Abstract
Cardiovascular diseases, particularly ischemic heart disease (IHD), are the leading cause of mortality globally, accounting for 16% of deaths. Effective management of ischemic cardiomyopathy (ICM) is crucial, as outlined in the latest European Society of Cardiology (ESC) guidelines for chronic coronary syndrome (CCS). The guidelines emphasize a structured approach comprising four key steps: a general clinical evaluation to exclude non-cardiac causes, cardiac examination, and likelihood estimation using echocardiography, diagnostic testing such as stress echocardiography and coronary CT angiography, and treatment involving lifestyle changes and medication, alongside potential revascularization. The review underscores the importance of coronary angiography and functional assessments in diagnosing ischemic heart failure (IHF) and guiding treatment strategies. Non-invasive imaging techniques, including stress echocardiography and myocardial perfusion scintigraphy, are valuable for assessing ischemia and myocardial viability while reducing unnecessary invasive procedures. Coronary CT angiography is also examined for its procedural advantages and risks. A comparative analysis of diagnostic modalities highlights the strengths and limitations of each technique, emphasizing the need for individualized approaches based on patient characteristics. The ESC 2024 guidelines advocate for a patient-centered imaging strategy based on the likelihood of coronary artery disease (CAD) while addressing the economic and environmental impacts of imaging practices. Overall, implementing these guidelines and leveraging diverse imaging modalities can optimize management strategies for IHD, ultimately improving patient outcomes.
Keywords
Coronary angiography, stress echocardiography, myocardial scintigraphy, coronary computed tomography angiography (CCTA), fractional flow reserve CT (FFR-CT)Introduction
Cardiovascular diseases are the leading cause of death globally, with ischemic heart disease (IHD) accounting for approximately 16% of all deaths worldwide [1, 2]. Effective prevention and management of ischemic cardiomyopathy (ICM) are essential for reducing its prevalence and impact. The primary strategies are summarized in Table 1.
Key strategies for preventing and managing ischemic cardiomyopathy
| Strategy | Description |
|---|---|
| Lifestyle modifications | |
| Healthy diet | Consuming a balanced diet rich in fruits, vegetables, whole grains, and lean proteins |
| Regular physical activity | Engaging in at least 150 minutes of moderate-intensity aerobic exercise per week |
| Smoking cessation | Quitting smoking significantly lowers the risk of developing ischemic cardiomyopathy |
| Management of risk factors | |
| Blood pressure control | Maintaining healthy blood pressure levels through lifestyle changes and medication |
| Diabetes management | Controlling blood sugar levels to reduce the risk of cardiovascular complications |
| Cholesterol reduction | Managing cholesterol levels with diet, exercise, and medications such as statins |
| Pharmacological therapies | |
| Aspirin | Low-dose aspirin to reduce the risk of blood clots and heart attacks in certain individuals |
| Statins and other anti-LDL drugs | Medications to lower cholesterol levels and reduce cardiovascular risk |
| Beta-blockers | Drugs to manage hypertension and prevent further cardiac events in individuals with ischemic cardiomyopathy |
| Medical interventions | |
| Angioplasty | Procedure to open narrowed or blocked coronary arteries, improving blood flow to the heart muscle |
| Coronary artery bypass surgery | Surgery to bypass blocked arteries and restore adequate blood flow to the heart in severe cases |
The updated European Society of Cardiology (ESC) guidelines [3] for managing suspected chronic coronary syndrome (CCS) outline a structured, four-main-step approach:
General clinical evaluation: Assess symptoms, rule out non-cardiac causes of chest pain, and exclude acute coronary syndrome (ACS) through a basic examination, which includes electrocardiogram (ECG) and selected tests (blood work, chest X-ray).
Cardiac examination and likelihood estimation: Conduct echocardiography to assess left ventricular function and estimate the likelihood of obstructive coronary artery disease (CAD), which helps guide further diagnostic testing.
Diagnostic testing: Perform tests such as stress echocardiography (SE) or coronary computed tomography angiography (CCTA) to confirm the CCS diagnosis and assess future cardiovascular risk.
Treatment and risk modification: Implement lifestyle changes and pharmacological therapies. If needed, consider coronary revascularization and assess for microvascular disease if no obstructive CAD is detected.
Coronary angiography and functional assessment
Invasive coronary angiography (ICA) results are categorized as follows:
Mild or none: coronary stenosis 0–39%.
Intermediate: coronary stenosis 40–69%.
Obstructive: coronary stenosis ≥ 70% or left main stenosis ≥ 50%.
Invasive physiological testing, such as fractional flow reserve (FFR), provides results on a continuous scale from 0 to 1, with lower values indicating more severe ischemia. However, high values do not necessarily rule out ischemia, and these tests may not fully assess microvascular dysfunction [4].
For patients with a very high (≥ 85%) likelihood of obstructive CAD, symptoms unresponsive to medical therapy, low-exertion angina, or high event risk based on initial assessments (e.g., echocardiogram, exercise ECG), direct ICA without additional testing is a reasonable approach. FFR should guide revascularization decisions for stenoses < 90%. Functional imaging is preferred when evaluating myocardial ischemia, viability, or microvascular disease, and it is often superior to CCTA for patients with moderate to high CAD risk and in specific populations. Functional imaging avoids radiation exposure and bypasses some limitations of CCTA [3].
Although advancements in ICA have reduced procedural complications, there are still inherent risks, particularly when femoral access is used. ICA should be avoided in patients unwilling to undergo invasive procedures, those who are not suitable candidates for revascularization, or when revascularization is unlikely to improve the patient’s quality of life. Coronary angiography is essential for managing ischemic heart failure (IHF) by diagnosing CAD, visualizing arterial stenoses, and assessing their severity. This procedure informs therapeutic decisions, including revascularization via percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), and allows for monitoring treatment efficacy. Additionally, coronary angiography evaluates collateral circulation and provides prognostic insights to tailor long-term management strategies for better patient outcomes [5, 6].
