or without reduced RV flow [2]. In the affected lung area, there is anatomical obstruction and hypoxic vasoconstriction, which lead to an increase in peripheral vascular resistance and decrease in arterial compliance. The result of this pathophysiological process is almost always fatal, if not treated acutely. Two out of three patients die within 2 h of presentation [3]. It is an immediately life-threatening situation, high-risk PE requires an expedited diagnostic and therapeutic strategy with the aim to restore circulation to the pulmonary bed.
The incidence of PE is estimated to be 60–70 per 100,000 of the general population [4]. Cross-sectional data from autopsy reports shows that the incidence of venous thromboembolism is almost eight times higher in patients of ages 80 years or more compared to the fifth decade of life. It is responsible for ≤ 300,000 deaths per year in the US, ranking high among the causes of cardiovascular mortality [5].
PE can be subclassified based on severity into massive, sub-massive, and non-massive or low risk categories. Massive PE causes hemodynamic compromise which is defined as cardiac arrest, systolic blood pressure less than 90 mmHg or vasopressors required despite adequate filling status along with end-organ damage or persistent hypotension [3]. Sub-massive PE is heralded through imaging findings showing right ventricular dysfunction on echocardiography, through CT of the chest or serologically through elevated cardiac biomarkers. To the contrary, low risk or non-massive PE does not demonstrate these imaging findings or clinical evidence of hemodynamic compromise, with lower rates or mortality in patients with sub-massive PE than those with massive PE (58.3% in patients with massive PE versus 15.1% in sub-massive PE), as demonstrated through the International Cooperative Pulmonary Embolism Registry [6].
Multidetector CT pulmonary angiography (CTPA) is the imaging of choice in patients with suspected PE. The positive predictive value of a positive CTPA was 92–96% in patients with an intermediate or high clinical probability, but much lower (58%) in patients with a low pre-test likelihood of PE [7].
Therapeutic approaches
In order to determine the appropriate therapeutic strategy, it is essential to stratify risk based on PE severity. Patients are first divided into those with hemodynamic instability and those without hemodynamic instability. Patients without hemodynamic instability are then further stratified based on clinical, imaging and laboratory findings indicative of PE severity and comorbidities that may contribute to an adverse prognosis [3].
Acute RV failure, defined as a rapidly progressive syndrome with systemic congestion resulting from impaired RV filling and/or reduced RV flow output, is a critical determinant of outcome in acute PE. Tachycardia, low systolic BP, respiratory insufficiency (tachypnea and/or low oxygen saturation), and syncope, alone or in combination, have been associated with an unfavorable short-term prognosis in acute PE [3].
Treatment modalities
American College of Cardiology current treatment guidelines currently recommend systemic thrombolytic therapy when there is high suspicion for imminent cardiopulmonary decline.
Anticoagulation in the form of subcutaneous, weight adjusted low molecular weight heparin, fondaparinux, intravenous unfractionated heparin (UFH) or non-vitamin K antagonist oral anticoagulant (NOAC), should be initiated in patients with high or intermediate clinical probability of PE [8].
However, it has been shown that thrombolytic therapy leads to accelerated improvement compared to UFH alone with improvements in RV dilatation. Thrombolysis must be initiated within 48 h of symptoms onset but may be effective up to 6–14 days after symptom onset [9]. This benefit was proven through a meta-analysis of trials of thrombolysis for PE showing a significant reduction in the combined outcome of mortality and recurrent PE [10]. To note, this benefit was achieved with a 9.9% rate of severe bleeding and a 1.7% rate of intracranial hemorrhage [11]. Intravenous administration of recombinant tissue-type plasminogen activator (rtPA) is superior to streptokinase and urokinase, first generations thrombolytic agents [12].
Absolute contraindications to the use of thrombolytics include active bleeding, recent cerebrovascular accident or transient ischemic attack, recent neurosurgical procedure, or recent intracranial trauma [1]. Thrombolytic therapy is also contraindicated in patients in whom anticoagulation is contraindicated for any reason. Relative contraindications include recent requirement for cardiopulmonary resuscitation, abdominal, ophthalmic, or obstetric surgery, extracranial trauma, intracranial tumor or vascular abnormality, systolic blood pressure greater than 180 mmHg or diastolic blood pressure greater than 110 mmHg, or recent gastrointestinal bleed. A comprehensive list of relative contraindications is provided in the table below (Table 1).
