Abstract
Aim:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), or COVID-19, infection resulting in acute respiratory distress syndrome (ARDS) requiring veno-venous or veno-arterial extracorporeal membrane oxygenation (VV or VA-ECMO) support is a life-threatening disease process that requires prolonged intubation and has a high risk of mortality.
Methods:
In this retrospective, observational, single-center cohort study, we attempt to better understand the role of extubation in the course of treatment by dichotomizing groups into those extubated early while remaining on ECMO treatment (group A), compared to patients who remained intubated for the entirety of their ECMO treatment (group B).
Results:
The data indicate that early extubation of patients with COVID-19-associated ARDS requiring ECMO support leads to improved survival rates for group A (93%) compared to prolonged intubation (group B) throughout the course of ECMO therapy (64%) (p = 0.13). Additionally, patients extubated earlier (19 days vs. 59 days; p = 0.012) required significantly fewer vasoactive drugs (norepinephrine dosing: 0.03 mcg/kg/min vs. 0.093 mcg/kg/min; p = 0.04), and were less likely to require a tracheostomy (0 vs. 4, p = 0.026).
Conclusions:
Although the utility of ECMO in severe ARDS patients remains a contentious topic, early extubation seems to increase survival rates and overall patient outcomes in patients with COVID-19-associated ARDS requiring ECMO support.
Keywords
COVID-19-associated ARDS, ECMO, ECLS, extubation, intubation, survival rate, mortality rate, decannulationIntroduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), or COVID-19, pandemic has resulted in many patients being hospitalized with severe hypoxemia, requiring endotracheal intubation and mechanical ventilation support [1]. COVID-19-associated acute respiratory distress syndrome (ARDS) requiring mechanical ventilation has a mortality rate ranging from 31% to 65%. The use of mechanical ventilation in patients with COVID-19 ARDS increases the risk of adverse outcomes, including readmission to the hospital and all-cause mortality compared to non-mechanically ventilated patients [2–4]. Veno-venous extracorporeal membrane oxygenation (VV-ECMO) has increasingly been used as a life-sustaining treatment option in patients with severe ARDS since the 2009 H1N1 pandemic [5, 6]. Even though the type of ECMO cannulation was determined by the ICU attending, most patients were cannulated with either bicaval-right internal jugular and superior vena cava-VV-ECMO support (Figure 1) or with a Protek Duo® right ventricular assist device (RVAD) support (Figure 2) [7]. In the bicaval cannulation setup, blood is removed via a 50 cm cannula inserted in the femoral vein from the inferior vena cava and placed back into the circulation by a right internal jugular cannula terminating at the right atrium/superior vena cava junction [4]. In the dual lumen, single cannula Protek Duo® setup blood is removed from the drainage holes that are in the right atrium and returned into the pulmonary artery [5]. We had one patient cannulated by veno-arterial (VA)-ECMO. Patients with ARDS requiring ECMO have a high mortality rate ranging between 37–40%, with five-year post-ECMO treatment mortality being as high as 64% [8–10]. In general, ARDS is a very severe condition that has a death rate of 39.4% [11]. Further research has shown that non-intubated patients experienced less severe respiratory symptoms compared to intubated patients; however, the death rate in the two patient populations was the same (36%) after 60 days [12]. Despite advancements in ECMO circuitry and a growing body of research, little is understood about the appropriate timing of extubation throughout treatment in this population. Therefore, this paper provides a relationship between ARDS requiring ECMO support and the role of extubation throughout the treatment process.