SE, myocardial scintigraphy, and cardiac computed tomography (CT) are valuable for identifying patients who may benefit from coronary angiography. These imaging modalities help determine the presence and extent of IHD and guide subsequent diagnostic and therapeutic interventions [7–9].
This review provides an overview of SE, cardiac CT, and myocardial perfusion scintigraphy, examining their advantages and limitations in selecting candidates for coronary angiography. These modalities improve the diagnosis and management of CAD by refining patient selection for invasive procedures, ultimately enhancing patient outcomes.
Overview and clinical significance of coronary angiography: technique, indications, and risks
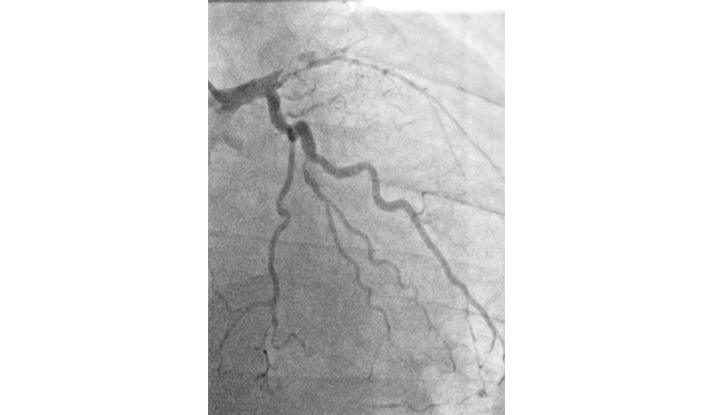
During coronary angiography (Figure 1), characteristics and anomalies of coronary circulation can be assessed. Coronary circulation is classified by the artery giving rise to the posterior descending artery. While coronary dominance itself is not clinically significant, anomalies are identified in 1.0–1.5% of cases, with most being benign and of little clinical consequence. However, certain anomalies, such as an abnormal origin of the left anterior descending artery from the right sinus of Valsalva, are clinically relevant, as they may cause compression between the aorta and pulmonary artery during systole, leading to symptoms.
Contraindications for coronary angiography include both absolute and relative considerations. The only absolute contraindication is the patient’s refusal to provide informed consent for the procedure. Relative contraindications include correctable electrolyte imbalances, drug toxicity (e.g., hyperkalemia or digitalis toxicity), fever, acute renal failure, heart failure, severe allergies to iodinated contrast agents, ongoing oral anticoagulant therapy, severe bleeding disorders, uncontrolled hypertension, and pregnancy.
Coronary angiography’s limitations include its two-dimensional nature, which shows the vascular lumen but not the vessel walls. This limitation may lead to underestimating the severity and extent of atherosclerotic plaques when normal segments are absent. Intraluminal thrombi and ulcerated plaques are assessed indirectly based on coronary filling patterns. To improve diagnostic accuracy, coronary angiography can be supplemented with other invasive techniques such as intravascular ultrasound (IVUS), optical coherence tomography (OCT), or FFR.
Coronary angiography is a critical tool in cardiology, providing detailed and precise information that guides the diagnosis and treatment of heart disease. However, the procedure carries several risks, as highlighted in Table 2.
Risks associated with coronary angiography
| Risk | Description |
|---|---|
| Bleeding and hematoma | The insertion site may bleed or develop a hematoma. |
| Infection | Infection can occur at the catheter insertion site, although rare. |
| Allergic reactions | Patients may experience allergic reactions to the contrast dye. |
| Arrhythmias | The procedure can sometimes trigger abnormal heart rhythms. |
| Contrast-induced nephropathy | The contrast dye can cause kidney damage, particularly in patients with existing kidney conditions. |
| Radiation exposure | Repeated exposure to X-ray radiation may increase the risk of cancer. |
| Vascular complications | Injury to the artery used for catheter insertion can occur, which may lead to pseudoaneurysm or arteriovenous fistula. |
| Heart attack or stroke | The procedure can dislodge plaque, potentially leading to a heart attack or stroke, although this is rare. |
| Death | In very rare cases, serious complications from coronary angiography can be fatal. |
Given these risks, it is advisable to conduct non-invasive diagnostic tests such as SE, CT, and myocardial scintigraphy before referring a patient for coronary angiography.
SE is a valuable tool for evaluating myocardial ischemia. This technique uses ultrasound imaging to assess heart function under stress conditions induced by exercise or pharmacological agents. SE has proven effective in diagnosing CAD and predicting patient outcomes.
Cardiac CT, particularly CCTA, provides detailed images of the coronary arteries and can accurately detect significant stenoses. This non-invasive imaging modality has been shown to be highly effective in diagnosing CAD, reducing the need for invasive procedures in many patients. Mowatt et al. [10] highlighted the diagnostic accuracy of cardiac CT and its role in assessing CAD, emphasizing its potential to rule out significant coronary obstructions with high accuracy.
Myocardial scintigraphy, or nuclear stress testing, evaluates myocardial perfusion and identifies areas with reduced blood flow to the heart muscle. This test is particularly useful for diagnosing CAD and determining the severity and extent of ischemia.
Cosmai and Heller [11] discussed the role of myocardial scintigraphy in assessing CAD, underscoring its diagnostic capabilities and its utility in guiding therapeutic decisions.
