Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
Email: sahajwilkhoo@gmail.com
ORCID: https://orcid.org/0009-0000-2943-6404
Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
ORCID: https://orcid.org/0000-0003-3943-8934
Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
ORCID: https://orcid.org/0009-0007-4855-6226
Affiliation:
2American MD Program, Tbilisi State Medical University, Tbilisi 0186, Georgia
ORCID: https://orcid.org/0000-0002-1075-9606
Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
ORCID: https://orcid.org/0009-0007-2054-0169
Explor Cardiol. 2025;3:101250 DOI: https://doi.org/10.37349/ec.2025.101250
Received: October 23, 2024 Accepted: January 16, 2025 Published: February 10, 2025
Academic Editor: Cristina Sena, University of Coimbra, Portugal
L-Carnitine (LC) is integral to energy production and fatty acid metabolism, facilitating the transport of long-chain fatty acids into mitochondria for β-oxidation. It modulates metabolic pathways, including pyruvate dehydrogenase activity, proteolysis, and protein synthesis, while also having anti-inflammatory and antioxidant characteristics. LC can be commonly applied to win the battle against HIV and cancer cachexia. Also, it can be recruited with the aim of improving physical and cognitive functions in athletes and the elderly. Despite these benefits, long-term LC administration has been associated to cardiovascular risks due its conversion to trimethylamine-N-oxide (TMAO) by the gut microbiota. Elevated TMAO levels are linked to atherosclerosis, oxidative stress, and an increased risk of cardiovascular disease, diabetes, and chronic kidney disease. Managing TMAO levels using dietary treatments and gut microbiota-targeting techniques, such as probiotics, may reduce these risks. This comprehensive review presents the state-of-the-art information on LC’s dual role, emphasizing the balance between its therapeutic potential and the risks of prolonged supplementation. It aims to guide clinicians and researchers in optimizing LC’s benefits while addressing its long term cardiovascular safety concerns.
L-Carnitine (LC) is a medically significant nutrient that contributes to the production of energy and the metabolism of fatty acids, by transporting long-chain fatty acids into the mitochondrial matrix for their conversion into energy, via the β-oxidation process [1, 2]. Additionally, LC regulates pyruvate dehydrogenase activity through its reaction with acetyl-CoA, as well as proteolysis and protein synthesis necessary for maintaining the protein balance in skeletal muscle. It also functions as an anti-inflammatory and antioxidant substance, which may decrease the damage that exercise causes to the muscles [2].
Acute and long-term LC supplementation is beneficial in several pathologic conditions, including infectious diseases (including HIV), hemodialysis, cancer cachexia, and dystonia. Several categories of healthy people could benefit from LC supplementation: healthy adults and the elderly, overweight subjects, and athletes. It has been reported that the consumption of LC can present some positive effects on healthy subjects by improving physical, mental, and cognitive functions, and also decreasing the feeling of fatigue in the elderly. It may speed up the healing process, improve oxygen and blood flow to muscle tissue in athletes, and mitigate muscle damage and lower indicators of cellular deterioration, thereby reducing the generation of free radicals [3].
The results of short-term supplementation studies are primarily responsible for the belief that LC supplementation does not affect metabolism. Long-term supplementation, particularly when combined with carbohydrates, has been shown in recent studies to raise skeletal muscle’s total carnitine (TC); this can have significant positive effects as discussed above. However, it has been suggested that LC found in red meat, is what promotes atherosclerosis and that there may be a connection between eating red meat and a higher risk of cardiovascular disease [2].
