Abstract
The oxidation of lipoproteins has a key role in the development of atherosclerosis, a condition where plaque builds up in artery walls. Research shows that when low-density lipoprotein (LDL) oxidizes, it speeds up atherosclerosis. Oxidized LDL (Ox-LDL) causes many pathologic scenarios that lead to atherosclerosis. It was suggested as a fundamental player in endothelial dysfunction, creating foam cells, and triggering inflammation in artery walls. How Ox-LDL contributes and interacts with specific receptors on endothelial cells is crucial to these effects. This article aims to shed light on LDL oxidation, the stages of the process, and how Ox-LDL promotes atherosclerosis. A comprehensive search was conducted across various databases, including PubMed, Google Scholar, Scopus, and Ovid, to identify literature and studies that discuss Ox-LDL and their involvement in the pathogenesis of atherosclerosis and cardiovascular diseases, thereby establishing a well-defined perspective on the subject. This review will provide a closer look at the Ox-LDL particle, the different forms and stages of oxidation, and the role of various LDL receptors involved in LDL uptake and breakdown focusing on how they contribute to atherosclerosis. Then, it will discuss the role of scavenger receptors and their contribution to the uptake of Ox-LDL and how this contributes to the development of atherosclerosis.
Keywords
Cardiovascular diseases, scavenger receptors, foam cell, oxidative stressIntroduction
Cardiovascular diseases continue to be a predominant cause of mortality worldwide, and their prevalence is anticipated to increase in the forthcoming years [1]. In the fight against cardiovascular disease to reduce its effects on health outcomes, a crucial approach centers on implementing primary and secondary prevention strategies, along with effectively managing modifiable risk factors. One promising avenue deserving exploration is the role of oxidized low-density lipoproteins (Ox-LDLs), the primary component of plaque formation in atherosclerosis [2].
Atherosclerosis is a persistent inflammatory condition that significantly influences the emergence of cardiovascular diseases [3]. The idea that inflammation plays a crucial role in the development of atherosclerosis and its associated complications has attracted significant interest; however, it has not yet been integrated into clinical practice [4]. The hallmark of atherosclerosis is the buildup of Ox-LDL within the walls of arteries, which leads to plaque development [5]. Ox-LDL’s presence sets off an immune reaction that causes oxidative stress and inflammation in the arteries. Such stress harms the endothelial cells that line the blood vessels and leads to the attraction of white blood cells, which starts the creation of foam cells. These cells add to the growth and buildup of plaque. Consequently, the involved arteries lose flexibility and become narrow, which hinders the flow of blood to essential organs such as the heart and brain [6]. Without treatment, atherosclerosis may result in serious pathologic scenarios, including heart attacks and strokes. Hence, it’s vital to explore the role of Ox-LDL and comprehend the processes of Ox-LDL accumulation and oxidative stress to devise effective prevention or treatment methods for cardiovascular diseases. The discovery and categorization of different classes of scavenger receptors have shed light on their essential role in facilitating the uptake of Ox-LDL and the advancement of atherosclerosis [5, 7]. These receptors assume a critical function in the uptake of Ox-LDL by macrophages. This intricate process lies at the core of comprehending the genesis and progression of atherosclerosis.
LDLs: structure, biochemistry, and measurement
LDL serves as the primary transporter of cholesterol to cells. An increase in dietary fat contributes to its elevated levels, which can lead to pathological complications once a certain threshold is surpassed. LDL is part of a diverse group of particles, with a mass of approximately 3,000 kDa and a diameter of 220 nm. Its hydrophobic core consists of roughly 170 triglycerides and 1,500 cholesterol esters, while its hydrophilic surface is formed by around 700 phospholipid molecules, approximately 500 unesterified cholesterol molecules, and a single large molecule of ApoB weighing 500 kDa [8]. Numerous expert panels have concluded that the assessment of ApoB is sufficiently standardized for clinical practice. Furthermore, ApoB can be measured cost-effectively through commonly accessible automated techniques, offering greater accuracy, precision, and selectivity compared to LDL cholesterol or non-high-density lipoprotein cholesterol [9].