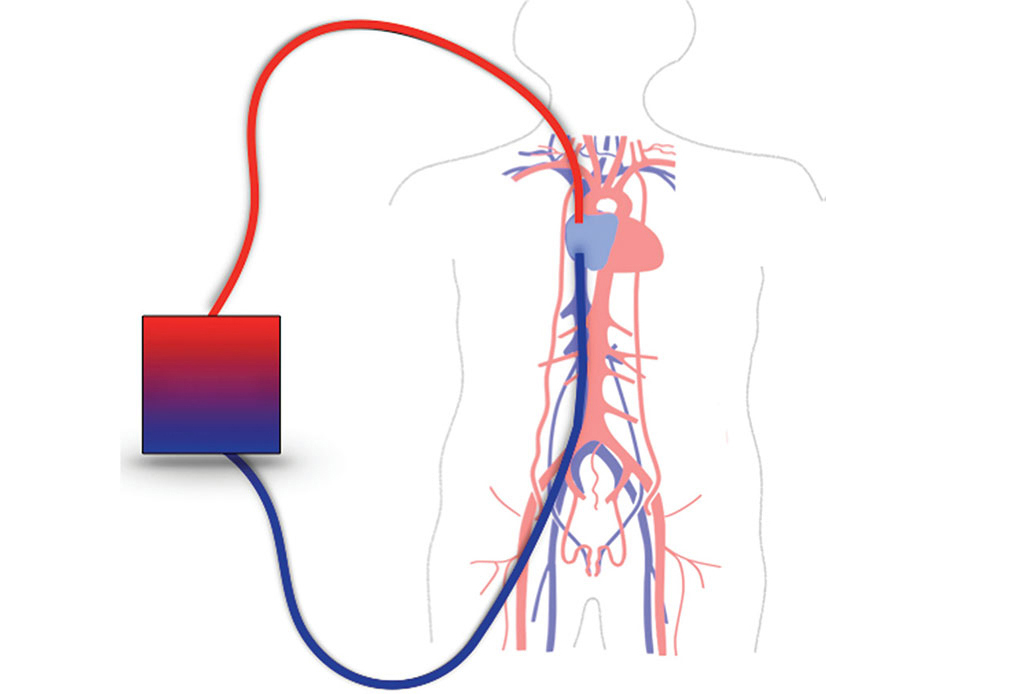
Conventional VV-ECMO configuration. A multistage cannula is placed in the right femoral vein tip at or below the IVC/RA junction to drain blood into the ECMO circuit. A membrane lung provides oxygenation and carbon dioxide removal. The blood is then pumped and returned back to the patient through a cannula in the right internal jugular vein at or above the SVC/RA junction. VV-ECMO: veno-venous extracorporeal membrane oxygenation; IVC: inferior vena cava; RA: right atrium; SVC: superior vena cava
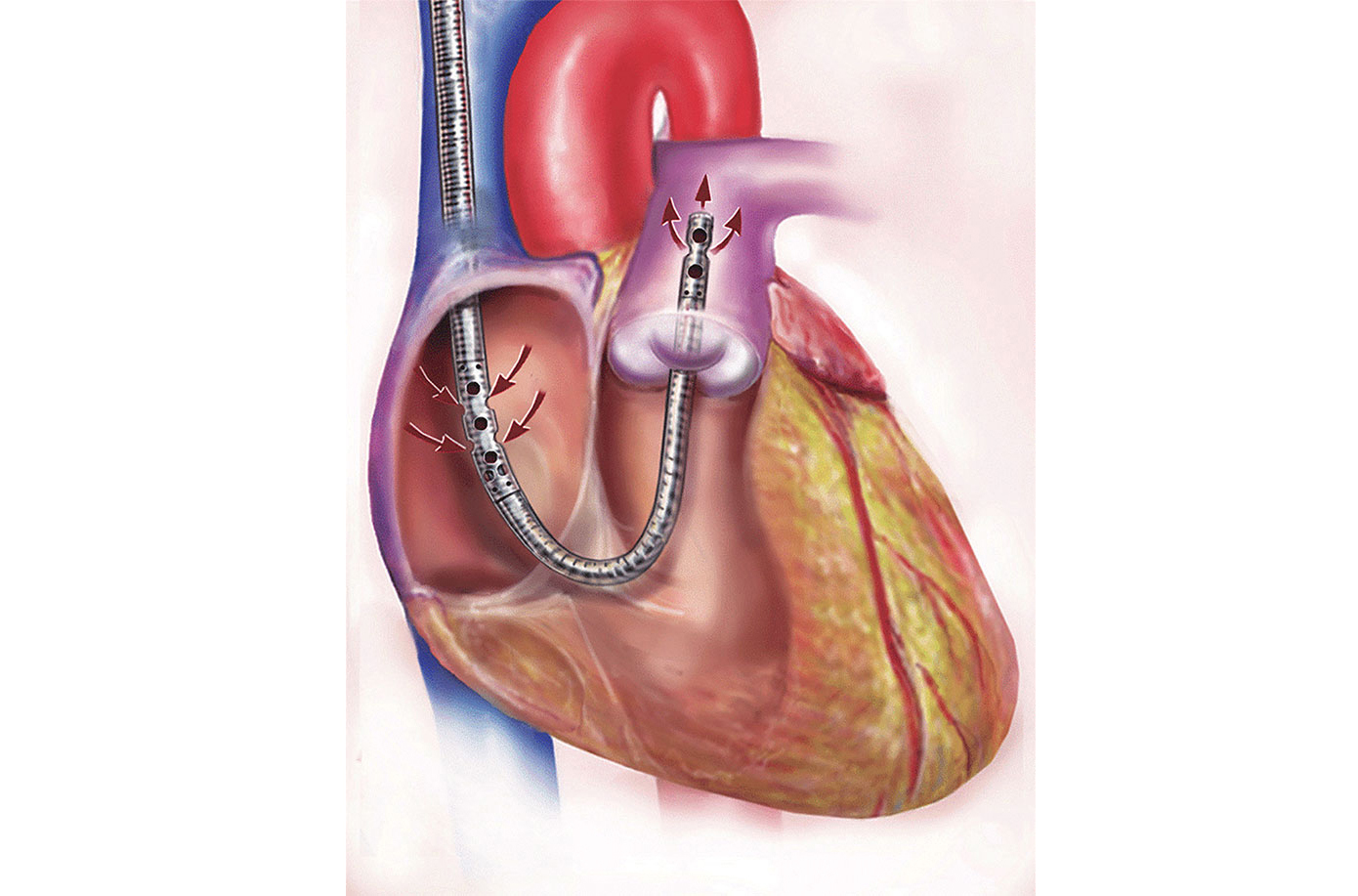
Dual-lumen Protek Duo® RVAD cannula in the correct position and inserted through the jugular vein. RVAD: right ventricular assist device. Adapted with permission from [7], © 2024 Elsevier Inc.
Materials and methods
This observational, retrospective, single-center cohort study includes 25 patients admitted to the Penn State Milton S. Hershey Medical Center Heart and Vascular Institute Critical Care Unit (HVICCU) who suffered COVID-19-associated ARDS requiring ECMO support. Patients with COVID-19-associated ARDS were placed on extracorporeal life support (ECLS) rescue therapy, using either bicaval approach with 2 cannulas or single, dual lumen cannula Protek Duo® RVAD percutaneous support, or VA-ECMO support. Approval of the study was obtained through the Penn State Health Institutional Review Board and the study ID number was STUDY00021063.
Patients were placed on ECMO according to the Penn State Hershey HVICCU Interim Adult ECLS Guidelines for COVID-19, combined with expert clinical judgment (Table 1). The guidelines are separated into three steps. The criteria for the first step include age (18–65 