Recent studies have revealed an obligatory role of gut microbiota in the production of trimethylamine-N-oxide (TMAO) from ingested LC in humans [4]. TMAO is a low molecular weight member of the amine oxide class of compounds. It is created when the hepatic flavin monooxygenases (FMOs; FMO-1 and FMO-3) oxidize trimethylamine (TMA). The primary nutritional substrates used by intestinal microflora in the colon to metabolize phosphatidylcholine/choline, carnitine, betaine, dimethylglycine, and ergothioneine are what form TMAO. Numerous factors influence its level, including kidney function, age, gender, diet, composition of the intestinal microbiota, and liver FMO activity. Numerous studies show a positive correlation between the level of TMAO concentration and the onset of several illnesses, including diabetes mellitus, hypertension, ischemic stroke, atrial fibrillation (AF), heart failure (HF), acute myocardial infarction (MI), and chronic kidney disease (CKD) [5]. The use and supplementation of LC have experienced a notable surge in developed nations. It has yet to be determined whether the recent widespread and quickly expanding use of carnitine supplements poses any health risks [4]. This review aims to evaluate the link between oral LC consumption and the development of adverse cardiac events, mediated by increased TMAO production linked to LC use.
In the context of the metabolism of fatty acids and carbohydrates, LC is a molecule that facilitates the transfer of acyl groups, hence boosting the oxidation of fatty acids and the nonoxidative disposal of glucose. The IUPAC name for LC is (3R)-3-hydroxy-4-(trimethylazaniumyl)butanoate and was initially named vitamin BT (Figure 1). LC binds acyl groups by establishing ester bonds with carboxylic acids at its 3-hydroxyl position, serving as a carrier for fatty acids. Carnitine acyltransferases and LC transmit acyl groups between cell organelles and into the mitochondrial matrix, where β-oxidation takes place, helping to contribute to the oxidation of fatty acids [6]. The products of the β-oxidation are then used by the Krebs cycle to produce adenosine triphosphate (ATP) as a form of energy [7]. Furthermore, LC marginally increases glucose tolerance while promoting nonoxidative glucose metabolism [6].
It has been estimated that the total L-carnitine content (TLC) content in the human body is about 300 mg/kg, and a person’s body composition, gender, and diet all affect their carnitine levels [1, 6]. LC is either consumed in the form of animal-based food products or derived endogenously [7]. In the absence of dietary intake, two necessary amino acids methionine and lysine are converted into the amino acid carnitine. This can happen in the kidneys, liver, and brain. The largest amounts are found in the skeletal and cardiac muscles, which cannot produce their carnitine and must instead get it from plasma. Plasma carnitine levels and the amount of carnitine consumed through diet have a positive correlation. Most unabsorbed LC has been described to be broken down by digestive tract microbes [1]. Carnitine is found almost exclusively (99%) intracellularly, existing either as free LC or as several species of acyl-carnitine esters, predominantly in the muscle and liver. LC contents of the extracellular fluid, liver, skeletal muscle, and kidney were 0.5, 1.3, 1.27, and 0.2 mmol/L, in that order [6].
Hereditary or acquired defects in the transport mechanisms are the major cause of LC deficiency, leading to pathologies such as cardiomyopathy and skeletal muscle myopathy. Regulatory feedback mechanisms leading to an increase in the dietary absorption of LC, and/or de novo synthesis may take place to overcome the LC deficiency and reduce the loss by urinary excretion. Such possible adaptation has been reported in vegetarians [7]. Diabetes complications, trauma, hemodialysis, hunger, obesity, cardiomyopathy, fasting, endocrine imbalances, drug interactions, and other disorders have all been linked to anomalies in the regulation of carnitine [1].