Oxidized low-density lipoproteins
Oxidation of LDLs, commonly known as the “bad cholesterol”, is intricately linked to the development of atherosclerosis. To comprehend the underlying mechanisms of this process, it is imperative to delve into the processes of modification and internalization of these particles. The LDL receptor, commonly referred to as LDLR, serves as a receptor located on the cell surface that is crucial for the identification and uptake of native LDL (nLDL) particles (Figure 1). This receptor is primarily found in hepatocytes and is essential for the removal of circulating LDL from the bloodstream. The mechanism by which LDLR facilitates the endocytosis of nLDL involves the interaction of ApoB (ApoB100), which is the protein part of LDL, with the receptor. Following this interaction, nLDL is taken up via clathrin-coated pits and subsequently transported to endosomes [10]. In the endosomes, where the medium is acidic, the LDLR experiences conformational changes that facilitate the release of LDL from the complex of ligands and receptors. Subsequently, the liberated LDL is transported to lysosomes for degradation, resulting in the release of its content including the free cholesterol.
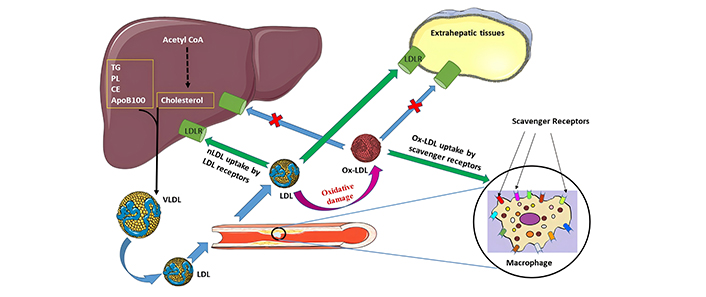
Ox-LDL in atherosclerosis [5]. CE: cholesteryl esters; LDL: low-density lipoproteins; LDLR: LDL receptor; nLDL: native LDL; Ox-LDL: oxidized LDL; PL: phospholipids; TG: triglycerides; VLDL: very LDL
Note. Reprinted from “Scavenger receptors: different classes and their role in the uptake of oxidized low-density lipoproteins” by Babakr AT. Biomed Pharmacol J. 2024;17:699–712 (https://dx.doi.org/10.13005/bpj/2897). CC BY.
The regulation of LDLR expression is subject to feedback mechanisms, at equilibrium, the quantity of LDLRs is modulated to facilitate the uptake of adequate cholesterol necessary for cellular proliferation while also compensating for losses. This regulatory feedback is mediated by membrane-associated transcription factors referred to as sterol regulatory element binding proteins (SREBPs) [11]. In conditions of low cellular cholesterol levels, SREBP-2 becomes activated and moves to the nucleus, where it binds to the promoter region of the LDLR gene, initiating its transcription. This process results in an upsurge in LDLR expression, leading to increased uptake of circulating LDL. Conversely, high cellular cholesterol levels inhibit SREBP-2, causing a reduction in LDLR expression. This feedback mechanism plays a crucial role in maintaining cholesterol balance by decreasing LDL uptake when cellular cholesterol levels are adequate.
Factors such as inflammation, hyperglycemia, and a deficiency in dietary antioxidants can induce oxidative stress, which can subsequently lead to the oxidation of LDLs [12]. These altered lipoproteins are recognized by scavenger receptors, which are encoded by a specific group of genes. Clearance of Ox-LDL primarily occurs in macrophages, dendritic cells, and Kupffer cells in the liver. Nevertheless, scavenger receptors are also present in other cell types, including endothelial cells [13, 14]. If the efficient degradation of Ox-LDL is impeded and scavenger receptor expression is unregulated, it can result in cellular dysfunction, apoptosis, and the formation of foam cells [15]. The wide array of diseases linked to lipoprotein oxidation may have a common root cause. This underscores the significance of understanding the intricate interplay between LDLR regulation, oxidative stress, and scavenger receptor function in various pathologies [16].