By utilizing these non-invasive tests, clinicians can gather essential diagnostic information while minimizing the risks associated with coronary angiography. These tests help determine which patients genuinely need invasive evaluation and intervention, thereby optimizing patient outcomes and reducing the likelihood of complications. Strategic use of non-invasive imaging ensures that coronary angiography is reserved for patients who will benefit most, thereby improving overall safety and effectiveness of care.
SE methods and applications
SE provides a precise, comprehensive cardiac evaluation beyond coronary artery stenosis. The advanced ABCDE approach evaluates regional wall motion abnormalities (A), pulmonary congestion and diastolic function (B), left ventricular reserve (C), coronary microvascular function (D), and cardiac autonomic balance (E). Ultrasound contrast agents (UCAs) enhance image quality, while artificial intelligence (AI) reduces operator dependence. SE’s limitations in detecting non-obstructive coronary lesions are mitigated by simultaneous coronary flow and perfusion imaging. It is a cost-effective, widely available, radiation-free, and environmentally sustainable procedure [12].
Exercise SE
There are two main types of exercise SE: isometric exercises (e.g., handgrip exercises) and isotonic exercises (e.g., treadmill and ergometer exercises). Exercise SE is preferred for its dynamic evaluation under physiological stress conditions.
Treadmill exercise echocardiography:
Methods: The workload is incrementally increased using protocols such as the Bruce protocol. ECG and blood pressure are monitored, and echocardiograms are recorded immediately after exercise.
Image acquisition: Efficient imaging is crucial, requiring patient cooperation to hold their breath and breathe shallowly post-exercise to improve image quality.
Supine ergometer exercise echocardiography:
Methods: Patients exercise on a bicycle in a semi-sitting position, with workload increased progressively. ECG and blood pressure are continuously monitored.
Precautions: Similar to treadmill echocardiography, images should be acquired during exhalation for optimal image quality.
Handgrip SE:
This isometric exercise involves sustained handgrip to increase pressure load on the left ventricle (LV) without significantly increasing heart rate.
Six-minute walk test:
This test involves walking as far as possible in six minutes, with an echocardiographic assessment performed post-exercise to evaluate cardiac function [13].
Interpreting SE requires expertise in evaluating regional wall motion under stress. The wall motion score index is often used to quantify the extent of ischemia, with higher scores indicating more severe diseases. Advanced techniques like Doppler tissue imaging and strain rate imaging can enhance sensitivity in detecting myocardial ischemia, although these are not yet widely adopted in clinical practice [13]. SE assesses IHD using exercise (treadmill or bicycle) or pharmacological method. It has applications even in pediatric populations and offers excellent feasibility with modern technology and contrast agents. It is used to diagnose IHD, assess myocardial viability, and evaluate prognosis. Advances like strain imaging, myocardial perfusion, and 3D imaging continue to enhance its role in cardiac imaging and stress testing [14].
Exercise SE is contraindicated in patients with ACS, poorly controlled heart or respiratory failure, severe hypertension, severe aortic stenosis, severe obstructive hypertrophic cardiomyopathy, fatal arrhythmias, acute aortic dissection, inability to exercise, and lack of patient consent. SE has limitations, including the subjective nature of image interpretation, potential underestimation of multi-vessel disease, and challenges in patients with obesity or lung disease. Test sensitivity depends on the level of stress achieved, with lower sensitivity under suboptimal stress [15].
Exercise SE is generally safe, with low incidences of severe complications such as arrhythmias or myocardial infarctions (MIs). However, the presence of emergency equipment and monitoring systems is essential.
Dipyridamole SE
The dipyridamole test [16] plays a critical role in the evaluation of IHD by leveraging its vasodilatory properties to induce hyperemia and, in certain conditions, ischemia. The outcome of the test largely depends on the dose of dipyridamole and the patient’s coronary anatomy. Low doses typically highlight the hyperemic effect in patients with no to moderate CAD whereas higher doses induce ischemia, particularly in those with moderate to severe CAD.
Dipyridamole works by increasing endogenous adenosine levels, which in turn activate A2A receptors, leading to vasodilation. This mechanism is particularly useful for identifying ischemic areas through induced flow maldistribution, known as the “steal” phenomenon. This phenomenon can be vertical (between subepicardial and subendocardial layers) or horizontal (between different vascular beds).
The test starts with an intravenous dipyridamole infusion, followed by echocardiography to assess wall motion and coronary flow reserve (CFR). High doses (up to 0.84 mg/kg) and accelerated protocols have improved sensitivity, especially in detecting minor CADs and in patients on antianginal therapy.
Dipyridamole SE offers high diagnostic accuracy, comparable to dobutamine SE (DSE) with high-dose protocols. The test is generally well-tolerated, with minor side effects resolving with aminophylline.
Furthermore, it allows for simultaneous assessment of myocardial function and coronary flow, providing comprehensive diagnostic and prognostic information. It is suitable for various patient groups, including those with microvascular dysfunction and conditions like left bundle branch block or non-obstructive CAD.
The ABCDE protocol enhances the prognostic value of dipyridamole SE by assessing multiple cardiac functions. Overall, dipyridamole SE is a robust, versatile, and safer choice for evaluating IHD, especially in specific patient populations and settings with limited resources.
Low-dose dipyridamole: At lower doses, particularly in patients with absent to moderate CAD, the hyperemic (vasodilation) effect of dipyridamole is predominant. This means that increased blood flow is achieved without significant ischemia.
High-dose dipyridamole: In cases of moderate-to-severe CAD, higher doses of dipyridamole tend to produce an ischemic response. The drug induces myocardial ischemia by causing a steal phenomenon, where blood is redirected away from areas supplied by stenotic arteries.
Echocardiography imaging detects ischemia by observing wall motion abnormalities. A positive test shows areas of the heart muscle that do not contract normally under stress, indicating compromised blood flow.