The body requires approximately 15 mg of carnitine per day, which is produced both internally and through nutrition. The majority of the carnitine in American diets comes from foods of animal origin. For a 165-pound person, a typical omnivorous diet would give 24 mg to 145 mg of carnitine per day. A vegan diet, on the other hand, offers roughly 1.2 mg of carnitine. Supplements containing simply carnitine or a mix of carnitine and other substances are available for purchase. LC and acetyl-L-carnitine (ALC), the two primary types of carnitine used in dietary supplements, come in dosages ranging from roughly 3 mg to 5,000 mg. The absorption of supplementary LC is significantly lower than that of dietary LC, ranging from 14% to 18%. In 1989, the Food and Nutrition Board (FNB) of the National Academies of Sciences, Engineering, and Medicine concluded that carnitine is not an essential nutrient. Therefore, the FNB did not establish Dietary Reference Intakes (DRIs) for carnitine. Several types of medications have the potential to interact with carnitine supplements, including pivalate-conjugated antibiotics, valproic acid, and other anticonvulsants. There isn’t a known upper limit of acceptable carnitine intake. On the other hand, taking supplements containing about 3 g of carnitine daily may result in diarrhea, cramping in the abdomen, nausea, vomiting, and a fishy body odor [8, 9]. In addition, it can result in seizures in individuals with seizure disorders and muscle weakness in those with uremia [10]. Readers are kindly referred the Figure 1 for an illustrated overview of LC mentioning its sources, functions in metabolism, distributions, requirements, absorption, potential side effects of excessive intake and drug interactions.
TMAO is a metabolite produced by gut microbiota. Microbiota derives it from dietary sources like choline, carnitine, and betaine [11]. It is oxidized in the liver and its plasma levels are greatly influenced by diet, and liver enzymatic activity [12]. Recent research has shown a positive correlation between increased TMAO levels and increased risk of cardiovascular events, CKD, and events [5, 13]. The potential of TMAO to result in atherogenic effects is linked to alterations in cholesterol and bile acid metabolism, inflammatory pathway activation, and foam cell formation promotion. However, the exact link between TMAO and these diseases remains unclear. Therapeutic strategies to reduce TMAO levels are being studied including antibiotics and targeted molecules [11, 12]. TMAO might serve as a precise biomarker for conditions like mortality, cardiovascular diseases, diabetes, cancer, and kidney function [7]. The gut microbiota plays a crucial role in host physiology, influencing the gut-brain axis and metabolizing dietary components into compounds like TMAO. Elevated TMAO levels are linked to cardiovascular, metabolic, and neuropsychiatric disorders, impacting aging, oxidative stress, and brain function. Therapeutic strategies target TMAO levels through microbiota modulation. Clinically TMAO is a very significant molecule that plays a crucial role in determining the risk and even early manifestations of certain conditions. TMAO levels impact a set of functioning in the body. Ranging from oxidative stress, and microglial activation to even apoptosis of neurons. This level is also seen to be associated with an increased risk of first stroke. Along with this, TMAO levels have other neurodegenerative properties as well [14].
Genetics, food, illness conditions, and environmental variables all influence the composition of the gut microbiota. In CKD, uremic dysbiosis causes a decrease in microbial diversity, favoring the growth of pathogenic microbes that raise levels of compounds such as TMAO. Increased TMAO levels are caused by lower clearance and increased synthesis as a result of gut dysbiosis, a weakened intestinal barrier, and increased hepatic enzyme activity. TMAO is plentiful in animal-derived meals such as red meat, egg yolk, and fish. While TMAO has been associated with vascular damage and cardiovascular risk in CKD patients, the evidence is inconsistent. A balanced diet could help avoid dysbiosis and lower TMAO levels [15, 16]. Increased plasma TMAO levels are linked to an increased risk of severe cardiovascular events and death. Its atherogenic effects include abnormalities in cholesterol and bile acid metabolism, which cause inflammation and foam cell production. TMAO levels also rise as kidney function degrades, which correlates with mortality in CKD patients [12].
A research of 163 patients with idiopathic/hereditable pulmonary hypertension (PH), congenital heart disease-associated PH, and chronic thromboembolic PH examined plasma TMAO levels as a possible biomarker in PH. Elevated TMAO levels were connected to severe illness states and a poor prognosis, with higher levels indicating worse outcomes even when controlling for confounders. TMAO levels were reduced in individuals who improved with treatment, underscoring the importance of monitoring PH severity and prognosis [17].