Oxidation of LDL
It becomes evident that exploring how oxidative stress transforms nLDL particles into proatherogenic molecules is paramount in understanding atherosclerosis pathogenesis.
The oxidation of LDL is a critical step in the development and progression of atherosclerosis and many pathological conditions. A key statistic that highlights this importance is that Ox-LDL can induce endothelial dysfunction [17, 18], which is an early event in atherosclerosis. Endothelial dysfunction refers to impaired function of the cells lining blood vessels, leading to reduced vasodilation and increased inflammation. The oxidative modification leads to structural changes in LDL, making it more prone to uptake by macrophages [19, 20]. Once engulfed by macrophages, these Ox-LDL particles trigger an inflammatory response and contribute to foam cell formation, a hallmark feature of early atherosclerotic lesions. There are two forms of Ox-LDL: minimally modified and extensive Ox-LDLs. Both have been shown to contribute to the formation of fatty plaques in arteries. The physiological oxidizing agents responsible for this process remain an active area of research [21].
An increase in reactive oxygen species (ROS) production leads to the oxidation of various components of LDLs, such as phospholipids, cholesterol esters, and polyunsaturated fatty acids, culminating in the formation of Ox-LDLs. The oxidation of LDL is a complex, multi-step process. The initial phase, known as initiation, involves the attack of free radicals on the polyunsaturated fatty acids present in LDL particles, resulting in the generation of lipid peroxyl radicals, followed by the propagation phase, during which these radicals engage in further reactions with additional fatty acids, thereby instigating a chain reaction that leads to widespread lipid peroxidation. The process concludes with the termination phase, wherein antioxidants or other molecules neutralize the lipid radicals, effectively ceasing the oxidation process [8, 22]. In scenarios characterized by inadequate antioxidant defenses, Ox-LDL is recognized by macrophages, which subsequently leads to the formation of foam cells. Numerous investigations have employed in vitro-generated Ox-LDLs as a model for studying circulating Ox-LDLs in the context of atherosclerosis, and several chemically modified LDLs have been proposed to mimic Ox-LDLs [6]. While minimally modified LDL (mmLDL) do not exhibit strong binding affinity to macrophage scavenger receptors, they can enhance the expression of receptors such as CD36, SR-A, and macrosialin, mmLDLs arise from the oxidative modification of the lipid components of LDL, primarily phospholipids, without altering the apolipoprotein ApoB100. The oxidation of ApoB100 occurs at a later stage, resulting in the formation of highly Ox-LDLs [8, 23, 24].
Minimally modified LDL
mmLDL refers to the early stages of oxidation, where only small changes have occurred in the structure of LDL particles. These modifications can result from various physiological processes such as enzymatic or non-enzymatic reactions. Despite their relatively mild nature, these modifications are still recognized by specific receptors on macrophages, leading to the uptake of cholesterol and subsequent foam cell formation within arterial walls.
mmLDL plays a bio-vital role in atherosclerosis through various mechanisms and contributes to the initiation and propagation of this pathological process. One mechanism by which mmLDL promotes atherosclerosis is through its interaction with Ox-LDLRs on macrophages [25], leading to foam cell formation. In addition, previous works suggest that mmLDL can also bind to these receptors, albeit with lower affinity compared to fully Ox-LDL [26]. This binding triggers intracellular signaling pathways that promote inflammation and oxidative stress, further exacerbating endothelial dysfunction and promoting plaque formation. Moreover, mmLDL can undergo additional modifications within the arterial wall itself. Enzymes such as myeloperoxidase and lipoxygenases are present in abundance at sites of inflammation, where they catalyze the oxidation of mmLDL into extensive Ox-LDL, as described in Figure 2. The resulting Ox-LDL has been shown to be more pro-inflammatory and cytotoxic than mmLDL. Thus, while mmLDL serves as an initial trigger for atherosclerosis development, it can also serve as a precursor for the production of highly detrimental Ox-LDL species [27, 28].