The typical protocol involves an initial low dose of dipyridamole followed by higher doses or co-administration with atropine to enhance sensitivity.
CFR is an important measure obtained during dipyridamole stress testing. A normal CFR (≥ 2.0) indicates adequate coronary blood flow reserve, while a reduced CFR suggests significant epicardial or microvascular disease.
Measurements of diastolic flow velocity in the left anterior descending artery are often used for this purpose.
Vertical steal: Blood is diverted from the subendocardial to the subepicardial layers, particularly in single-vessel disease.
Horizontal steal: Occurs in multi-vessel disease or chronic occlusions with collateral circulation, where blood flow is diverted from ischemic regions due to the vasodilation of vessels supplying collateral flow.
Diagnostic accuracy: High-dose dipyridamole echocardiography is highly accurate for detecting CAD, with sensitivity and specificity comparable to other stress tests like DSE.
Safety profile: Dipyridamole is generally well-tolerated, with minor side effects such as headache and flushing. Major complications are rare.
Dipyridamole SE provides significant prognostic information in various patient subsets, including those with chronic CAD, recent MI, and those undergoing non-cardiac surgery.
Patients with severe carotid disease, second or third-degree atrioventricular (AV) block without a pacemaker, or bronchial asthma should avoid dipyridamole.
Withdrawal of theophylline or caffeine is required before testing to ensure adenosine receptor availability.
The use of advanced imaging technologies, such as coronary flow velocity imaging allows for the simultaneous assessment of coronary flow and myocardial function, improving diagnostic accuracy and patient management.
In summary, dipyridamole stress testing, particularly when combined with echocardiography, is a valuable tool in the diagnosis and management of IHD. It allows for the assessment of both CFR and myocardial ischemia, offering critical information for patient care and prognostication.
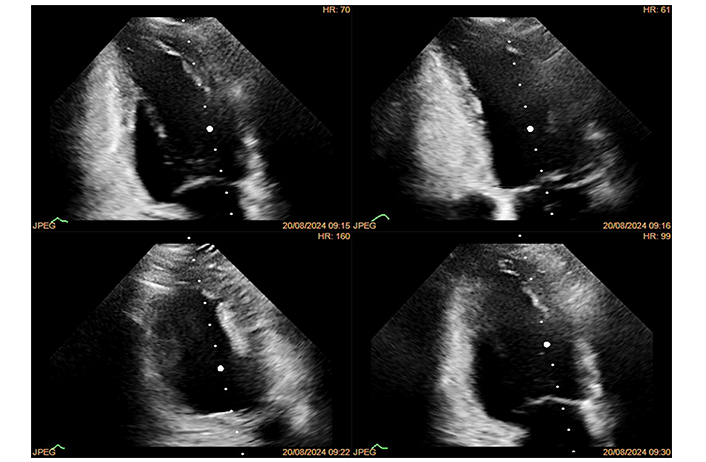
DSE
DSE [17] is effective for diagnosing IHD (Figure 2). However, side effects can limit achieving maximal pharmacological stress in about 10% of patients. Common side effects include complex ventricular tachyarrhythmias, hypotension, bradycardia, and supraventricular tachyarrhythmias. Despite these potential issues, side effects usually subside after stopping the drug, given dobutamine’s short half-life. Major complications are rare, occurring in approximately 1 out of 300–350 cases. It’s crucial to have proper resuscitation facilities and experienced personnel present during the test.
DSE is highly accurate for detecting CAD, with a sensitivity and specificity of 81% and 84%, respectively. It is comparable to other stress testing methods like exercise echocardiography and stress scintigraphy. DSE is also effective for assessing myocardial viability, differentiating between viable myocardium and scar tissue, and predicting functional recovery post-revascularization.
Dobutamine can occasionally induce coronary vasospasm, which needs to be recognized due to its clinical implications. Vasospasm can enhance DSE sensitivity but also cause false positives. It can occur during dobutamine infusion or after beta-blocker administration. Recognizing and treating coronary vasospasm appropriately, such as with nitrates instead of beta-blockers, is crucial to avoid adverse outcomes.
DSE has excellent prognostic value, comparable to other stress tests. The comprehensive assessment of regional wall motion, left ventricular contractile reserve, coronary flow velocity reserve, and heart rate reserve during DSE adds incremental prognostic value, making it a robust tool for evaluating IHD.
DSE’s limitations include side effects in 5–10% of tests and challenges in echocardiographic interpretation at high heart rates. Specific contraindications include severe arterial hypertension, uncontrolled tachycardia, hypertrophic cardiomyopathy, and recent MI. Utilizing ultrasound-enhancing agents can improve accuracy when visualizing endocardial borders is difficult.
DSE is considered a key diagnostic tool for IHD. It is also used for evaluating myocardial viability, assessing valvular heart diseases, and in heart transplant recipients to detect cardiac allograft vasculopathy. The method is validated and recommended by major cardiology guidelines for various clinical settings.
Overall, while DSE carries risks, its benefits in diagnosing and managing IHD are significant, provided it is performed under controlled and well-monitored conditions.
SE is a versatile and effective tool for diagnosing and managing CAD. Its ability to provide diagnostic and prognostic information at a relatively low cost and no radiation exposure makes it a preferred choice in many clinical scenarios. Continued technological advancements are likely to enhance its accuracy and expand its role in cardiovascular care. Several recent studies underscore the diagnostic value of strain measurements in assessing CAD and MI [18–21]. The global circumferential strain (GCS) and the ratio of GCS to global longitudinal strain (GLS) were significantly reduced in patients with CAD, particularly those with more extensive disease, indicating that GCS can serve as an effective supplementary diagnostic tool when traditional methods are inconclusive [20]. However, limitations such as small sample sizes and a lack of long-term follow-up raise concerns about the generalizability of these findings [18].