years old), EOLIA trial criteria (< 50 mmHg or 80 mmHg for 3–6 h respectively, or PaCO2 > 60 mmHg with pH < 7.25 for at least 6 hours), pH (< 7.2 with plateau pressure > 30 cmH2O, ventilator status (10 days or less of ventilator support), failed prone positioning strategies, and if intubated for 4 hours and still unable to maintain established criteria (SaO2 ≥ 88%, paO2 > 55 mmHg, pCO2 < 100 mmHg, pH > 7.2), (1 mmHg = 0.133322 kPa, 1 cmH2O = 0.0980665 kPa) [13]. Step two requires the determination of a respiratory ECMO survival prediction (RESP) score for acute respiratory failure, survival after VA-ECMO (SAVE) score for cardiogenic shock, and sequential organ failure assessment (SOFA) severity of illness score for hospital mortality [14, 15]. Step three requires laboratory findings, including platelet counts, C-reactive protein, and D-dimer, among other clinically relevant values.
Penn State Health COVID-19 ECMO inclusion criteria based on capacity
| Capacity | Inclusion criteria |
|---|---|
| Overall inclusion |
|
| < 50% capacity |
|
| 50–80% capacity |
|
| > 80% capacity |
|
1 mmHg = 0.133322 kPa, 1 cmH2O = 0.0980665 kPa. paO2: arterial partial pressure of oxygen; paCO2: arterial partial pressure of carbon dioxide
Our exclusion criteria included absolute markers [terminal illness, DNR/DNI status, cancer history with life expectancy < 5 years, and intracranial bleed (recent or active)] and relative markers [COVID-19-related cytokine storm with multi-organ failure and acute renal failure requiring continuous renal replacement therapy (CRRT)]. Five patients were excluded from the final analysis. Only one patient in the final analysis received the COVID-19 vaccine.