Elevated TMAO levels have been linked to a number of diseases, including cardiovascular and neurological disorders, renal disease, metabolic syndrome, obesity, type 2 diabetes (T2D), non-alcoholic fatty liver disease (NAFLD), cancer, and severe COVID-19. In CKD, reduced renal function limits TMAO clearance, whereas excessive levels in metabolic syndrome increase the risk of T2D and cardiovascular diseases. Obesity, a global health concern marked by excessive fat accumulation, has increased fivefold since 1997. It not only serves as a major risk factor for chronic diseases such as cardiovascular disease and diabetes but also exacerbates these conditions, further complicating their management and outcomes. Higher TMAO levels are linked to obesity and vitamin D deficiency, while causality is unknown. Dietary implications on TMAO are clear; a study of overweight participants found that those who ate cod had greater TMAO levels than those who ate salmon or a control diet. Exercise combined with a low-calorie diet was connected to decreased TMAO levels in obese people, however, bone mineral loss must be considered in interventions. The function of TMAO in causing endoplasmic reticulum (ER) stress and insulin resistance in diabetes is well-documented, with TMAO levels greater in type 2 diabetics. According to experimental findings, TMAO has an impact on glucose metabolism and insulin resistance. Metabolic-dysfunction-associated fatty liver disease (MAFLD), which is linked to TMAO, can proceed to severe liver disorders and is a known risk factor for several malignancies. TMAO’s effects extend to systemic arterial hypertension and vascular disorders, where elevated levels are associated with unfavorable cardiovascular events. TMAO’s role in neurological illnesses, such as Alzheimer’s, and cancer, particularly colorectal cancer, is becoming more widely acknowledged. Finally, while TMAO’s involvement in severe COVID-19 is postulated, more research is needed to determine its significance [18–22].
LC has garnered attention for its role in increasing TMAO levels in the bloodstream [23]. This relationship is particularly significant given TMAO’s association with cardiovascular diseases [24]. It is crucial to understand the pathway through which LC elevates TMAO levels and its implications for health conditions.
When LC is ingested, it is metabolized by specific gut bacteria into TMA as seen in Figure 2. This process involves several bacterial species capable of utilizing LC as a substrate. Once produced, TMA is absorbed into the bloodstream and transported to the liver, where it undergoes further oxidation to form TMAO via the enzyme FMO-3 [23, 25].
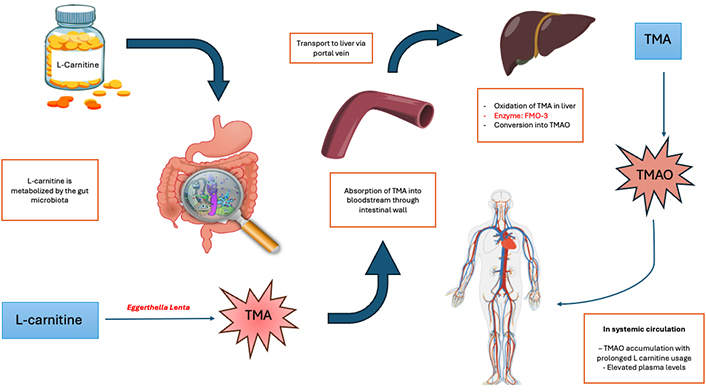
Pathway of TMAO production from LC. TMA: trimethylamine; TMAO: trimethylamine-N-oxide; FMO-3: flavin monooxygenase 3
Research suggests that LC supplementation can significantly elevate plasma TMAO levels. For example, a study has documented up to a tenfold rise in TMAO levels in healthy individuals after LC supplementation [24]. This elevation is not limited to healthy populations; patients with certain metabolic disorders also exhibit marked increases in TMAO levels when supplemented with LC [26]. Refer to Figure 2 for a better understanding of the pathway leading to TMAO production from LC.