Other studies have highlighted that GLS at rest was less negative in CAD patients, with substantial overlap with non-CAD patients, which reduces its specificity [21]. The incorporation of stress testing, such as dobutamine echocardiography, has shown potential for enhancing diagnostic accuracy, emphasizing the need for standardized strain measurement techniques. Additionally, some research indicates that 3D GCS offers superior accuracy in diagnosing large MIs compared to traditional two-dimensional methods. This aligns with evidence suggesting that both GLS and transverse longitudinal strain (TLS) are strong predictors of myocardial non-viability in patients experiencing cardiogenic shock [19].
While strain measurements, particularly GCS and 3D GCS, show considerable promise for diagnosing CAD and assessing the severity of MI, these studies reveal a need for improved standardization, larger sample sizes, and long-term follow-up. Addressing these gaps could enhance diagnostic accuracy and ultimately improve patient outcomes in cardiovascular care.
Myocardial scintigraphy: imaging protocol
The myocardial scintigraphy study [21–24] was conducted by a specialist blinded to other diagnostic data. The imaging protocol involved a rest phase using technetium-99m methoxyisobutylisonitrile (99mTc-MIBI). Patients were instructed to fast after midnight, except for medications and water. Patients were advised to wear comfortable clothing and avoid caffeine or methylxanthines for 12–24 hours. Patients were instructed to remove any metals to prevent imaging artifacts. An injection of 740 MBq of 99mTc-MIBI was dispensed intravenously while the patient was at rest, and scintigraphy was performed 45 minutes later.
Common radiopharmaceuticals in myocardial scintigraphy include 99mTc-sestamibi and 99mTc-tetrofosmin. These agents have a 65% extraction efficiency, proportional to blood flow. Compared to tetrofosmin, sestamibi has less hepatobiliary excretion, which simplifies the evaluation of the inferior wall of the LV [22]. Various protocols exist for the use of 99mTc, including different doses administered on the same day or on different days, tailored to patient convenience and needs.
Identification of IHD via scintigraphy
IHD is typically identified using myocardial perfusion imaging (MPI), which can be part of a nuclear stress test or involve pharmacologic stress. A positive result indicates insufficient blood flow to the heart, which may only become apparent during the exercise phase of the stress test. Positron emission tomography (PET) plays a key role here by detecting photons emitted from injected radiolabeled tracers with a PET scanner.
PET MPI
PET acquires dynamic rest and stress images to quantify myocardial blood flow and flow reserve. This imaging technique provides both qualitative and quantitative assessments. By comparing stress and rest images, PET can identify perfusion defects, describe affected segments, assess defect severity, and determine the extent of LV involvement.
Single-photon emission CT MPI
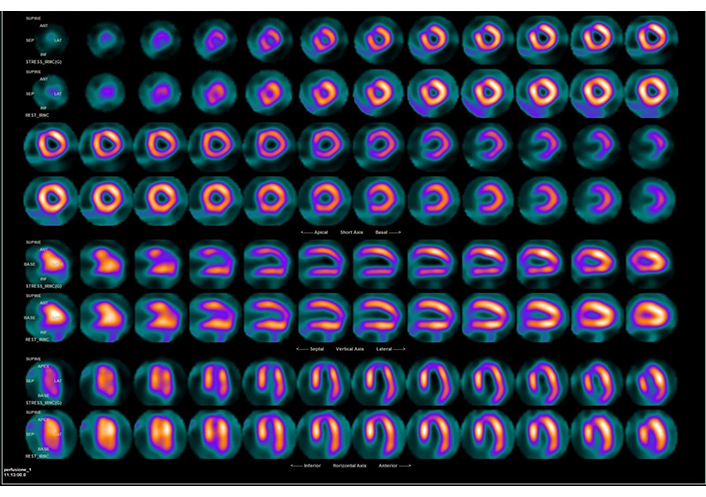
Single-photon emission CT (SPECT) is another method used in MPI. It detects gamma rays emitted by radionuclides such as 201Tl and 99mTc-sestamibi (Figure 3). By comparing ECG-gated stress and rest images, SPECT helps in identifying perfusion defects. Although SPECT is widely available and has well-established protocols, it has limitations, including lower spatial resolution compared to PET.
Hibernating myocardium
Hibernating myocardium is a state of chronic ischemia that may recover after revascularization. PET is valuable in this context as it produces metabolic images that, when compared to perfusion images, help identify hibernating myocardium. Metabolic tracers such as fluorine-18 (18F) fluorodeoxyglucose and carbon-11 (11C) acetate are used. If a matched defect is observed on PET perfusion images along with metabolic activity, indicates viable myocardium.
Indications and contraindications [22, 24]:
Indications:
Known or suspected stable angina;
Assessment of the physiological significance of known coronary artery stenosis.
Contraindications:
Unstable angina or acute MI;
Decompensated heart failure;
Acute illnesses like acute pulmonary embolism, acute myocarditis and/or pericarditis, aortic dissection, or acute cerebrovascular accident;
Severe pulmonary hypertension;
Critical left ventricular outflow obstruction or critical aortic stenosis;
Uncontrolled severe hypertension (> 200/110 mmHg);
Uncontrolled arrhythmias, including atrial fibrillation with rapid ventricular response, ventricular tachycardia, and second- or third-degree heart block without a functioning pacemaker.