The criteria standards became more stringent based on the capacity and number of patients on ECMO support. The age criteria and days on mechanical ventilation decreased from less than 65 years old and < 10 days on the ventilator to < 50 years and < 5 days on the ventilator when the capacity went from < 50% to greater than 80%, respectively fully (Table 1). The type of cannulation, bicaval vs. Protek Duo® vs. bifemoral vs. VA-ECMO was determined by the HVICCU intensivist. The primary determinant was the stability of the patient, as a Protek Duo® had to be done in the interventional catheterization lab and required the patient to be transported from the ICU to the lab, while the bicaval cannulation and VA-ECMO support could be performed at the bedside.
After institutional review board approval, we identified 25 patients from our database who underwent ECMO support for COVID-19. These patients were stratified to either be extubated on ECMO or not. We identified 14 patients who were extubated while on ECMO (group A) and 11 patients who were left intubated on ECMO support (group B) (Figure 3). We then obtained clinical data that included patient demographics, pre-ECMO laboratory valve, pre-ECMO maximum ventilator settings, medications including dosages during ECMO; time on ventilator during ECMO, type of ECMO, and tidal volumes on day 14 and 28 on ECMO along with ECMO settings; the need for non-invasive supplemental oxygen; and days off ventilator or ECMO support, need for reintubation and death (Table 2, 3, 4, 5).
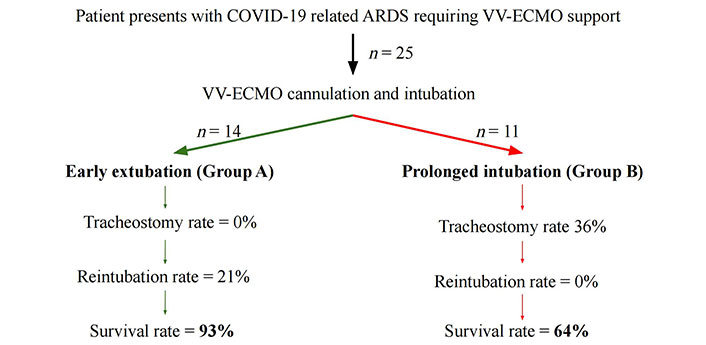
Flow chart demonstrating patient presentation and treatment course with relevant outcomes. ARDS: acute respiratory distress syndrome; VV-ECMO: veno-venous extracorporeal membrane oxygenation
Demographic and background information prior to ECMO cannulation
| Demographics | Extubated on ECMO (N = 14) | Remained intubated on ECMO (N = 11) | p-value |
|---|---|---|---|
| Age | 37 ± 9.7 | 46 ± 12 | 0.084 |
| Sex | 0.99 | ||
| Female | 3 (21%) | 2 (18%) | |
| Male | 11 (79%) | 9 (82%) | |
| Height (cm) | 174 ± 19 | 169 ± 24 | 0.62 |
| Weight (kg) | 115 ± 26 | 122 ± 26 | 0.72 |
| BMI | 39 ± 15 | 49 ± 37 | 0.29 |
| Hx prior lung disease | 0.39 | ||
| Asthma | 4 (29%) | 2 (18%) | |
| None | 10 (71%) | 7 (64%) | |
| OSA | 0 | 2 (18%) | |
| Time on Non-Invasive Ventilation (days) | 3.4 ± 5.3 | 3.2 ± 3.6 | 0.68 |
| Time of symptoms before intubation (days) | 12 ± 6.2 | 9.8 ± 6.8 | 0.47 |
| Prone | 0.66 | ||
| No | 4 (29%) | 2 (18%) | |
| Yes | 10 (71%) | 9 (82%) | |
| Days on ventilator before initiation of ECMO | 1.9 ± 2 | 4 ± 3.9 | 0.078 |
| Ventilator settings prior to ECMO | |||
| Peak pressure (cmH2O) | 30 ± 4.2 | 32 ± 5.3 | 0.31 |
| PEEP (cmH2O) | 16 ± 3 | 16 ± 2.8 | 0.65 |
| FiO2 (%) | 98 ± 4.3 | 96 ± 9.2 | 0.97 |
| Tidal volume (mL) | 467 ± 94 | 416 ± 91 | 0.27 |
1 cmH2O = 0.0980665 kPa. ECMO: extracorporeal membrane oxygenation; BMI: body mass index; OSA: obstructive sleep apnea; PEEP: positive end expiratory pressure; FiO2: fraction inspired