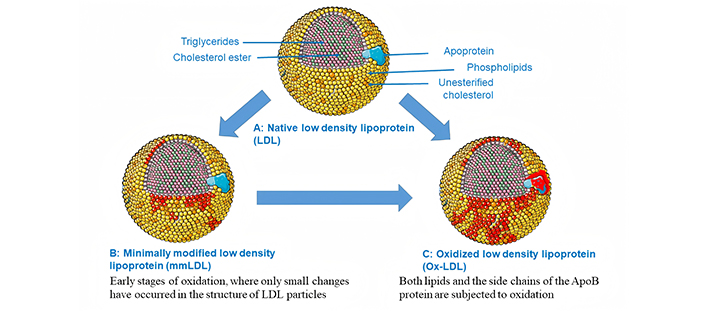
Native LDL, mmLDL, and Ox-LDL. Extensive Ox-LDL occurs when both lipids and the side chains of the ApoB protein are subjected to oxidation. The illustration was partially created utilizing Servier Medical Art, which is supplied by Servier and is licensed under a Creative Commons Attribution 4.0 unported license (https://creativecommons.org/licenses/by/4.0/)
In summary, mmLDL represents an important player in the pathogenesis of atherosclerosis. Its ability to interact with Ox-LDLRs on macrophages and induce inflammatory responses highlights its contribution to early lesion formation. Additionally, its susceptibility to further modification within the arterial wall underscores its strong association with the formation of extensive Ox-LDL species.
Extensively Ox-LDL
Extensively Ox-LDL is considered as the key player in the whole process of atherosclerosis [29]. Various mechanisms have been discovered that can result in the oxidation of LDL in the body. When LDLs undergo oxidation, both lipids and the side chains of the ApoB protein are subjected to oxidation through radical chain reactions [6]. Lipid oxidation products, which consist of reactive aldehydes, form covalent bonds with ApoB proteins to create adducts. The progression of atherosclerotic vascular disease and other pathologic scenarios such as type-2 diabetes [30, 31] may potentially be linked to Ox-LDL, as indicated by serum autoantibody levels, based on clinical and epidemiological research. Extensively Ox-LDL represents a more advanced stage of modification, characterized by multiple oxidative events that significantly alter its composition and function. This heavily oxidized form of LDL not only loses its ability to bind efficiently to normal LDLRs but also gains new properties that promote inflammation and endothelial dysfunction. As a result, extensive Ox-LDL becomes highly proatherogenic and immunogenic, contributing to plaque formation and progression [32]. LDLR is the primary receptor responsible for the uptake of nLDL by cells. However, when gets oxidized, nLDL will no longer be internalized by normal LDLR, in fact, they will be targets of the many scavenger receptors of macrophages. For example, unlike LDLR, the lectin-like Ox-LDLR-1 (LOX-1) is a major receptor in endothelial cells that recognizes and binds to Ox-LDL specifically [33]. The binding of Ox-LDL to LOX-1 triggers a cascade of cellular events, including the internalization of Ox-LDL, the generation of ROS, and the activation of inflammatory pathways.
In summary, the shift from minimally modified to extensively Ox-LDL marks a critical step in the scenario of atherosclerosis. The altered composition and function of extensive Ox-LDL make it a potent agent in promoting inflammation, endothelial dysfunction, and plaque formation. Understanding the precise physiological oxidizing agents that drive this process is essential for developing targeted therapeutic strategies to mitigate the impact of extensive Ox-LDL on cardiovascular health.
