Abstract
Papillary muscle rupture is a rare but fatal complication of acute myocardial infarction. The incidence of this complication has been reduced to less than 0.1% of patients due to the advent of primary percutaneous coronary intervention. Cardiogenic shock and acute pulmonary edema are the most common clinical presentations. In this review, we describe the role of transthoracic and transesophageal echocardiography in identifying the type, location, and hemodynamic consequences of the three different echocardiographic patterns of papillary muscle rupture. This information is crucial for managing medical therapy and determining the appropriate surgical or percutaneous treatment.
Keywords
Papillary muscle rupture, acute myocardial infarction, echocardiography, mitral regurgitation, therapyIntroduction
The most dramatic and fatal complication of acute myocardial infarction is the rupture or tearing of infarcted myocardial tissue. Although rare, these complications are associated with very high in-hospital mortality rates. Papillary muscle rupture (PMR), along with interventricular septum rupture and left ventricular free wall rupture, is one of these severe complications.
The decline in the incidence of PMR in recent decades can be attributed to the widespread use of primary percutaneous coronary reperfusion interventions. The current incidence rate ranges from 0.05% to 0.26%, depending on diagnostic criteria, the quality of diagnostic techniques, and the echocardiographic patterns used to detect PMR.
However, despite the reduced incidence, the in-hospital mortality rate (5%) remains high, particularly in patients managed conservatively (80%) and even in those undergoing surgery. Furthermore, in about half of the cases, the presence of hemodynamic instability, such as cardiogenic shock, often results in the decision to refrain from cardiac surgery during the acute phase due to the high surgical risk [1–4].
PMR accounts for more than half of the cases of acute severe mitral regurgitation following myocardial infarction. The remaining cases are secondary to left ventricular dysfunction, which leads to leaflet displacement and tethering of leaflets.
A recent Seminar, published by leaders in each field, provided an update on the acute mechanical complications of myocardial infarction, including right ventricular infarction, severe mitral regurgitation, ventricular septal rupture, and free wall rupture [5]. One section of this seminar focused specifically on the management of severe mitral regurgitation risk [6].
Clinical presentation and pathophysiology
PMR typically occurs within the first week after an acute ischemic event, most commonly between the 2nd and 6th day. It is more frequent in myocardial infarctions with ST-segment elevation and less frequent in non-ST-segment elevation infarctions.
PMR is usually a complication of a first infarction, particularly in patients with delayed presentation or without revascularization, and accounts for 36.3% of in-hospital deaths associated with acute myocardial infarction [7].
Patients with PMR are more likely to be older females with hypertension and chronic kidney disease, and less likely to have diabetes [6]. In patients with PMR, single-vessel occlusion is common, with occlusion of the right coronary artery (RCA) being more frequent than that of the left circumflex artery (LCX), and occlusion of the left anterior descending artery (LAD) being the least likely.
Rupture of the posteromedial papillary muscle (PPM) is more frequent than rupture of the anterolateral papillary muscle (APM) due to its single-vessel perfusion from the dominant RCA or LCX. This occurs 3 to 12 times more often than rupture of the APM, which is protected by a dual blood supply from both the LAD and LCX, reducing the risk of single-vessel occlusion [8–11].
Normal mitral valve closure depends on the correct apposition of both leaflets, whose symmetrical overlap ensures proper systolic coaptation. This mechanism, which prevents mitral regurgitation, is supported by the appropriate traction of the chordae tendineae and the papillary muscle complex. Both papillary muscles distribute the chordae tendineae to both the anterior and posterior leaflets. PMR significantly disrupts the mitral valve competency mechanism [7, 12–15].
PMR can be complete, involving rupture at the papillary trunk, or partial, when the lesion affects one or more heads of the papillary muscle, or rarely at the insertion of the chordae tendineae. Some authors define a rupture as “complete” when it involves the larger head of the papillary muscle. The severity of mitral regurgitation depends on the type of rupture and the group of chordae tendineae involved, considering that a single papillary muscle distributes chordae to both the anterior and posterior leaflets (Figure 1).
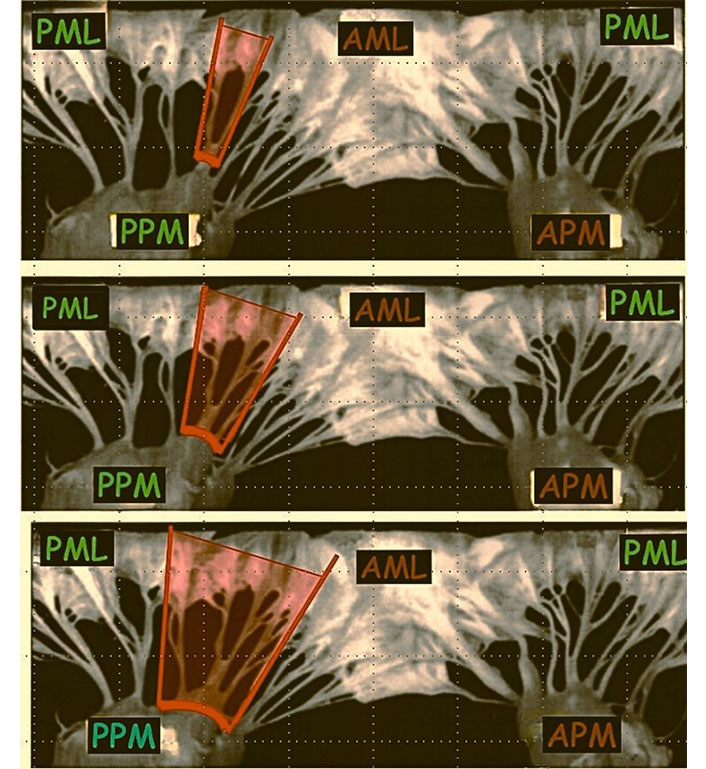
Mitral valve leaflet involvement in relation to the different types of the PPM rupture. AML: anterior mitral leaflet; PML: posterior mitral leaflet; APM: anterolateral papillary muscle; PPM: posteromedial papillary muscle
Partial rupture is the more common cause of varying degrees of mitral regurgitation, while complete rupture, which causes prolapse of both leaflets, leads to severe mitral regurgitation, often presenting clinically with pulmonary edema and cardiogenic shock [12, 14, 16, 17].