Lab values prior to ECMO cannulation
| Laboratory tests | Extubated on ECMO (N = 14) | Remained Intubated on ECMO (N = 11) | p-value |
|---|---|---|---|
| Arterial blood gas | |||
| pH | 7.3 ± 0.1 | 7.3 ± 0.18 | 0.54 |
| paO2 (mmHg) | 70 ± 22 | 72 ± 33 | 0.68 |
| paCO2 (mmHg) | 57 ± 11 | 68 ± 30 | 0.45 |
| Lactate (mmol/L) | 2 ± 1.1 | 2.6 ± 3.2 | 0.76 |
| Creatinine (mg/dL) | 0.93 ± 0.32 | 0.93 ± 0.47 | 0.58 |
| AST (unit/L) | 67 ± 31 | 70 ± 53 | 0.56 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.58 ± 0.36 | 0.22 |
| ALT (unit/L) | 88 ± 44 | 86 ± 78 | 0.46 |
| WBC (K/μL) | 16 ± 11 | 15 ± 8.1 | 0.81 |
| Hgb (g/dL) | 12 ± 2.7 | 12 ± 4.1 | 0.81 |
| Platelets (K/μL) | 306 ± 127 | 228 ± 63 | 0.051 |
| INR | 1.2 ± 0.18 | 1.2 ± 0.12 | 0.51 |
ECMO: extracorporeal membrane oxygenation; paO2: arterial oxygen pressure; paCO2: arterial carbon dioxide pressure; AST: aspartate aminotransferase; ALT: alanine transaminase; WBC: white blood cell count; Hgb: hemoglobin level; INR: international normalized ratio
Medications used, ventilator settings, and ECMO settings throughout the course of treatment
| Clinical characteristics | Extubated on ECMO (N = 14) | Remain Intubated on ECMO (N = 11) | p-value |
|---|---|---|---|
| Sedation medications dosages | |||
| Propofol (mcg/kg/min) | 55 ± 14 | 59 ± 15 | 0.42 |
| Versed (mg/kg) | 0.079 ± 0.033 | 0.11 ± 0.077 | 0.56 |
| Fentanyl (mcg) | 184 ± 57 | 175 ± 77 | 0.92 |
| Paralyzed | 0.99 | ||
| No | 3 (21%) | 2 (18%) | |
| Yes | 11 (79%) | 9 (82%) | |
| Vasoactive drugs dosages | |||
| Norepinephrine (mcg/kg/min) | 0.034 ± 0.022 | 0.12 ± 0.093 | 0.04 |
| Epinephrine (mcg/kg/min) | 0.16 ± 0.05 | 0.1 ± 0.03 | 0.99 |
| Days on ventilator | 19 ± 14 | 59 ± 41 | 0.012 |
| Type ECMO | 0.42 | ||
| VV-bicaval | 2 (14%) | 2 (18%) | |
| VV-bifemoral | 0 | 1 (9%) | |
| VV-protek duo | 12 (86%) | 7 (64%) | |
| VA | 0 | 1 (9%) | |
| Total days on ECMO | 32 ± 14 | 51 ± 37 | 0.31 |
| Tidal Volume day 14 (mL) | 233 ± 119 | 166 ± 140 | 0.22 |
| Tidal Volume day 28 (mL) | 212 ± 73 | 239 ± 127 | 0.95 |
| ECMO settings at time extubation for group A | |||
| Flow (L/min) | 4.8 ± 0.77 | 4.4 ± 0.95 | |
| FiO2 (%) | 88 ± 16 | 80 ± 34 | |
| Sweep (L) | 3.8 ± 3.7 | 6 ± 4.9 | |
| Ventilator (oxygen) settings at the time of decannulation for group B (group A extubated) | |||
| FiO2 (%) | 44 ± 12 | 41 ± 3.2 | |
| Pressure support (cmH2O) | N/A | 14 ± 3.5 | |
| PEEP (cmH2O) | N/A | 7.9 ± 2.2 |
1 cmH2O = 0.0980665 kPa. ECMO: extracorporeal membrane oxygenation; VV: veno-venous; VA: veno-arterial; PEEP: positive end expiratory pressure; FiO2: fraction inspired; N/A: not applicable
Patient outcomes
| Outcome | Extubated on ECMO (N = 14) | Remained intubated on ECMO (N = 11) | p-value |
|---|---|---|---|
| Tracheotomy | 0.026 | ||
| No | 14 (100%) | 7 (64%) | |
| Yes | 0 | 4 (36%) | |
| Mode of non-invasive respiratory support at time of decannulation | |||
| High flow | 9 (64%) | N/A | |
| NC | 5 (36%) | N/A | |
| Ventilator settings | |||
| PCV | N/A | 6 (54%) | |