Relative contraindications for thrombolytic therapy
| Relative contraindications |
|---|
| Recent cardiopulmonary resuscitation |
| Recent abdominal, ophthalmic, or obstetric surgery |
| Recent trauma (other than intracranial) |
| Known intracranial tumor or vascular abnormality |
| Uncontrolled hypertension: systolic BP > 180 mmHg, diastolic BP > 110 mmHg |
| Recent gastrointestinal bleeding |
| Known severe allergy or adverse reaction to thrombolytic agent or contrast media (not controlled by steroid/antihistamine therapy) |
| Severe thrombocytopenia |
| Known right-to-left cardiac or pulmonary shunt |
| Suspected intracardiac thrombus |
| Suspected infected venous thrombus |
| Chronic kidney disease |
| Active pregnancy |
| Severe liver disease |
Another point to consider is that a large segment of patients with acute deep venous tomography (DVT) or PE go on to develop disabling sequelae such as post thrombotic syndrome, recurrent venous thromboembolism, or chronic thromboembolic pulmonary hypertension (CTEPH) [6].
Full dose systemic thrombolysis is not recommended for intermediate risk PE due to the high risk of life threatening bleeding in comparison to the expected benefits of thrombolysis [12].
Limitations of both anticoagulation and systemic thrombolysis and the complexity and risk associated with open surgical embolectomy in some patients with PE have led to the development of percutaneous catheter based thrombectomy techniques.
Catheter-based mechanical thrombectomy
The indications of the use of catheter based thrombectomy are driven by the severity of the PE as well by specific patient related factors such risk for bleeding and other comorbidities [1]. Systemic thrombolysis has a reduction in both mortality as well as improvement in hemodynamic function but carries an increased risk of severe intracranial and extracranial bleeding as was demonstrated in the PEITHO (Pulmonary Embolism Thrombolysis) trial. Due to bleeding related adverse events as well as treatment failure in certain subsets of patients, catheter-based thrombus removal have emerged as a therapeutic option.
Given these limitations, endovascular therapy has been gaining momentum over the past 20 years. In general, catheter based thrombectomy for PE is considered when there are significant contraindications to thrombolytic therapy or when there are risk factors indicating an increased likelihood for mortality.
The American College of Cardiology/American Heart Association guidelines suggest that catheter thrombectomy can be considered when there is suspicion of cardiopulmonary worsening in sub-massive PE or evident cardiopulmonary deterioration [6].
Catheter based thrombectomy is indicated for patient with a high bleeding risk, failed thrombolysis or chock, as per current guidelines [13]. Patients who have low risk PE are generally not candidates for endovascular therapy—the exception being those with a saddle embolus without hemodynamic change or effect on the RV [14].
Patient selection and risk stratification
In clinical practice, patient should be carefully selected for an individualized endovascular strategy. There are three main considerations when selecting a patient for endovascular therapy: disease severity and acuity, risk of major bleeding, and patient specific factors. Thrombectomy techniques that have been developed include: thrombus fragmentation, thrombectomy with clot aspiration, hemolytic thrombectomy, and suction thrombectomy. With improper patient positioning, there is always a risk of distal embolization of thrombus and potential catastrophic vessel injury. Therefore, it is always advised to perform the procedure in conjunction with CT imaging.
Despite this, there are still multiple technical difficulties that users have encountered, many of which are due to catheter size. The newer devices, such as the FlowTriever System (Inari Medical, Irvine, CA) and the Indigo System (Penumbra Inc., Alameda, CA) have developed for patients with absolute contraindications to thrombolytic therapy.