The increased production of TMAO has been linked to various health conditions, like cardiovascular diseases, atherosclerosis and stroke as mentioned above [24]. However, some studies suggest that while LC supplementation raises TMAO levels, it does not necessarily correlate with adverse cardiovascular outcomes in all populations. For example, one study found no negative impact on cardiovascular health markers in older women supplemented with LC [4].
Additionally, there are implications for patients with CKD and those undergoing hemodialysis. In these populations, while LC supplementation increased TMAO levels, it was also associated with reduced markers of vascular injury and oxidative stress [27]. This suggests that the relationship between LC, TMAO, and health outcomes may be complex and influenced by underlying health conditions.
Moreover, recent studies have explored the potential link between elevated TMAO levels and various cancers. For instance, higher circulating levels of TMAO have been associated with gastric cancer risk, particularly among males [4, 28]. This indicates that the metabolic pathways involving LC and TMAO may play a role not only in cardiovascular health but also in cancer pathogenesis.
The gut microbiota is a complex ecosystem that has a major impact on human health via interactions between microbial composition and metabolic activity. Its use has an impact on the gut microbiota, causing significant alterations in microbial diversity and metabolic pathways that can have far-reaching consequences for overall health. Prior to LC use, microbial diversity is impacted by diet, genetics, and lifestyle, with the Firmicutes-to-Bacteroidetes (F/B) ratio serving as an important indicator of gut health. A larger F/B ratio is frequently associated with dysbiosis, which is connected to metabolic diseases such as obesity and insulin resistance, whereas a balanced F/B ratio indicates microbial balance [6, 27, 29].
LC is a substrate for gut microbiota, and its metabolism affects both microbial composition and human metabolic pathways. Following consumption, gut bacteria convert LC into TMA, which is aided by microbial enzymes such as TMA-lyase. This process is mostly carried out by taxa such as Escherichia coli, Clostridium sporogenes, and some Lactobacillus species. FMOs in the liver metabolize TMA into TMAO once it is taken into the bloodstream. This population tend to grow in abundance after consumption [28]. These microbial changes are correlated with fluctuations in beta diversity, which represent structural changes in the microbial community, although alpha diversity is frequently stable. Alpha diversity assesses the diversity of species within a single microbial community, indicating its richness and uniformity. In contrast, beta diversity evaluates the variations in microbial composition of separate communities, reflecting the degree of similarity or dissimilarity between them [30, 31].
Long-term LC use can worsen gut dysbiosis by increasing the F/B ratio, resulting in increased TMAO formation and systemic inflammation as mentioned in Table 1. This dysbiotic state may compromise gut barrier integrity, creating a pro-inflammatory state. However, several species involved in LC metabolism also produce short-chain fatty acids (SCFAs), which are necessary for intestinal health. SCFAs including acetate, propionate, and butyrate are created by gut bacteria during the fermentation of dietary fiber and play an important role in maintaining intestinal health and lowering inflammation. The SCFA-producing species, including Faecalibacterium prausnitzii, Bacteroides spp., and Clostridium spp., are well-known bacterial genera for synthesizing butyrate, which plays a vital role in maintaining intestinal barrier integrity and decreasing inflammation. Bacteroides spp. produce acetate and propionate, which help to control immunological responses and reduce intestinal inflammation. Clostridium spp., including Clostridium butyricum, produce butyrate, which helps to maintain gut epithelial cells and has anti-inflammatory properties [32–35].