| CPAP/PS | N/A | 5 (46%) | |
| Death | 0.13 | ||
| No | 13 (93%) | 7 (64%) | |
| Yes | 1 (7%) | 4 (36%) | |
| Reintubated | 0.23 | ||
| No | 11 (79%) | 11 (100%) | |
| Yes | 3 (21%) | 0 | |
| Days off Ventilator but on ECMO (group A) | 14 ± 8.5 | N/A | |
| Days off ECMO but on the ventilator (group B) | N/A | 8.3 ± 10 |
NC: nasal cannula; PCV: pressure control ventilation; CPAP/PS: continuous positive airway pressure/pressure support; ECMO: extracorporeal membrane oxygenation; N/A: not applicable
Patients were dichotomized into two treatment care groups: those who were extubated first while remaining on ECMO (group A) or not (group B). Summary statistics were calculated for continuous variables, including mean and standard deviation. Frequency counts and percentages were provided for categorical variables. We used the Wilcoxon rank-sum test to compare the continuous variables and the Fisher exact test to study the association between the treatment care and clinical factors. Kaplan-Meier methods and log-rank test were applied for time-to-event outcomes. Statistical Software R version 4.0.3 was used for statistical calculation. A p-value < 0.05 was considered statistically significant.
Results
Twenty-five COVID-19 patients on ECMO support who failed conventional care are included in the analysis (Figure 3). Fourteen patients were extubated first (group A; N = 14), and 11 patients were left intubated on ECMO (group B; N = 11). Clinical and demographic data by each group are summarized in Tables 1–4. No significant differences were seen in patient demographics or pre-ECMO clinical data.
The average age of patients in group A was 37 years compared to 46 years in group B. Group B also had a higher body mass index (BMI) than group A, but the time on non-invasive ventilation and ventilator support was virtually the same. Even though one of our inclusion criteria for ECMO support was failed proning, this was only done in 71% of cases for group A and 82% in group B. This was most likely due to the medical ICU team feeling the patient was either unstable or it was unsafe to prone the patient.
Prior to ECMO support, there was no difference in arterial pH, paCO2, or paO2 between either group. There was also no difference between the creatinine and liver enzyme levels between the two groups, and the lactate level was only slightly increased which shows end organ function was preserved in both groups.
The average number of days on a ventilator was 19 days among group A patients, compared with 59 days on average among group B patients (p = 0.012). None of the patients (0%) in group A received tracheotomy, and 4 (36%) patients in group B received it (p = 0.026). There was a significant difference in the dose of norepinephrine: patients in group A received 0.034 mcg/kg/min, and those in group B received 0.12 mcg/kg/min (p = 0.04). The Kaplan-Meier method (Figure 4) was used to determine overall survival between the two groups: 93% for the extubated group versus 64% for the intubated group (p = 0.54).