The FlowTriever system is a large bore catheter system which received U.S. Food and Drug Administration clearance for use in PE in May 2018. A 20-French catheter is advanced over a guide wire to the level of the right or left pulmonary artery proximal to the location of the occlusive thrombus. The FlowTriever Catheter is then advanced through the over the guidewire. The clot is aspirated through the 20-French catheter initially placed and removed. This catheter, also known as the aspiration guide catheter, can be removed and reinserted. This whole procedure can be repeated multiple times as needed. The FlowTriever Pulmonary Embolectomy Clinical Study (FLARE) was a prospective, single-arm, multicenter investigational device exemption trial in which patients with acute intermediate-risk PE were treated with the FlowTriever Retrieval/Aspiration System. The results of this trial demonstrated a reduction in RV/LV ratio of 25% [15], with a 0.9% major bleeding rate (with no intracranial hemorrhages), which suggests an excellent safety profile. In addition, there were no major vascular complications or device related cardiac injury events that were noted [16].
The benefits of percutaneous mechanical thrombectomy can be summarized into the following points—rapid hemodynamic stabilization, use in patient cohort who are at high risk for bleeding and its potential to improve hospital costs, given the reduced need for intensive care unit (ICU) stay and short hospital stay, as illustrated in the FLARE study [16].
Current guidelines recommend anticoagulation alone, with catheter intervention reserved for patients who fail to respond to conservative therapy. Currently known complications of catheter thrombectomy for PE include perforation or dissection or cardiovascular structures, pericardial tamponade, distal thrombus embolization, and can also potentially lead to arrhythmias and vascular access complications. Thrombectomy should be performed only in the main and lobar pulmonary arteries, not in the segmental pulmonary arteries to minimize some of these risks. Once hemodynamic improvement has resulted, the procedure should be terminated [17].
A rapid, accurate risk stratification to determine an appropriate treatment plan is the first step in treating acute PE. Thrombolysis, catheter intervention, and ongoing collaboration with cardiac surgeons are strategies that can maximize the likelihood of complete recovery for these devastatingly ill patients.
Conclusion
Acute PE remains a significant cause of morbidity and requires prompt diagnosis and management. Patients with a life-threatening PE require additional treatment beyond anticoagulation. Percutaneous thrombectomy using the FlowTriever system with a large bore design has shown promising results in terms of reducing clot burden and improving hemodynamics. As it is technically more demanding to navigate through RV and pulmonary vasculature, there is risk of pulmonary artery perforation which can lead to pericardial tamponade and is frequently catastrophic. There are very few resources that compile all known complications of percutaneous mechanical thrombectomy for the treatment of PE, which is the need of the hour, and should be based upon large, multi-center settings. Attention must be paid to life threatening complications such as cardiac tamponade which can be precipitated by the use of these devices. With this case report and review of literature, we also hope to spur specialized operator training to minimize these adverse outcomes.
Abbreviations
| ACLS: | advanced cardiac life support |
| BNP: | brain natriuretic peptide |
| CT: | computed tomography |
| CTA: | computed tomography angiography |
| CTEPH: | chronic thromboembolic pulmonary hypertension |
| DVT: | deep venous tomography |
| EKG: | electrocardiogram |
| FLARE: | FlowTriever Pulmonary Embolectomy Clinical Study |
| Hb: | hemoglobin |
| ICU: | intensive care unit |
| LV: | left ventricle |
| NOAC: | non-vitamin K antagonist oral anticoagulant |
| PE: | pulmonary embolism |
| PEITHO: | Pulmonary Embolism Thrombolysis |
| rtPA: | recombinant tissue-type plasminogen activator |
| RV: | right ventricle |
Declarations
Author contributions
AG: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. SS: Conceptualization, Writing—review & editing. AK: Writing—review & editing. WAP: Validation, Writing—review & editing. MS: Writing—review & editing. EN: Investigation, Writing—review & editing. AA: Validation, Writing—review & editing. SGH: Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Per hospital ethics regulations, ethics review is exempt for (see page 23 NYC HHC Human Subject Research Protections Program) which states: “The Research involves the collection or study of existing data, documents, records, pathological specimens or diagnostic specimens, and these sources are either publically available or if the information is recorded by the Principal Investigator in such manner that Human Subjects cannot be identified, directly or through identifiers linked to the Human Subjects.”
Consent to participate
Informed consent to participate in the study was obtained from the participants’ family.
Consent to publication
Not applicable.
Availability of data and materials
The raw data supporting the conclusions of this manuscript will be made available by the authors for reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2024.