Clinical suspicion for PMR should be high in patients with acute myocardial infarction who, within the first week (typically on the third or fourth day), experience a sudden worsening of symptoms, such as rapidly progressive pulmonary edema or cardiogenic shock with hypotension. In such cases, the presence of a midsystolic or holosystolic murmur on auscultation is not consistent due to the equalization of left atrial and left ventricular pressures, a phenomenon known as “silent” mitral regurgitation.
Given the wide range of clinical presentations and the challenge in categorizing patients for optimal treatment selection, a clinical classification has been proposed to establish a common language within the Heart Team. The clinical presentation is divided into four types, ranging from type 1 (the most severe) to type 4 (the least severe). A type 1 patient presents with shock, pulmonary edema, or cardiac arrest. Type 2 includes patients with severe heart failure or pulmonary edema but with preserved perfusion pressure. Type 3 patients experience episodes of pulmonary congestion or flash pulmonary edema. The least severe, type 4, is characterized by moderate or mild heart failure [18].
Acute mitral insufficiency due to PMR: echocardiographic diagnosis
Echocardiography is the preferred diagnostic method for accurately diagnosing PMR. Transthoracic echocardiography (TTE) has been the first-line diagnostic tool for PMR since the 1980s [19–21].
With the introduction of transesophageal echocardiography (TEE), the diagnosis of PMR has become more precise. Although advancements in TTE probes now allow for good diagnostic images in most cases, TEE provides detailed anatomical information that can guide therapeutic decisions.
The anatomy of PMR is complex and highly variable, depending on the extent of necrosis and the subsequent anatomical and functional changes, which influence the echocardiographic appearance.
In 1996, Nanda’s group published an echocardiographic classification based on transesophageal evidence, correlating echocardiographic features with the main types of PMR [22].
In clinical practice, and in our experience, this classification is both practical and valuable for diagnostic and prognostic purposes:
Partial-incomplete rupture. In partial-incomplete rupture, the partially ruptured papillary muscle remains adherent to the ventricular wall and exhibits chaotic movement.
Partial-complete rupture. In partial-complete rupture, the ruptured portion of the papillary muscle is freely mobile within the left ventricle but does not protrude into the left atrium during systole.
Subtotal/total (incomplete/complete) rupture. In subtotal or total rupture, the heads or the entire trunk of the papillary muscle protrudes into the left atrium during systole, pulling the chordae tendineae and mitral leaflets along.
In the case of partial-incomplete rupture, echocardiography shows a poorly defined echo at the site of the papillary muscle, with a structure of variable size still attached to the ventricular wall but exhibiting erratic, uncoordinated movement (out of phase with the ventricular wall motion). The partially ruptured papillary muscle often displays a more or less pronounced vibrating motion. This echocardiographic finding corresponds to the tearing of a limited portion of the papillary muscle, which remains attached to the muscle body.
This type of rupture is the most challenging to detect for several reasons: the small size of the torn portion, its proximity to the ventricular wall making it difficult to distinguish the ruptured from the healthy tissue, and the vibrating motion, which is often misinterpreted as normal (Figure 2, Movie S1).
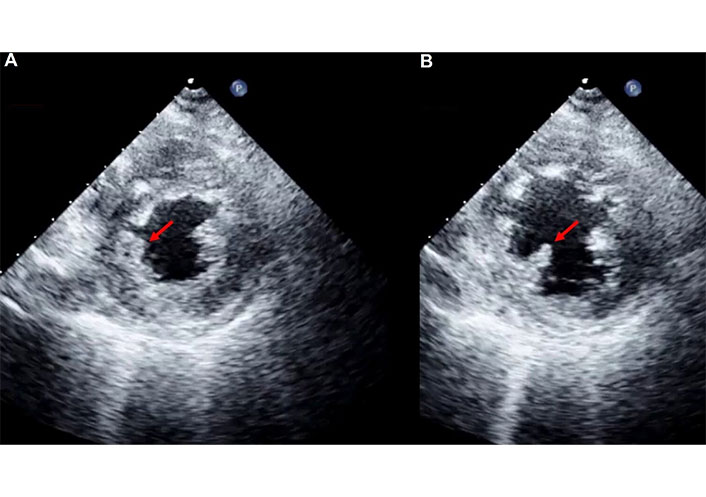
Bidimensional (2D) transthoracic echocardiography (TTE). Parasternal short-axis view at the papillary muscle level, in systole (A) and in diastole (B) in a patient with partial-incomplete rupture of the postero-medial papillary muscle. The red arrow indicates the torn portion of the postero-medial papillary muscle that is better recognizable in B. See Movie S1
In the case of a partial-complete rupture, echocardiography reveals a mobile echo density of variable size, detached from the ventricular wall and exhibiting chaotic movement within the left ventricle. This finding corresponds to the partially detached head of the papillary muscle, which remains connected to the trunk by a thin strip of tissue. Although this portion of the papillary muscle shows erratic and disordered movement, it stays within the left ventricle and does not prolapse into the left atrium.
This type of rupture presents an intermediate level of diagnostic difficulty, as the torn portion is typically large enough to be easily detected and interpreted. Occasionally, the torn segment of the papillary muscle is attached to the ventricular wall by tertiary chordae tendineae (Figures 3, 4, and 5, Movies S2 and 3).