Modality or vasodilator-specific contraindications:
Asthma or emphysema with active wheezing (for adenosine, dipyridamole, regadenoson);
Resting systolic blood pressure < 90 mmHg (for adenosine, dipyridamole, regadenoson);
Typical contraindications for magnetic resonance imaging (MRI);
Renal insufficiency [estimated glomerular filtration rate (GFR) < 30 mL/min per 1.73 m² for MRI and CT contrast];
Known hypersensitivity to the contrast material or vasodilator agent being considered;
History of asthma or emphysema (for adenosine, dipyridamole); supplies for respiratory emergencies should be available if using regadenoson;
Caffeine intake within the last 12 hours.
Warnings or other relative contraindications:
Hypertrophic cardiomyopathy with severe left ventricular outflow tract obstruction;
Significant arrhythmias; heart rate > 100 beats per minute that cannot be controlled with β-blockers;
Moderate renal insufficiency (GFR 30–60 mL/min/body surface area);
Morbid obesity (body mass index > 40);
Recent seizure or stroke;
Electrolyte level abnormalities.
CCTA for IHD: procedure, advantages, and risks
CCTA [25–27] is crucial for diagnosing IHD, providing anatomical insights and functional assessment through techniques like FFR-CT. The procedure, while highly beneficial, involves specific steps and considerations.
Procedure
Beta-blockers or ivabradine are used to lower the heart rate to below 60 beats per minute to reduce motion artifacts during the scan.
During the imaging session, a CT scanner, ideally with at least 64 slices-captures detailed images of the coronary arteries. A non-contrast scan first measures the Agatston score to evaluate coronary calcification. Following this, a contrast agent is injected into the bloodstream to enhance visualization of the arteries. The scan is synchronized with the ECG to capture images during minimal heart motion.
The resulting images are reconstructed into a three-dimensional model of the coronary arteries. Cardiologists analyze the images to assess vessel narrowing and identify any significant stenosis.
Advantages
One of the primary advantages of CCTA is its ability to accurately exclude the presence of CAD in symptomatic patients. A normal CCTA result is associated with a good prognosis and may eliminate further invasive testing. This aspect contributes significantly to streamlined patient management and reduces the frequency of unnecessary procedures.
CCTA is also valuable in identifying non-obstructive CAD, which allows for early intervention and preventive therapy that might be overlooked by other non-invasive tests. Early identification improves cardiovascular risk management and enables timely treatments.
While CCTA may increase the total number of catheterizations, it reduces unnecessary invasive procedures by accurately identifying patients with non-obstructive CAD. This precise identification ensures that resources are focused on patients who genuinely need invasive testing.
Moreover, CCTA has been associated with increased rates of revascularization and a reduction in MIs. By identifying significant lesions that require intervention, it facilitates more effective treatment and improved patient outcomes.
The integration of CCTA with FFR-CT further enhances diagnostic accuracy by evaluating the functional significance of coronary lesions. FFR-CT provides a high level of accuracy in assessing the physiological impact of stenosis, which guides treatment decisions more effectively.
Risks
Despite its advantages, CCTA presents certain risks. One notable risk is radiation exposure, which, while minimized by advancements in technology and imaging protocols, remains a concern. The potential long-term effects of ionizing radiation must be considered, and efforts must be made to apply the lowest effective dose.
Contrast agents can cause allergic reactions or adverse effects. While such reactions are rare, they require careful monitoring and management during the procedure.
Additionally, CCTA primarily provides anatomical information and does not directly assess the functional significance of coronary lesions. This limitation can lead to discrepancies between the anatomical findings and their functional implications. To address this, FFR-CT can be used in conjunction to provide a more comprehensive evaluation of stenosis.
Image quality can be affected by factors such as severe coronary calcification, high heart rates, or patient motion, which can impact diagnostic accuracy. In cases where image quality is compromised, additional testing or the use of FFR-CT may be necessary to achieve a complete assessment.
Lastly, CCTA can sometimes lead to overdiagnosis or false positives, where non-significant lesions are incorrectly identified as significant. This can result in unnecessary follow-up tests or interventions, emphasizing the need for careful interpretation and management.
FFR-CT: FFR-CT provides a non-invasive approach for assessing the functional significance of coronary stenosis, integrating both anatomical and physiological data from coronary CT angiography. This helps clinicians determine whether a stenosis is hemodynamically significant, which is a key factor in deciding the need for interventions such as coronary revascularization.
One advantage of FFR-CT over traditional CT alone is its ability to offer more detailed insight into blood flow and pressure differences across a coronary lesion, enabling more personalized decision-making. However, its limitations include the need for high-quality imaging data and computational resources, as well as a time delay for analysis, since the data often have to be sent to specialized centers for interpretation.
FFR-CT has high sensitivity for significant stenosis, but its lower specificity can cause false positives, leading to unnecessary invasive procedures. As a result, FFR-CT is increasingly seen as a complementary tool that helps reduce the number of unnecessary catheterizations but should be integrated with clinical judgment and possibly other non-invasive tests like SE or MPI to improve overall diagnostic accuracy. It offers high negative predictive value and sensitivity but has moderate positive predictive value (60–70%). A value ≤ 0.80 indicates significant stenosis and typically leads to coronary angiography, while values > 0.80 suggest follow-up or stress testing. FFR-CT combines anatomical and physiological data, improving diagnostic accuracy but requiring high-quality imaging and advanced analysis.
Ongoing advances, including AI and machine learning, are expected to further refine FFR-CT analysis, making it faster and more accurate, and potentially improving its positive predictive value in the future [28].
A comparative analysis of diagnostic techniques
Cardiac evaluation requires precise techniques to assess function and identify conditions. SE, myocardial scintigraphy, and CCTA offer unique approaches to cardiac function and perfusion, each with specific indications, benefits, and limitations. Table 3 compares the methods, advantages, and risks of these techniques to support informed clinical decisions.