Dual role of LC in human health, insights of beneficial short term usage and potentially harmful long term complications
| Aspect | Description |
|---|---|
| Gut microbiota composition |
|
| Gut dysbiosis |
|
| SCFAs production |
|
| Role of TMAO in inflammation |
|
| Fatty acid metabolism |
|
| Amino acid metabolism |
|
| Mitochondrial activity |
|
| Dual role in health |
|
F/B: Firmicutes-to-Bacteroidetes; LC: L-carnitine; TMA: trimethylamine; TMAO: trimethylamine-N-oxide; ATP: adenosine triphosphate; BCAAs: branched-chain amino acids; SCFAs: short-chain fatty acids
Gut microbial metabolites generated from LC, such as SCFAs, act as signaling molecules, regulating host immunity and metabolism. SCFAs attach to immune cells’ G-protein-coupled receptors (GPCRs), which regulates cytokine production and reduces inflammation. SCFA regulate cytokines such as TNF-α, interleukin 6 (IL-6), and IL-10, which affect inflammation and immune cell activity. SCFAs also stimulate the development of regulatory T cells (Tregs), which are critical for immunological tolerance and limiting excessive inflammation. These interactions underscore SCFAs’ essential role in immune regulation and their potential therapeutic implications in inflammatory disorders [36, 37]. They also enhance intestinal barrier integrity and mitochondrial health. However, LC is metabolized by particular gut bacteria to form TMA, which is then oxidized to TMAO, adding complexity. TMAO causes oxidative stress and inflammation, which may offset the benefits of LC. This dual role shows the delicate balance between LC’s positive and detrimental effects, which is influenced by microbiota composition and metabolic pathways [18, 26, 38].
LC is essential in various metabolic pathways, including fatty acid metabolism, amino acid catabolism, and mitochondrial activity as mentioned in Table 1. It serves as a crucial component in fatty acid transport, stimulates the transfer of long-chain fatty acids into the mitochondria, where they undergo β-oxidation to create acetyl-CoA, a precursor for ATP synthesis via the tricarboxylic acid cycle (TCA cycle) [5, 6, 26]. This occurs via the carnitine palmitoyltransferase (CPT) system, with CPT1 aiding the transport of fatty acids into the mitochondria and CPT2 facilitating their release once processed. This process is critical for regulating energy levels, particularly during fasting or prolonged physical exercise [4, 39, 40].
In addition to its role in lipid metabolism, LC is implicated in amino acid metabolism, namely the transamination of branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine. It also contributes to nitrogen balance and efficient energy generation by converting BCAAs into their respective keto acids. This pathway is critical for limiting the build up of harmful intermediates and maximizing protein turnover over short term [41–43].
LC also affects mitochondrial activity by boosting mitochondrial biogenesis and decreasing oxidative stress. It strengthens mitochondrial membranes, improving their integrity and function. It’s functions help to increase cellular metabolism and energy production while reducing the negative consequences of oxidative damage. Furthermore, LC’s ability to regulate fatty acid oxidation affects systemic inflammation and immunological responses, as metabolites such as SCFAs can influence immune cell activity and gut barrier integrity [9, 44–47]. Refer Table 1 for a summarized tabulation of the dual role of LC in human health.
ALC, propionyl-L-carnitine, L-carnitine L-tartrate, glycine propionyl-L-carnitine (GPLC), and L-carnitine fumarate all have different impacts on gut microbiota and TMAO synthesis, but ALC stands out due to its unique features. While gut bacteria metabolize LC into TMA, which is then converted to TMAO in the liver, these derivatives have distinct metabolic pathways and bioavailability, which may influence the amount of TMAO levels [32, 48, 49].
ALC has been demonstrated to be more easily absorbed and metabolized, and it may have a lower influence on TMAO production than LC. This could be due to ALC’s quick absorption and capacity to cross the blood-brain barrier, potentially altering the gut microbiota composition and limiting the abundance of TMA-producing bacteria. While ALC may influence microbial diversity, its exact impact on TMAO levels is less well studied than LC [8, 28].
Another derivative, propionyl-L-carnitine, has been linked to enhanced cardiovascular health and exercise performance, while its effects on gut microbiota and TMAO formation are less well understood [50–52].
Similarly, L-carnitine L-tartrate is largely utilized to aid in muscle rehabilitation and has not been thoroughly connected to TMAO synthesis [53, 54].