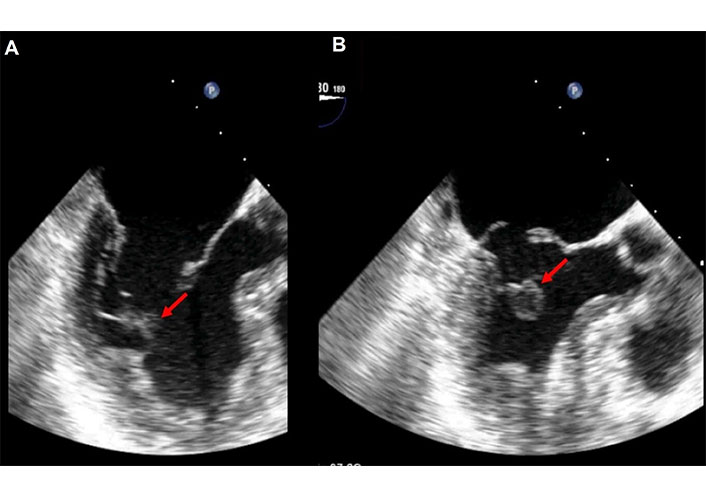
Bidimensional (2D) transesophageal echocardiography (TEE). The transducer is in mid/upper esophageal position with an angle of 180°. Long axis view in diastole (A) and in systole (B) in a patient with partial-complete rupture of the posteromedial papillary muscle. The red arrow indicates the ruptured portion of the postero-medial papillary muscle that is better recognizable in B. See Movie S2
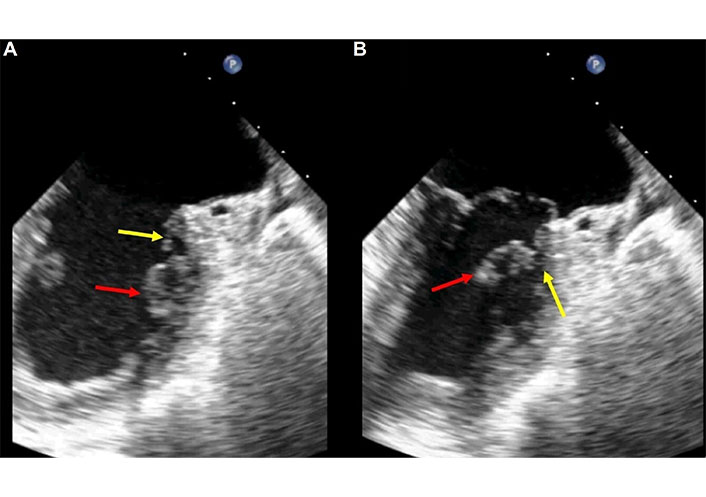
2D-TEE in the same patient of Figure 3. The transducer is in mid esophageal position with an angle of 66°. Two chamber (commissural) view in diastole (A) and in systole (B). The red arrows indicate the ruptured portion of the postero-medial papillary muscle that is better recognizable in B. The yellow arrows indicate the thin strip of tissue and the tertiary chordae tendineae that grasp the ruptured portion of the papillary muscle to the left ventricle wall. TEE: transesophageal echocardiography. See Movie S3
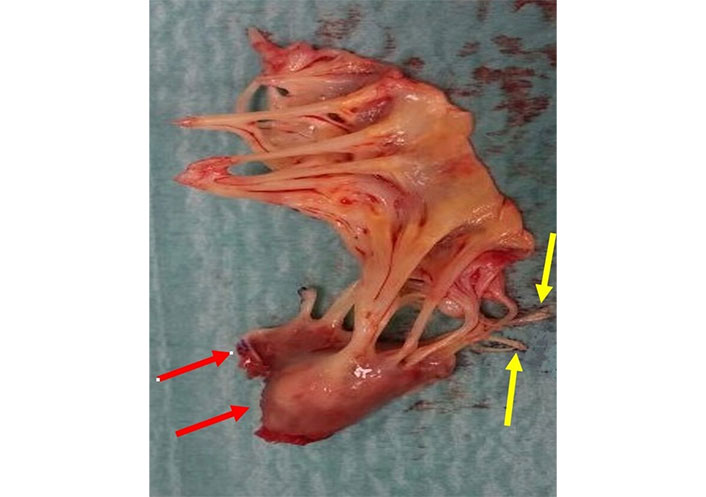
In case of a complete subtotal/total (incomplete/complete) rupture, echocardiographic examination reveals a mobile echo density in the left atrium during systole, consistent with the head/heads or trunk of the ruptured papillary muscle. The size and density of the detached muscle vary widely depending on the degree of rupture and potential fibro-sclerotic changes. Sometimes the systolic mobile echo in the left atrium has an irregular shape because of tangled ruptured chordae around the muscle. Occasionally, the systolic mobile echo in the left atrium appears irregular due to tangled ruptured chordae around the muscle. This form of PMR is the easiest to diagnose, as the mobile echo, representing the papillary head, is clearly identifiable by its characteristic back-and-forth movement from the ventricle to the left atrium (Figure 6, Movie S4).
2D-TEE in a patient with complete total rupture of the posteromedial papillary muscle. The transducer is in mid oesophageal position. Two chamber (commissural) view in diastole (left) and in systole (right). In systole the trunk of the posterior papillary muscle is seen in the left atrium. TEE: transesophageal echocardiography. See Movie S4
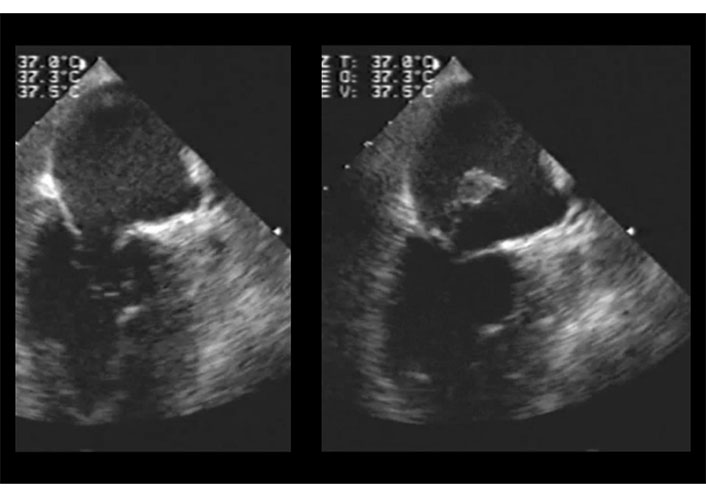
If the ruptured papillary muscle partially protrudes into the mitral orifice without fully prolapsing into the left atrium, TTE may be negative, while TEE provides a definitive diagnosis (Figures 7, 8, and 9 and Movies S5–8).