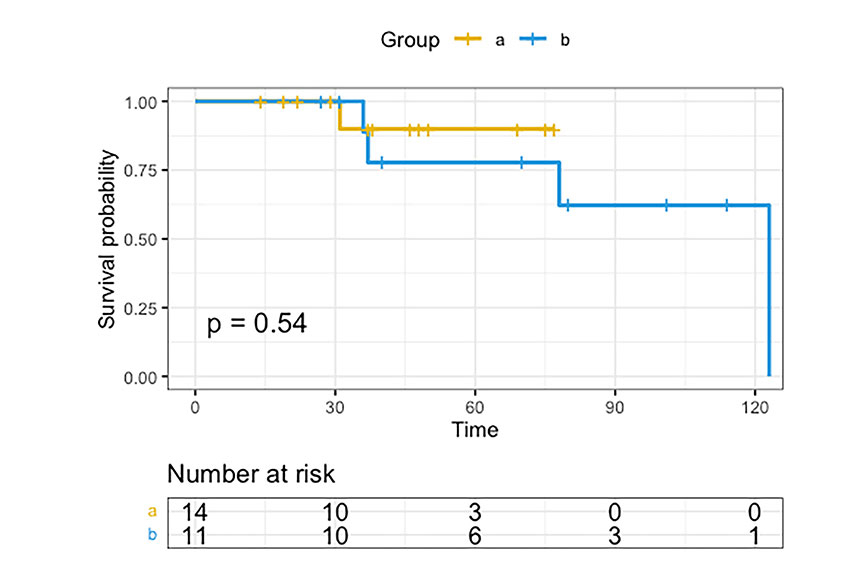
The Kaplan-Meier method was used to evaluate the overall survival for each group. Group A: extubated while on ECMO; group B: continuously intubated on ECMO. ECMO: extracorporeal membrane oxygenation
Furthermore, in group A we had 3 patients reintubated while in group B no one was reintubated. In group B, of the 7 surviving patients, 4 underwent tracheotomy and 3 were successfully extubated after decannulation. Two of the three patients were decannulated first for safety issues as their families said they would not be safely extubated on ECMO support due to mental capacity reasons. In group A, the only death was due to an intracranial hemorrhage shortly after extubation.
Discussion
The central finding of this retrospective, single-center cohort study is that early extubation is vital in patients placed on ECMO due to severe ARDS from COVID-19 infection. Our data show that early extubation during ECMO treatment is associated with a higher survival rate compared to those with prolonged intubation, even though it was not statistically significant. When the Kaplan-Meier analysis method was used, the survival remained statistically insignificant (Figure 4). However, it can be reasoned that the survival would reach significance if we had more patients in both groups because, on day 75, the survival rate for group A was significantly higher than that of group B. Furthermore, after day 75, the survival decreased dramatically for group B. There were statistically significant differences in that patients extubated earlier required significantly lower amounts of norepinephrine and were less likely to require a tracheotomy. This novel data provides a potentially crucial clinical recommendation that can improve survival rates in this highly vulnerable patient population [5].
The use of ECMO in patients with severe ARDS remains controversial and not well-understood, but advancements continue to occur [16]. Due to improved ECMO circuits and technology and a better overall understanding of its physiological mechanics, ECMO use in ARDS patients has increased since the Influenza A H1N1 pandemic in 2009 [17–20]. At the beginning of the COVID-19 pandemic, the initial survival data on ECMO patients were not encouraging. The first single-center, retrospective study in Wuhan, China, shows 83% mortality in COVID-19 patients on ECMO [21]; other early reports suggest that the mortality could be as high as 94.1% [22].
Since those poor initial findings, more recent data has been more encouraging. A multi-center cohort study of 269 patients with COVID-19-associated ARDS treated with ECMO found a 60-day survival rate after ECMO of 56% [23]. Another systematic review and meta-analysis that reviewed data from 773 patients showed that VV-ECMO treatment in adults with severe ARDS was associated with reduced 60-day mortality rates [24]. Furthermore, a multi-center emulated trial of 4,244 patients with COVID-19-associated ARDS treated with ECMO found better patient outcomes for patients treated in high-volume ECMO centers [23]. Despite this improvement in outcomes, using ECMO for COVID-19 ARDS patients remains controversial, and more research is required [24].
Other single-center retrospective studies have observed the importance of intubation times in COVID-19 patients requiring ECMO. These studies show that an increase in the amount of time a patient spent intubated prior to receiving ECMO plays a critical role in the mortality rate, with those with less time intubated before ECMO initiation having an improved survival [8]. Our data show that group A patients spent a mean of 1.9 days on mechanical ventilation before starting ECMO, while group B spent 4 days. This data supports the key recommendation from the Giraud study [8] that those patients who receive mechanical ventilation for a shorter amount of time prior to ECMO have improved survival rates. Therefore, we propose that there exists a supportive relationship regarding mechanical ventilation in ECMO in those with COVID-19 ARDS. Not only would it be sensible to limit the amount of mechanical ventilation prior to ECMO initiation, but a primary goal of clinical management should be to extubate these patients as soon as clinically reasonable once on ECMO support.