The physiological oxidizing agents in vivo
The specific physiological oxidizing agents responsible for LDL oxidation in vivo remain an active area of research. Understanding these agents and their mechanisms is critical for better understanding the pathogenesis of atherosclerosis and potentially may suggest novel therapeutic interventions [19]. ROS can be produced by various enzymatic systems, including NADPH oxidases, xanthine oxidases, and lipoxygenases [34]. Additionally, myeloperoxidase, an enzyme released by activated immune cells catalyzes the production of hypochlorous acid, which further contributes to LDL oxidation [35]. Moreover, transition metal ions like copper and iron play crucial roles in mediating LDL oxidation. These metals can generate reactive hydroxyl radicals when they interact with H2O2 or other ROS present within arterial walls [36]. Furthermore, pro-inflammatory cytokines released during inflammation can enhance LDL oxidation indirectly via ROS generation or facilitating metal ion release from storage proteins [37]. Overall, it is evident that multiple physiological factors contribute to LDL oxidation in the body and Ox-LDLRs significantly impact the progression of atherosclerosis. The identification and understanding of physiological oxidizing agents involved in this process are critical for developing effective preventive strategies against cardiovascular diseases. Further investigations focusing on elucidating the complex interplay between these agents and their underlying mechanisms will contribute not only to our knowledge of atherosclerosis but also offer potential avenues for therapeutic intervention aimed at reducing the burden of this prevalent disease.
Ox-LDL: proatherogenic effects
Ox-LDL results from the oxidative modification of LDL due to ROS, nitrogen species, or other reactive molecules [38]. This process occurs predominantly under conditions of oxidative stress. The impact of Ox-LDL on different pathological conditions including coronary heart disease (CHD), diabetes mellitus, metabolic syndrome, hypertension, and kidney diseases was investigated in previous works [12, 38–40]. Poznyak et al. [41] underscores the evolving understanding of LDL modifications, particularly desialylation, as potential therapeutic targets, and the challenges faced in antioxidant therapies aimed at reducing atherosclerosis. The initiation stage of endothelial dysfunction is a vital and pinpoint step in the progress of various cardiovascular diseases. The inner lining of blood vessels is composed of endothelial cells, which are instrumental in maintaining vascular health. These cells regulate vascular tone and blood flow, and prevent clot formation, thereby ensuring the smooth functioning of the circulatory system. When this delicate balance is disrupted, as seen in the initiation of endothelial dysfunction, it sets the stage for the following pathologic scenarios including the development of atherosclerosis [39, 42, 43]. Whereas, in the progression stage, as endothelial dysfunction progresses, the confinement of LDL within the sub-endothelial space incites an immune response. This immunological reaction holds pivotal importance in the evolution of cardiovascular conditions. The immune cells detect Ox-LDL particles due to specific epitopes present on the LDL molecules [44]. The formed plaques may rupture, ultimately resulting in the development of blood clots that may obstruct the normal flow of blood, thereby leading to the occurrence of heart attacks or strokes [42]. LDL cholesterol is prone to oxidation, and Ox-LDL and its uptake by scavenger receptors play a central role in the formation of atherosclerotic plaques as illustrated in Figure 1.
Despite the association of Ox-LDL with adverse health outcomes and CVD, still, the use of this biomarker in clinical settings is hindered by many challenges, including poor knowledge of the pathogenesis, testing issues, and lack of standardization.
Ox-LDL roles in atherogenesis
Several mechanisms have been proposed for how Ox-LDL contributes to atherogenesis [40, 45]. These roles and mechanisms highlight the multifaceted impact of Ox-LDL in the pathogenesis of atherosclerosis, making it a critical target for therapeutic interventions for the prevention and treatment of cardiovascular diseases. Table 1 shows the different roles of Ox-LDL in atherosclerosis.