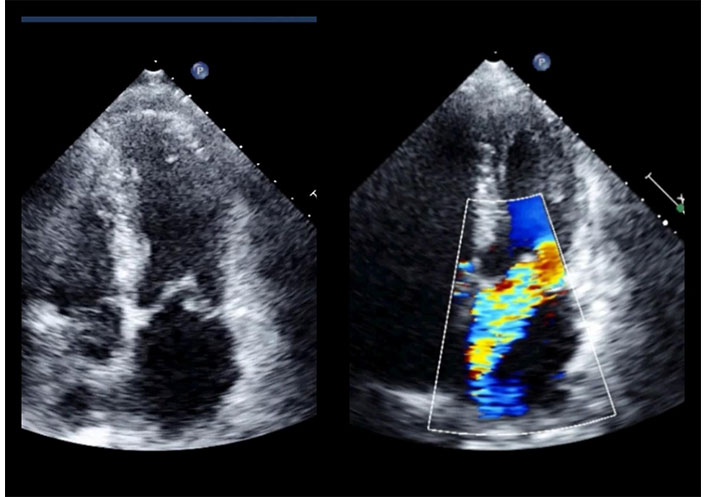
2D-TTE in a patient with inferior myocardial infarction. Apical four chamber view in systole. Posterior mitral leaflet prolapses into the left atrium (left); color Doppler assessment reveals severe mitral regurgitation directed towards the interatrial septum (right). TTE: transthoracic echocardiography. See Movies S5 and 6
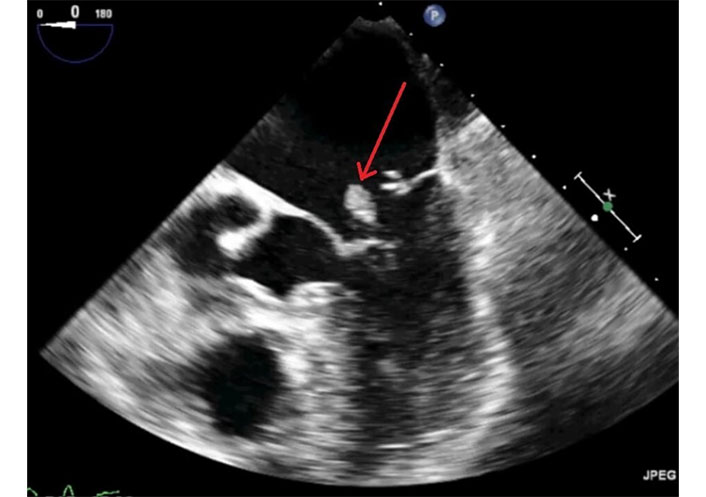
2D-TEE in the same patient of Figure 7. The transducer is in mid/upper esophageal position with an angle of 0°. Long axis view in systole. The red arrow indicates the head of the ruptured papillary muscle that is recognizable in the left atrium, near mitral orifice. TEE: transesophageal echocardiography. See Movie S7
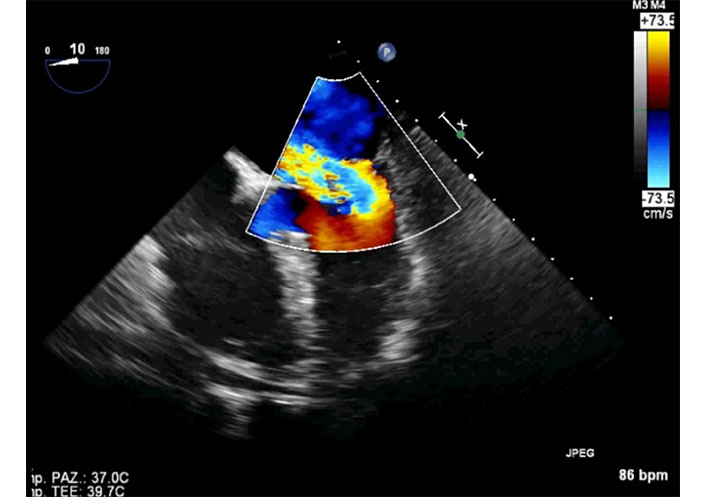
2D-TEE in the same patient of Figure 7. The transducer is in mid esophageal position with an angle of 10°. Four camber view in systole. The color Doppler assessment reveals severe mitral regurgitation directed towards the interatrial septum. A large convergence area is seen in the left ventricle (severe mitral regurgitation). TEE: transesophageal echocardiography. See Movie S8
The impact of the different types of PMR on the mitral leaflets is highly variable (Figure 1). In cases of partial, incomplete rupture, interference with leaflet motion may be minimal, allowing overall valve kinetics to be preserved. However, even in partial rupture, interference with a large portion of the leaflet may occur, leading to severe regurgitation. In partial-complete and subtotal/total ruptures, multiple scallops of both leaflets lose their support and prolapse into the left atrium (Figures 10 and 11, Movie S9). In these cases, regurgitation is always severe.