GPLC and L-carnitine fumarate, while employed in certain situations such as improving athletic performance and mitochondrial function, have yet to show substantial differences in microbiota-related benefits when compared to LC itself. These derivatives, however, may still have the potential to modulate inflammation and gut health due to their distinct biochemical features [55–57].
Overall, while LC derivatives, notably ALC, show promise in lowering TMAO production and modifying gut microbiota composition, additional studies are needed to completely understand their effects. Personalized interventions could help maximize the advantages of these compounds while reducing the dangers associated with TMAO generation [49, 58–60].
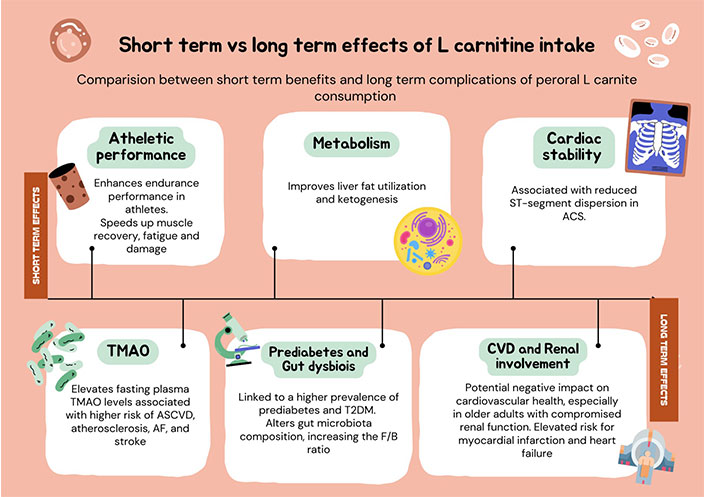
Recent investigations into the acute effects of LC supplementation have revealed significant physiological changes. For instance, acute intake of LC (3–4 g) before exercise has been shown to enhance endurance performance in athletes by increasing running speeds at specific lactate thresholds and reducing heart rate responses [61]. A lower dose (750 mg) of LC under fat-mobilizing conditions improved liver fat utilization and ketogenesis without stimulating energy expenditure [40]. In patients experiencing acute MI, elevated blood levels of carnitine were noted compared to healthy controls, likely due to impaired uptake or increased leakage from ischemic myocardium. Furthermore, LC supplementation in acute coronary syndrome (ASC) patients was associated with reduced QT dispersion, indicating improved myocardial electrical stability [62, 63].
In contrast, chronic elevations in TMAO, a metabolite derived from LC, have been linked to an increased risk of atherosclerotic cardiovascular disease (ASCVD), particularly in older adults with compromised renal function [64, 65]. Long-term LC supplementation has been shown to elevate fasting plasma TMAO levels, raising concerns about its implications for cardiovascular health, despite its potential benefits in improving muscle mass and physical performance. Notably, while TMAO is associated with a higher prevalence of prediabetes, it does not appear to significantly influence insulin resistance or longitudinal changes in fasting plasma glucose [24, 61, 66, 67].
Thus, while acute LC supplementation may confer immediate physiological benefits, chronic elevations in TMAO levels warrant careful risk evaluation due to their association with adverse cardiovascular outcomes, particularly in vulnerable populations. Readers are kindly referred to the Table 2 for understanding the comparison between the short-term and long-term effects of TMAO levels.