Roles of Ox-LDL in atherosclerosis
| Role | Description |
|---|---|
| Induction of endothelial cell dysfunction | Ox-LDL binds to its receptor, LOX-1, on endothelial cells, leading to a decrease in nitric oxide production, which is essential for maintaining vascular tone and preventing inflammation. This binding also increases the expression of adhesion molecules, promoting the adhesion of leukocytes to the endothelium, a key step in the initiation of atherosclerosis [17, 24]. |
| Trigger oxidative stress in vascular cells | Ox-LDL can also trigger oxidative stress in vascular cells, leading to further damage and contributing to the progression of atherosclerotic lesions [21]. |
| Macrophage activation and foam cell formation | Ox-LDL is taken up by macrophages through LOX-1, leading to the accumulation of lipids within the cells, forming foam cells. These foam cells contribute to the formation of atherosclerotic plaques [46]. |
| Stimulation of smooth muscle cell proliferation and migration | Ox-LDL promotes the proliferation and migration of smooth muscle cells, which are crucial for the development of atherosclerotic plaques and the thickening of the arterial wall [47]. |
| Induction of inflammation | Ox-LDL triggers inflammation by activating macrophages and other immune cells, leading to the release of cytokines and chemokines that further promote the recruitment of inflammatory cells to the atherosclerotic lesion [48]. |
| Modulation of inflammation-related pathways | Ox-LDL modulates inflammation-related molecular pathways, including the regulation of microRNAs and activation of the NLRP3 inflammasome [49]. |
| Plaque instability and thrombosis | Ox-LDL contributes to the instability of atherosclerotic plaques by inducing the production of metalloproteinases, which can lead to the rupture of plaques and the formation of thrombi, which can cause acute cardiovascular events [50]. |
| Regulation of macrophage polarization | Ox-LDL regulates macrophage polarization, it can induce macrophage polarization through various mechanisms, including cell signaling, metabolic reprogramming, epigenetics, and intercellular communication, which promotes vessel wall inflammation and aggravates the progression of atherosclerosis [51]. |
| Arterial stiffening | Ox-LDL may also play a role in arterial stiffening, which is associated with increased cardiovascular risk [52]. |
LOX-1: oxidized low-density lipoprotein receptor-1
In conjunction with Ox-LDL, anti-Ox-LDL antibodies also play a crucial role in identifying individuals at risk for cardiovascular diseases. These antibodies serve as potential serum biomarkers, enhancing the ability to pinpoint those who may be predisposed to cardiovascular diseases. The potential of anti-Ox-LDL antibodies as serum biomarkers for cardiovascular disease and atherosclerosis is significant. Their ability to identify individuals at risk and their emerging applications in imaging and therapy highlight the importance of continued research in this area [29]. As studies progress, these biomarkers may play a pivotal role in improving cardiovascular health outcomes and advancing clinical practices.
Ox-LDL has been acknowledged for an extended period as a significant contributor to the development of atherosclerosis. Nevertheless, it is only in recent years that investigation of the complex functions of these particles and their associated receptors within these pathologic scenarios has been explored. The discovery of scavenger receptors opens up new avenues for understanding and potentially treating atherosclerosis.
Scavenger receptors: role in atherosclerosis
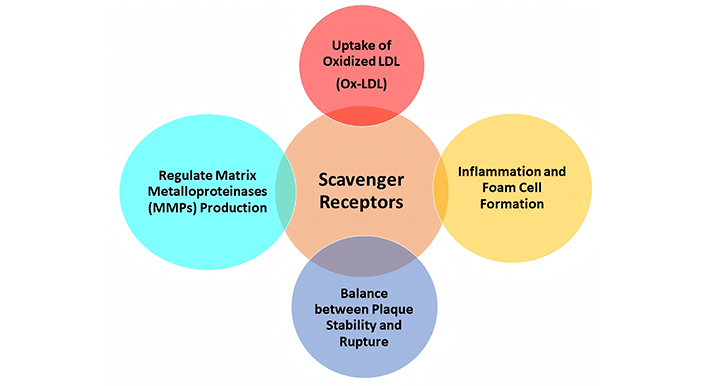
Scavenger receptors play a significant role in atherosclerosis progression. These receptors of different classes are primarily expressed in macrophages and endothelial cells [5]. Their contribution to atherosclerotic scenarios involves a variety of processes as shown in Table 2 and Figure 3.