In a panel of ECMO experts, there was a strong consensus that a weaning strategy from ECMO support should also include discontinuation from mechanical ventilation to allow for spontaneous breathing [25]. However, it has not been fully established whether ECMO or mechanical ventilation should be weaned first [26, 27]. Many clinicians do not consider awake ECMO a practical treatment option, with only 32% of providers responding that their center extubates from mechanical ventilation before weaning ECMO support [26]. That same survey found that ECMO decannulation occurs in 58% of centers before extubation from mechanical ventilation, with most ECMO centers extubating less than half of their patients on ECMO [26]. The practice of discontinuing mechanical ventilation during ECMO treatment has been increasing [28, 29]; however, a concrete weaning process for mechanical ventilation and ECMO has yet to be established [29, 30].
The proposed mechanism by which early extubation leads to improved patient outcomes is as follows: early extubation allows the patient to spend less time on sedation, thereby reducing the vasopressor requirements, allowing for ambulation, improving nutrition, and decreasing the likelihood of developing concurrent pneumonia. Prolonged ECMO treatment is not advised due to an increased risk of bleeding, renal failure, bacterial pneumonia, and sepsis, among other complications [31, 32]. In addition, prolonged ECMO with intubation requires complete bed rest, immobility, high sedation doses, and significant financial resources [33]. Earlier extubation requires less sedation, thereby reducing the incidence of ICU delirium, a condition that has been shown to increase mortality and long-term cognitive impairment [34, 35].
Our study showed these positive effects of extubation. Our patients who were extubated early had improved survival, less need for vasoactive drugs, and did not need a tracheotomy. The survival difference between our two groups was dramatic, even though it was not statistically significant. Further research is required to determine the role of extubation in the overall survival of ARDS patients on ECMO.
Our long-term results are limited due to follow up. At our one-year ECMO survival reunion, 6 patients that had COVID-19 associated ECMO support returned. Of these 6, only 2 still required oxygen, and all were able to complete their daily activities with minimal limitations. An additional 2 patients reached out and informed us they could not join; however, a reason was not given.
This retrospective, observational study has several limitations. First, the small patient sample sizes do not show statistically significant differences in mortality rates and our recommendations are not to be applied without caution. The small sample size was due to looking specifically at COVID-19 associated ECMO support and the survival benefit of extubating on ECMO support. Even though the results are not statistically significant and the sample size is small, the trend of our results shows a positive correlation between early extubation and survival. Second, this single-center patient information does not allow for external validation of our results. Third, all clinical decisions after ECMO initiation were made by the intensivists, and no standard weaning protocol was used. ECMO management is also a highly resource-intensive treatment option that should be conducted in centers with highly intensive patient volumes and managed by providers skilled and experienced in ECMO management [36]. Finally, no standard protocol was used to wean the patients from the ventilator, and extubating the patients was left at the discretion of the ICU attending. Despite these limitations, our data show significant promise in improving post-admission quality of life, reducing the incidence of pneumonia and neurological deficits, and, most importantly, improving survival rates.
Abbreviations
| ARDS: | acute respiratory distress syndrome |
| ECLS: | extracorporeal life support |
| ECMO: | extracorporeal membrane oxygenation |
| HVICCU: | Heart and Vascular Institute Critical Care Unit |
| RVAD: | right ventricular assist device |
| VA-ECMO: | veno-arterial extracorporeal membrane oxygenation |
| VV-ECMO: | veno-venous extracorporeal membrane oxygenation |
Declarations
Author contributions
SK, AP, and CB: Writing—original draft, Writing—review & editing. CD, CN, RW, MF, and OF: Data curation, Investigation. SZ: Formal analysis. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Penn State Health Institutional Review Board and the Study ID number is STUDY00021063.
Consent to participate
No consent was needed from participants of this study as this was an observational, retrospective study and this waiver of consent was accepted during the IRB review.
Consent to publication
Not applicable.
Availability of data and materials
The datasets generated for this study are available upon request to the corresponding author.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.