Comparison between SE, myocardial scintigraphy, and CCTA
| Aspect | SE | Myocardial scintigraphy | CCTA |
|---|---|---|---|
| Method | Evaluates cardiac function under stress using exercise or pharmacologic agents | Assesses blood flow to heart muscle using radiopharmaceuticals, combined with stress | Provides detailed anatomical images of the coronary arteries using CT technology |
| Population | Patients with moderate likelihood of CAD; able to exercise; evaluation of valvular disease or left ventricular dysfunction | Patients with suspected CAD, particularly those who cannot exercise or where functional information about myocardial perfusion is required | Patients with low/intermediate likelihood of CAD; unclear outcomes from other stress tests; need to assess coronary anatomy |
| Types/Procedure | Treadmill, supine ergometer, handgrip, six-minute walk test, dipyridamole, and dobutamine | Imaging includes rest and stress phases using PET or SPECT with radiopharmaceuticals. | Imaging includes pre-scan medications, contrast injection, and ECG-triggered scans. |
| Applications | Diagnosis of IHD, valvular disease, heart failure, cardiomyopathies | Diagnosis of cardiac ischemia, assessment of CFR, identification of hibernating myocardium | Exclusion of CAD in symptomatic patients, early identification of nonobstructive CAD |
| Imaging techniques | Real-time echocardiographic images during or immediately after stress | PET and SPECT imaging to visualize myocardial perfusion and viability | High-resolution CT images with optional FFR-CT for functional assessment |
| Safety | Generally safe with low risk; requires monitoring and emergency equipment | Low risk, with concerns primarily related to radiation and allergic reactions; contraindications for specific patient groups | Risks include radiation and contrast reactions; low-dose protocols are used. |
| Contraindications | Acute coronary syndrome, severe heart failure, severe hypertension, etc. | Unstable angina, severe heart failure, severe pulmonary hypertension | Severe renal insufficiency, uncontrolled hypertension, and contrast allergies |
| Advantages |
|
|
|
| Limitations |
|
|
|
| Indications | Ideal for patients who can exercise; used for functional cardiac assessments. | Ideal for evaluating myocardial perfusion, particularly when anatomical insights are needed. | Ideal for coronary anatomy evaluation and ruling out CAD |
| Sensitivity | 70–85% | PET: 90–95%SPECT: 85% | 95–99% |
| Specificity | 77–90% | PET: 80–90%SPECT: 70% | 64–85% |
| Wait times | Generally short, as SE is widely available. | Moderate to long due to limited availability, particularly for PET, which requires specialized facilities | Moderate, depending on scanner availability and patient preparation (e.g., heart rate control) |
| Technical details for the technician | Optimal transducer placement and continuous monitoring during stress rely on operator experience. | Precise radiotracer injection timing; correct patient positioning to avoid artifacts; longer post-processing time for PET | Requires heart rate control (beta-blockers); timely administration of contrast; avoiding artifacts |
| Details to consider for the patient | Ability to exercise or tolerate pharmacological stress | Radiation exposure, especially with SPECT; possible claustrophobia during scan; need for radiotracer injection | Kidney function (for contrast use); potential allergies to contrast; preparation to slow heart rate |
| Unique benefits | Real-time, non-invasive, and assesses the heart’s response to physiological stress | Excellent for detecting ischemia and assessing myocardial viability | High accuracy in visualizing coronary arteries and identifying significant lesions |
SE: stress echocardiography; CCTA: coronary computed tomography angiography; CT: computed tomography; CAD: coronary artery disease; PET: positron emission tomography; SPECT: single-photon emission CT; ECG: electrocardiogram; IHD: ischemic heart disease; CFR: coronary flow reserve; FFR-CT: fractional flow reserve CT
Each diagnostic technique has a significant role in cardiovascular evaluation, with SE being advantageous for real-time functional assessment without radiation, CCTA excelling in detailed coronary anatomy for CAD exclusion, and myocardial scintigraphy offering high sensitivity for perfusion assessment. Choosing the appropriate modality depends on patient characteristics, clinical scenarios, and the specific information required to guide management decisions.
Viability and ischemia detection
The detection of viable myocardium through imaging techniques is crucial in guiding revascularization decisions for patients with prior MI and significant left ventricular dysfunction or ICM. The presence of viable myocardium supports the need for revascularization, as it may improve survival and left ventricular function. Similarly, residual ischemia identified in imaging studies for patients with known CAD justifies the need for either PCI or CABG to restore blood flow and prevent future cardiac events.
In line with recent guidelines [29], a patient-centered approach is emphasized, taking into account anatomical findings and physiological testing results. CABG is typically the treatment of choice for patients with complex or multivessel disease, offering superior long-term outcomes. However, PCI remains a viable alternative for patients with less complex coronary disease or those who are not suitable candidates for surgery.
In summary, the selection between PCI and CABG depends on the complexity of the disease, ischemic burden, and the presence of viable myocardium as detected through imaging, ensuring the chosen revascularization strategy aligns with the patient’s risk profile and clinical condition.
What ESC 2024 guidelines say on imaging
The recent ESC 2024 guidelines on CCSs clearly describe the expanding domain of application of cardiac imaging as a necessary step for an efficient and accurate cardiac diagnosis [3]. In particular, some indications have been summarized in Table 4. Indications must be linked to the calculation of a risk-factor-weighted clinical likelihood score, that integrates the information on symptoms, age, and sex with the number of risk factors, so as to offer an integrated assessment of the likelihood of anatomic CAD. This makes life certainly easier for the practicing cardiologist. A summary of the main indications is provided in Table 4.