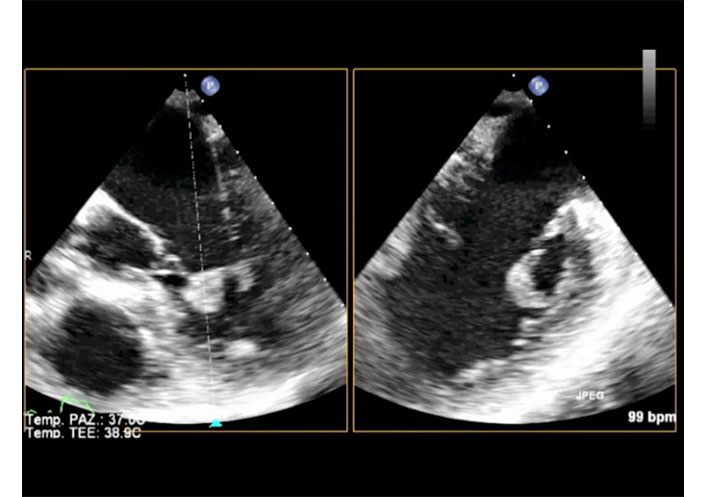
2D-TEE X-plane in a patient with complete total rupture of the anterior papillary muscle. The transducer is in mid esophageal position. Long axis view (left) and two chamber (commissural) view (right) in diastole. The ruptured trunk of the anterior papillary muscle is seen in the left ventricle in both views. TEE: transesophageal echocardiography. See Movie S9
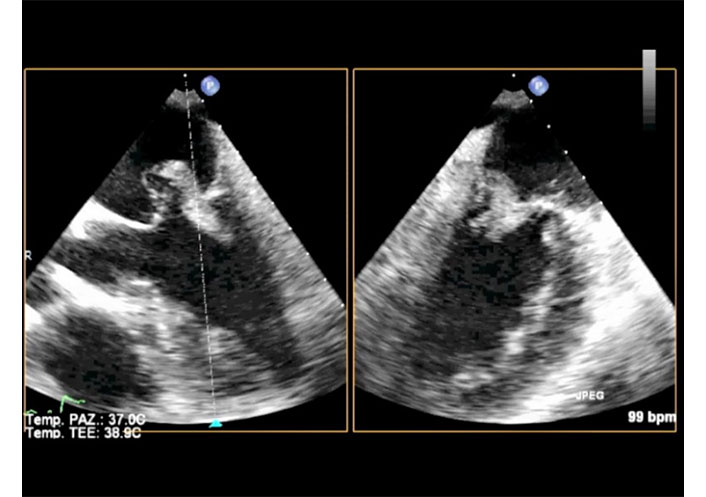
2D-TEE X-plane in the same patient of Figure 10. Long axis view (left) and two chamber (commissural) view (right) in systole. In both views part of the ruptured trunk of the anterior papillary muscle is seen in the left atrium and part remains in the ventricle. TEE: transesophageal echocardiography. See Movie S9
To understand the relationship between the type and extent of acute PMR and the severity of mitral regurgitation, a left ventricular simulator with a porcine mitral apparatus was used. The hemodynamic effects varied considerably depending on the location and extent of the rupture. Rupture of less than two-thirds of the cords from one papillary muscle head, or rupture of the PPM head, had less significant hemodynamic effects than rupture of more than two-thirds of the cords from one head or rupture of the APM head [23].
For an accurate transesophageal echocardiographic study of PMR anatomy, the probe positions and views recommended by guidelines are followed [24, 25].
Longitudinal and two-chamber views with the probe in the mid-esophageal position, as well as short-axis views with the probe in transgastric and deep transgastric positions, are the most informative in our experience. In particular, for partial incomplete rupture, the most challenging form to detect, ETT and TEE short-axis views allow the identification of even minimal portions of papillary muscle showing non-coordinated movement with the ventricular wall, suggesting partial rupture (Figure 2, Movie S1).
Mono-dimensional recording, with its high temporal resolution, reveals fluttering and vibration in the disanchored portion of the papillary muscle. Although this pattern is not an independent diagnostic criterion, it supports the diagnosis. In more advanced forms of rupture, longitudinal views enable visualization of the residual portion of the papillary muscle still attached to the left ventricular wall.
Transthoracic examination can also be diagnostic, though some details may not be well-defined. short-axis views may lack sufficient quality to diagnose partial and incomplete ruptures. The kinetic abnormalities of the left ventricle should be carefully examined, though it is not unusual for such abnormalities to be minimal or even absent. Limited necrosis affecting only the trunk or head of the papillary muscle can result in severe mitral regurgitation without impairing the motion of the left ventricular walls. Therefore, the absence of kinetic abnormalities does not exclude acute PMR due to ischemia [26–29] (Movies S1–3).
Mitral regurgitation arises from the systolic eversion of scallops no longer supported by the chordae tendineae, into the left atrium. In cases of PMR with displacement of the muscle head into the left atrium, the regurgitation is also caused by the dragging of chordae and leaflet portions that follow the movement of the ruptured papillary muscle. It is important to note that a papillary muscle head can supply chordae tendineae to different scallops of the same leaflet, or even to scallops of both leaflets. This depends on the location of the rupture and the extent of muscle involvement. Consequently, the regurgitant jet does not have a consistent direction, as it can be generated by altered kinetics in both leaflets. A jet directed towards an atrial wall may be seen in cases where only one leaflet prolapses. More frequently, the posterior leaflet is involved, and the regurgitant jet is directed towards the interatrial septum (Figure 7 and Movie S6, Figure 9 and Movie S8). In cases of more extensive ruptures involving scallops from both leaflets, the jet direction is central (Figure 12, Movie S10) or predominantly towards the interatrial septum or posterior wall of the atrium.
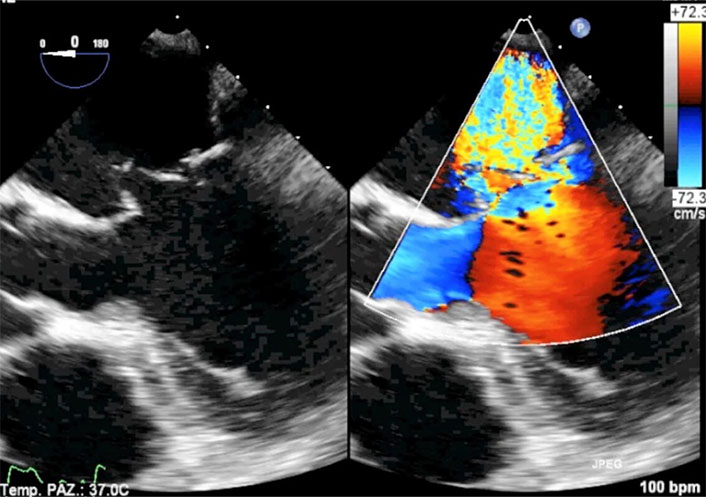
In this context, three-dimensional transesophageal reconstruction may be useful, though in our opinion [30], its role is limited and primarily supports the two-dimensional transesophageal method (Figure 13, Movies S11 and 12).