Roles of scavenger receptors in atherosclerosis
| Role | Description |
|---|---|
| Uptake of Ox-LDL | Scavenger receptors recognize and bind to modified LDL, particularly Ox-LDL. This uptake leads to the formation of foam cells within the arterial wall, a hallmark of atherosclerotic plaques [5]. |
| Inflammation and foam cell formation | Scavenger receptor-mediated uptake of Ox-LDL triggers an inflammatory response. Foam cells accumulate in the intima (inner layer) of blood vessels, contributing to plaque formation [53]. |
| Plaque stability and rupture | Scavenger receptors are involved in the balance between plaque stability and vulnerability. While they promote lipid accumulation, excessive scavenger receptor activity can lead to plaque rupture and thrombosis [54]. |
| Matrix metalloproteinases (MMPs) | Scavenger receptors regulate MMP production. MMPs degrade the extracellular matrix, weakening the fibrous cap of a plaque. A vulnerable plaque with a thin cap is more likely to rupture [55]. |
Scavenger receptors exhibit less selectivity and can bind other modified forms of lipoproteins [46]. These receptors are typically found on macrophages within arterial walls and contribute to foam cell formation, a hallmark characteristic of early lesions of atherosclerosis. One of the key roles of scavenger receptors is their ability to bind to a wide range of ligands and facilitate their removal.
The mechanisms through which scavenger receptors accomplish the clearance of different ligands involve processes such as phagocytosis, endocytosis, adhesion, and signaling [14]. Considerable efforts were made to discover innovative therapeutic approaches that can prevent or reverse the detrimental effects of cardiovascular disease by elucidating the complex interplay between oxidative stress, receptor function, and plaque formation. However, these efforts were hindered by many challenges including the poor knowledge regarding the structure, functionality, and mechanism of action of scavenger receptors. Therefore, further research and development of targeted interventions were strongly recommended for better outcomes in these pathologic conditions.
Conclusions
In conclusion, this review has explored the critical roles of Ox-LDLs in the pathogenesis of atherosclerosis, underscoring their involvement in foam cell formation, endothelial dysfunction, and inflammatory responses in the arterial walls. By examining the interactions between nLDL, Ox-LDL, and their respective receptors, as well as the pivotal function of scavenger receptors in the uptake of Ox-LDL, and the atherogenic effects of these receptors, the paper highlights the complex mechanisms driving atherosclerosis and its progression to cardiovascular diseases. Furthermore, the review emphasizes the importance of oxidative stress in transforming LDL into proatherogenic molecules and the transition from minimally modified to extensive Ox-LDL, which is crucial for disease development.
This review stresses the need for a deeper understanding of the interplay between oxidative stress, LDLR regulation, and scavenger receptor activity to identify potential therapeutic targets. Addressing these aspects through targeted interventions could mitigate the burden of cardiovascular diseases, which remain the leading cause of mortality and financial strain on healthcare systems worldwide. Finally, the challenges in utilizing Ox-LDL as a biomarker for clinical applications highlight an area needing further research, aiming to refine prevention and treatment strategies for atherosclerosis and associated cardiovascular conditions.
Future directions
Future work in the study of Ox-LDLs and their role in atherosclerosis should prioritize:
Identifying innovative therapeutic targets such as desialylation of LDL, and overcoming the significant challenges in utilizing Ox-LDL as a clinical biomarker.
Research that elucidates the exact contributions of minimally modified and extensive Ox-LDL to atherosclerosis can open promising avenues for potential interventions.
Current limitations include an insufficient understanding of Ox-LDL pathogenesis, standardization issues in testing, and a fragmented knowledge of scavenger receptor mechanisms. As such, more in-depth in vivo studies are necessary to establish causation and translate molecular insights into clinical practice.
Abbreviations
| LDLR: | low-density lipoprotein receptor |
| mmLDL: | minimally modified low-density lipoprotein |
| nLDL: | native low-density lipoprotein |
| Ox-LDLs: | oxidized low-density lipoproteins |
| ROS: | reactive oxygen species |
| SREBPs: | sterol regulatory element binding proteins |
Declarations
Acknowledgments
The author wishes to extend his heartfelt gratitude and sincere appreciation to Waddah Abdalla for the invaluable support and assistance he provided during the process of preparing and designing the figures.
Author contributions
ATB: Conceptualization, Visualization, Writing—original draft, Writing—review & editing.
Conflicts of interest
The author declares that there are no conflicts of interest regarding the publication of this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.