Indications for cardiac imaging in the ESC 2024 guidelines
| Pre-test likelihood | Imaging test | CoR | LoE |
|---|---|---|---|
| Any | Resting TTE | 1 | B |
| Very low (< 5%) | Only exercise ECG | 1 | C |
| Low (5–50%) | CCTA | 1 | A |
| Moderate-high (> 15–80%) | SE | 1 | B |
| Moderate-high (> 15–80%) | SE + CFVR | 2 | B |
| Moderate-high (> 15–80%) | Stress PET, SPECT; CMR | 1 | B |
| High (> 80%) | ICA | 1 | C |
ESC: European Society of Cardiology; CoR: class of recommendation; LoE: level of evidence; TTE: transthoracic echocardiography; ECG: electrocardiogram; CCTA: coronary computed tomography angiography; SE: stress echocardiography; CFVR: coronary flow velocity reserve; PET: positron emission tomography; SPECT: single photon emission computed tomography; CMR: cardiac magnetic resonance; ICA: invasive coronary angiography
What ESC guidelines do not say about imaging
Even more interesting is what guidelines do not say about cardiac imaging applications. Since the previous release of ESC 2019 guidelines, there has been an explosion of interest in the post-pandemic era for the issue of cost-containment in health care, at a time when the problem of overuse, overtesting, overdiagnosis and overtreatment is draining essential resources putting the universal access to health care at risk even in most affluent societies [30]. The radiation risk inherent in the use of ionizing radiation in medicine is now recognized as a top reason for the induction of cancer, especially considering that the exposure to medical radiation now equals exposure to natural radiation [31]. Third, the climate emergency is now a top priority in the political and healthcare agenda, and cardiac imaging explains at least 1% of the total carbon dioxide emissions [32]. Yet, guidelines ignore the issue of economic, radiological, and environmental sustainability of cardiac imaging. Different techniques with diagnostic equipoise have substantially different economic, radiological, and environmental costs (Table 5). These fundamental aspects of the practice of cardiology will become increasingly important in the era of economic crisis, cancer prevention, and climate emergency [12].
The relative economic, radiological, and environmental costs of cardiac imaging [30]
| Cost | SE | Stress CMR | Stress PET | Stress SPECT | CCTA |
|---|---|---|---|---|---|
| Economic cost | 1 | 3× | 10× | 4× | 2× |
| Radiologic cost (mSv) | 0 | 0 | 3 | 10 | 4 |
| Environmental cost from a single test (kg CO2) | 2 | 200–300 | Not mentioned | 20–30 | 20–30 |
SE: stress echocardiography; CMR: cardiac magnetic resonance; PET: positron emission tomography; SPECT: single photon emission computed tomography; CCTA: coronary computed tomography angiography; mSv: millisievert (1 millisievert = 50 chest X-rays); CO2: carbon dioxide
Conclusions
Cardiovascular diseases, particularly IHD, remain the leading cause of death globally, accounting for 16% of all deaths worldwide. The latest guidelines from the ESC provide a structured approach to managing IHD, with an emphasis on improving diagnostic accuracy and treatment precision. The guidelines recommend a personalized approach to care, where decisions about revascularization are informed by anatomical findings, functional tests, and the patient’s pre-test likelihood of CAD. This approach aims to identify which patients will benefit most from procedures like PCI or CABG.
Non-invasive imaging techniques, such as SE, CCTA, and MRI, play a crucial role in assessing myocardial viability and ischemia. These methods help accurately select patients for invasive interventions, reducing unnecessary procedures. However, while imaging is essential, the guidelines also highlight the growing concern over the economic, radiological, and environmental impacts associated with diagnostic imaging. Future guidelines will need to balance clinical effectiveness with sustainability considerations, addressing healthcare resource utilization, radiation exposure, and carbon emissions.
Technological advancements, such as strain imaging and AI-assisted echocardiography, are also enhancing diagnostic precision and reducing operator dependency, making these techniques more reliable and accessible. Overall, the management of IHD requires an evidence-based, balanced approach that leverages advanced imaging technologies while considering individual patient risk profiles, healthcare resource constraints, and long-term sustainability challenges.
Abbreviations
| 99mTc-MIB: | technetium-99m methoxyisobutylisonitrile |
| ACS: | acute coronary syndrome |
| AI: | artificial intelligence |
| CABG: | coronary artery bypass grafting |
| CAD: | coronary artery disease |
| CCS: | chronic coronary syndrome |
| CCTA: | coronary computed tomography angiography |
| CFR: | coronary flow reserve |
| CT: | computed tomography |
| DSE: | dobutamine stress echocardiography |
| ECG: | electrocardiogram |
| ESC: | European Society of Cardiology |
| FFR: | fractional flow reserve |
| FFR-CT: | fractional flow reserve computed tomography |
| GCS: | global circumferential strain |
| GFR: | glomerular filtration rate |
| GLS: | global longitudinal strain |
| ICA: | invasive coronary angiography |
| ICM: | ischemic cardiomyopathy |
| IHD: | ischemic heart disease |
| LV: | left ventricle |
| MIs: | myocardial infarctions |
| MPI: | myocardial perfusion imaging |
| MRI: | magnetic resonance imaging |
| PCI: | percutaneous coronary intervention |
| PET: | positron emission tomography |
| SE: | stress echocardiography |
| SPECT: | single-photon emission computed tomography |
Declarations
Author contributions
MFC: Conceptualization, Writing—original draft, Validation, Supervision. FC, GD, AN, and SM: Formal analysis, Investigation. LS: Formal analysis, Investigation, Methodology, Visualization, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The figures are part of the StressEcho 2030 study (from the general register of CEUR 34/2024—N. protocol of AOR San Carlo of Potenza referred to the application for study approval 20240025965—protocol date 25.06.2024).
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
Relevant data can be provided on reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.