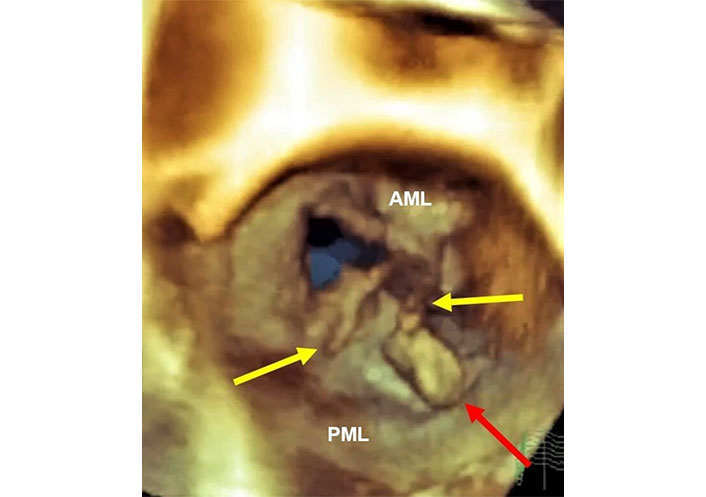
Three-dimensional transesophageal echocardiography (3D-TEE) in the patient of Figure 9 with complete total rupture of the anterior papillary muscle. Volumetric cropped image is rotated to obtain the atrial and surgical view. Still frame in systole. The red arrow indicates the head of the ruptured papillary muscle in the left atrium. The yellow arrows indicate the ruptured chordae tendineae and probably material from mitral leaflets. AML: anterior mitral leaflet; PML: posterior mitral leaflet. Movies S11 and 12
Traditional methods for quantifying mitral regurgitation, such as those based on vena contracta or the proximal isovelocity surface area (PISA), are not applicable to this specific type of regurgitation. However, these methods are typically unnecessary, as acute mitral regurgitation in patients with PMR is almost always classified as severe based on the anatomy of the mitral valve apparatus (Figure 7 and Movie S6, Figure 9 and Movie S8, Figure 12 and Movie S10), as outlined by current guidelines [31, 32].
Although severe, mitral regurgitation can have varying hemodynamic consequences and may be tolerated differently by patients [18].
The echocardiographic assessment should include a comprehensive evaluation of the right ventricle, pulmonary pressures, and any coexisting pathologies, in addition to defining the mitral pathology and left ventricular function.
Echocardiography and management of surgical and interventional therapy of acute PMR
Acute PMR is a catastrophic event, with case reports indicating that over 80% of patients experiencing this complication develop cardiogenic shock and die within the first 24 hours. Early diagnosis and appropriate initial treatment are crucial to stabilizing the patient for subsequent procedures. Poor preoperative conditions are known to contribute to negative surgical outcomes.
Given the high incidence of cardiogenic shock and massive pulmonary edema, mechanical circulatory support (MCS) should be used alongside medical therapy [15, 33–35].
The use of MCS, regardless of the specific device chosen for each case, should be short-term, serving as a bridge to surgery. Early surgical intervention is considered the cornerstone of treatment and the preferred option, as highlighted in current guidelines. However, questions regarding surgical options persist due to unsatisfactory outcomes. Despite advances in cardiac surgery, emergency or urgent mitral valve replacement still carries a high mortality rate. In some cases, surgery is not feasible due to unstable hemodynamics. MCS has been proposed as a means of stabilizing patients before surgery, potentially improving survival, especially when combined with aortocoronary bypass [36].
The types of PMR that may occur clinically show different degrees of hemodynamic impairment. However, no systematic studies exist that detail the relationship between PMR, mitral regurgitation, and left ventricular function, leaving the best methods for hemodynamic stabilization and bridging to surgery largely undetermined. Moreover, no studies have evaluated how frequently and over what time period a partial rupture, which is relatively well tolerated if it does not cause severe mitral regurgitation, progresses to more severe degrees of rupture.
Treatment decisions are often made on a case-by-case basis, considering clinical conditions and available resources. Some groups prefer to use MCS to stabilize patients before waiting for hemodynamic improvement and infarct consolidation, while others favor early surgery.
Surgical repair is challenging due to tissue friability, and reparative surgery is often difficult. Reports suggest that transcatheter mitral clips have been used to stabilize patients or as a bridge to surgery in selected cases. However, early surgery is sometimes preferred to address severe mitral regurgitation and revascularize the patient.
However, it should be noted that the choice of MCS depends on various factors such as the severity of the patient’s condition, the availability of resources, and the experience of the medical team. A multidisciplinary approach involving cardiologists, cardiac surgeons and critical care specialists is often necessary to plan the most appropriate therapy pathway.
The choice of MCS is influenced by factors such as the patient’s condition, available resources, and medical team expertise. Current guidelines recommend surgery when clinical stability cannot be achieved through medical therapy, inotropic support, or mechanical assistance. Data from a multicentre cohort study show that in general surgical treatment for mechanical complications of myocardial infarction are still associated with high in-hospital mortality rates. However, long-term survival in patients surviving the immediate post-operative period is encouraging [36].
For severely unstable patients with high surgical risk, it is debated whether mitral valve replacement is preferable to percutaneous repair, an attractive option which has shown promising results. If the PMR is partial, and adjacent tissues are less affected by ischemia, mitral valve repair may be feasible. The ACC/AHA guidelines (2013–2020) recommend mitral valve repair, if possible, particularly in cases of partial PMR where the tissue quality is suitable for repair. Factors such as surgeon experience and hemodynamic instability also play a role in determining the appropriate surgical procedure [31, 37].
Mitral valve replacement is more commonly performed, largely due to greater surgeon experience and the established reliability and durability of the procedure. It is generally mandatory in cases of papillary muscle trunk rupture when the tissue is not suitable for repair. In such cases, a biological valve is preferred over a mechanical one when mechanical support with ventricular assist devices (VAD) or extracorporeal membrane oxygenation (ECMO) is needed, due to the high risk of thrombosis [8, 16, 38–40].
In recent years, percutaneous mitral valve repair has emerged as a promising option for high-risk surgical patients, with positive outcomes reported in reviews focusing on ischemic mitral regurgitation, including cases of PMR [41–43].
The International Registry of MitraClip in Acute Mitral Regurgitation following Acute Myocardial Infarction (IREMMI Registry) included 93 patients from 2016 to 2020 with severe post-infarction mitral regurgitation, of whom 50 had cardiogenic shock. Patients treated with percutaneous mitral clips after clinical stabilization had similar outcomes [44].
The role of transcatheter edge-to-edge repair (TEER) was assessed in 3,797 patients with cardiogenic shock and mitral regurgitation who underwent transcatheter procedure between 2013–2021 in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry (STS/ACC/TVT registry). One-year follow-up showed that device success was associated with lower all-cause mortality and fewer incidences of heart failure [45].
Percutaneous repair with Clip implantation has now been introduced alongside surgery for patients with PMR. TEER with Clip has proven effective in high-risk surgical cases, and isolated cases of mitral regurgitation have been successfully treated using Clip devices [46–49].
The IREMMI Registry has recently evaluated the outcomes and safety of TEER for acute mitral regurgitation after myocardial infarction. In this analysis patients treated with TEER in the acute setting due to ischemic aetiology, including complete PMR, partial muscle rupture and chordal (head) rupture, were included. In this multicentre registry, TEER was a feasible therapy in critically ill patients with PMR due to a recent myocardial infarction. TEER may have a role as salvage treatment or bridge to surgery in this population [50].
In our experience, the application of transcatheter mitral repair with Clip/Clips is limited to cases where the regurgitation results from prolapse of part of the leaflets, without significant systolic prolapse of large amount of the papillary muscle (head or trunk) into the left atrium. In such cases (types 1 and 2 of the Moursi classification) the repair procedure targets stabilization of the prolapsed scallop/scallops, which can be effectively managed with Clip/Clips implantation.
Echocardiographic characterization of the precise mechanisms of mitral regurgitation is crucial in planning the percutaneous procedure. For example, in our personal case involving an 85-year-old woman with acute mitral regurgitation during an inferior myocardial infarction, echocardiography revealed partial rupture of the PPM and posterior mitral leaflet prolapse. Based on echocardiographic results, the Heart Team, recommended percutaneous mitral valve repair with Clip. The targeted lesion was considered the prolapsed leaflet that was successfully clipped (Figures 14, 15, 16, 17, 18, and 19, Movies S13–20).
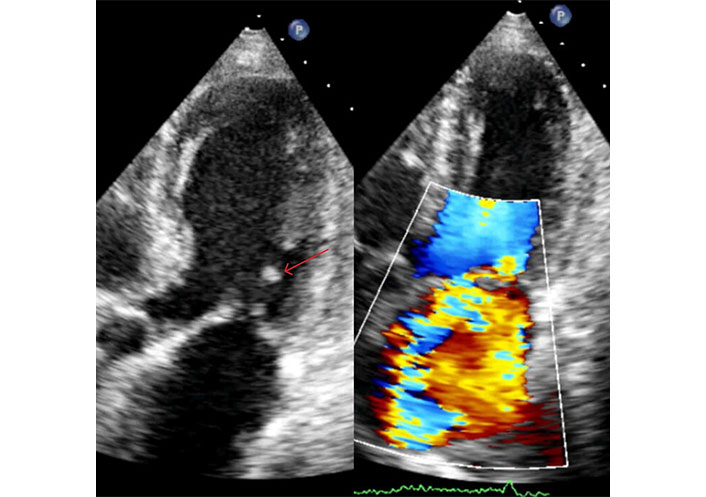
2D-TTE in a patient with inferior myocardial infarction. Apical long-axis view in systole. The red arrow indicates the head of posterior papillary muscle detached from its body. The echodensity of the ruptured head is increased due to fibrosclerotic evolution (left). Color Doppler assessment reveals severe mitral regurgitation directed towards the interatrial septum (right). TTE: transthoracic echocardiography. See Movies S13 and 14
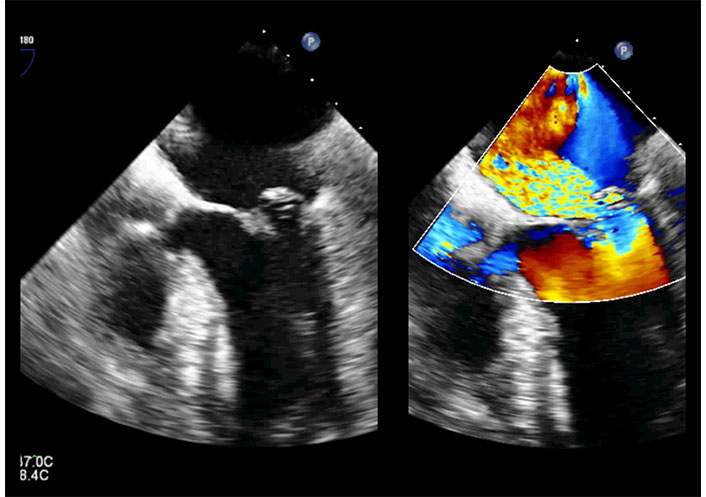
2D-TEE in the same patient of Figure 14. Mid esophageal position, 0°. Off-axis view (long axis/four camber view) in systole. The posterior leaflet prolapses into the left atrium (left). Color Doppler detects severe mitral regurgitation directed towards the interatrial septum (right). TEE: transesophageal echocardiography. See Movies S15 and 16
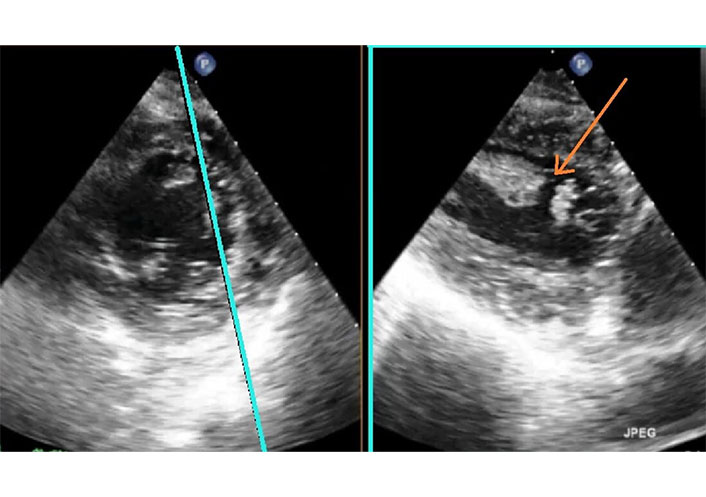
2D-TEE X-plane in the same patient of Figure 14. Short axis view (left) and two-chamber view (right) with the probe in deep trans-gastric position. The plane for the two-chamber view is selected on the short axis so that the papillary muscle is imaged longitudinally (blue line). The red arrow indicates the site of papillary muscle rupture. TEE: transesophageal echocardiography. See Movie S17
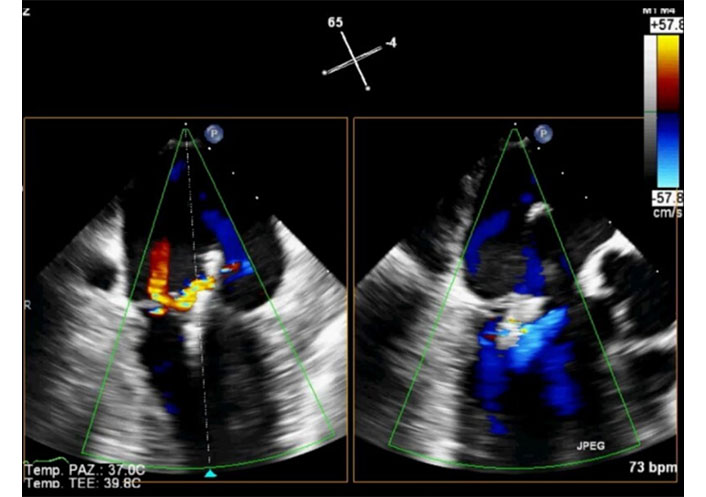
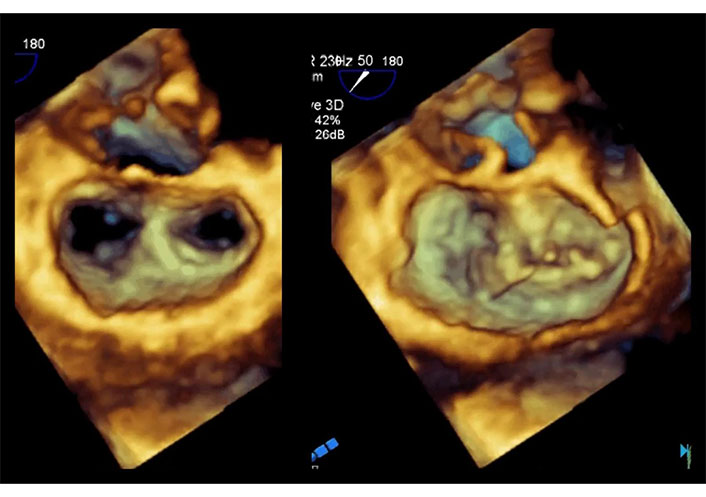
Intraprocedural 2D-TEE X-plane in the same patient of Figure 14. Color Doppler in systole. Two-chamber view (left) and long-axis view (right). Positioning and release of the Clip under echocardiographic guidance. The Clip is well positioned, and the degree of residual mitral regurgitation is considered satisfactory. TEE: transesophageal echocardiography. See Movie S19
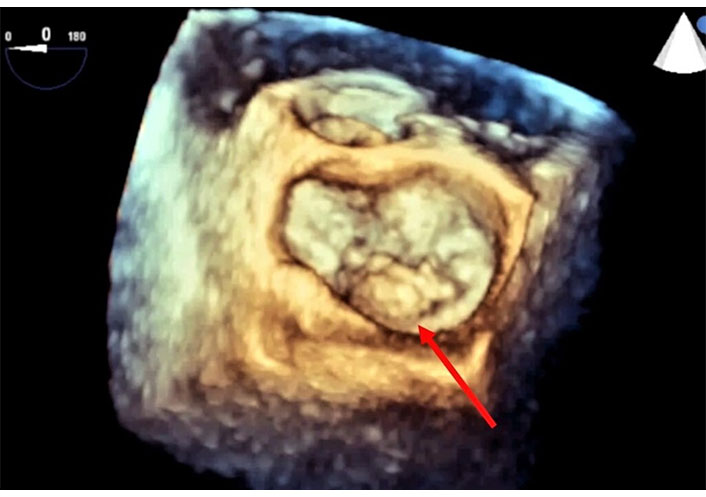
Intraprocedural 3D-TEE in the patient of Figure 14 after the deployment of the Clip and the delivery system and of the steerable guide catheter. Volumetric cropped image is rotated to obtain the atrial and surgical view. The double orifice is well defined in diastolic frame (left). In systolic frame it is possible identify two chordae protruding in medial orifice (right). TEE: transesophageal echocardiography. See Movie S20
Conclusions
PMR is a serious and potentially fatal complication that can occur after a myocardial infarction. Early and accurate echocardiographic diagnosis is essential for identifying the anatomical features of the rupture and for evaluating the severity of mitral regurgitation. Tailored management is critical, with treatment strategies varying according to the echocardiographic findings and the patient’s clinical condition. While some patients may require surgical intervention to repair or replace the mitral valve, less invasive percutaneous interventions may be suitable for carefully selected patients, as determined by echocardiographic assessment.
The involvement of a multidisciplinary heart team, including cardiologists, cardiac surgeons and other specialists, is crucial in formulating the most appropriate treatment strategy.
Despite advancements in both the diagnosis and management of PMR, further studies are needed to refine treatment protocol and guide optimal therapeutic approaches.
Abbreviations
| APM: | anterolateral papillary muscle |
| LCX: | left circumflex artery |
| MCS: | mechanical circulatory support |
| PMR: | papillary muscle rupture |
| PPM: | posteromedial papillary muscle |
| TEE: | transesophageal echocardiography |
| TEER: | transcatheter edge-to-edge repair |
| TTE: | transthoracic echocardiography |
Supplementary materials
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/101242_sup_1.pdf.
Declarations
Author contributions
PGP: Conceptualization, Writing—original draft, Validation, Supervision, Writing—review & editing. IP: Conceptualization, Writing—original draft. AM: Conceptualization, Writing—original draft. AT: Writing—review & editing. FN: Validation, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Ethics approval is not required for this study according to our local Ethics Committee since it is routine activity at the echocardiography lab.
Consent to participate
Informed consent to participate in the study was obtained from relevant participants.
Consent to publication
Not applicable.
Availability of data and materials
Requests for accessing the data sets should be directed to Paolo G. Pino, aries52@libero.it.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.