Comparison between long-term and short-term impact of increased TMAO levels
| Short-term impact | Long-term impact |
|---|---|
| Enhances endurance performance in athletes | Elevates fasting plasma TMAO levels |
| Improvs liver fat utilization and ketogenesis | Associated with a higher risk of ASCVD, Atherosclerosis, AF, and stroke |
| Higher levels observed in acute MI | Potential negative on cardiovascular health. Particularly concerned in older adults with compromised renal function |
| Associated with reduced ST-segment dispersion in ACS | Linked to a higher prevalence of prediabetes and T2DM |
| Requires careful assessment due to potential adverse effects on cardiovascular health |
MI: myocardial infarction; ACS: acute coronary syndrome; TMAO: trimethylamine-N-oxide; ASCVD: atherosclerotic cardiovascular disease; AF: atrial fibrillation; T2DM: type 2 diabetes mellitus
Recent research has revealed that TMAO may be a risk factor for the onset and progression of atherosclerosis, along with food and gut microbiota both of which may play a role in the development of cardiovascular disease, including atherosclerosis [68, 69]. A dietary source of TMAO, phosphatidylcholine, is the main risk factor for the development of atherosclerosis, HF, and an elevated risk of mortality [5, 69]. Furthermore, there is a correlation between elevated mortality and severe CVD symptoms and indications and a rise in TMAO levels. In another research, mice given a diet enriched with TMAO developed atherosclerotic lesions in their aortas [70]. Recently, it was reported that higher phosphatidylcholine intake is linked to an increased risk of fatal cardiovascular disease in US residents, especially in patients with diabetes independent of traditional risk factors. A recent study found that Americans with diabetes, in particular, who consume higher levels of phosphatidylcholine, have an elevated chance of mortality from cardiovascular disease even in the absence of risk factors [5, 55].
Elevated blood TMAO levels cause an imbalance in cholesterol homeostasis which leads to influenced macrophages, which in turn causes foam cell development and atherosclerotic plaques [68, 69, 71]. According to new research, TMAO both causes and raises the HF ratio. Increased TMAO levels in addition to endothelial dysfunction, vascular aging, and oxidative stresses have been shown to cause thrombotic events and platelet excitability in humans, which in turn induce atherosclerosis [69, 72]. Multiple studies have proved that for TMAO to induce inflammatory gene expression in leukocytes and both cell types, nuclear factor kappa B signaling has to be activated [69]. In addition, TMAO induces several inflammatory pathways, which cause the activation of pro-inflammatory cytokines such as IL-6 and TNF-α which causes chronic inflammation thereby causing plaque formation and plaque rupture leading to cardiovascular disease [71].
In the light of a recent cohort study by Tang and his colleagues, comprising 4,000 individuals for 3 years, it has been found that TMAO can be a new biomarker for cardiac mortality, MI, or cerebrovascular accidents in addition to increasing cardiac-related mortality by 2.5 times [73, 74]. Experimental studies reported that procedures like choline-induced fecal transplantation from one person to another can also transfer the risk of developing atherosclerosis [69, 75]. Additionally, TMAO has been demonstrated to worsen reduced glucose tolerance, limit hepatic insulin signaling, and incite inflammation in adipose tissue in vivo—all essential elements of the atherosclerotic pathway [76].
TMAO is linked to several additional conditions besides atherosclerosis, such as HF, cancer, CKD, PH, and T2D [5, 71]. Despite the complexity of TMAO’s role in atherosclerosis, studies have indicated that managing TMAO through nutrition, medication, or microbiome modification may help lessen its effects and even reverse its contribution to the advancement of the disease [73, 76].
The contributions of LC and TMAO to adverse cardiac events, such as MI, stroke, sudden cardiac death (SCD), and HF, have come under more and more scrutiny in research as seen in Table 3. The pathological process of cardiac remodeling, which involves alterations in the structure and function of the heart, is a major way that TMAO aggravates these effects [77]. Research also indicates that TMAO may function as a biomarker for major adverse cardiac events (MACE) as well as a contributing component [55]. These results highlight the significance of controlling LC intake because an excess of this dietary component raises TMAO levels, which in turn raises cardiovascular risk.
Adverse cardiac events associated with increased TMAO levels
| Cardiac event | Description | Mechanism |
|---|---|---|
| MI |
|
|
| Stroke |
|
|
| AF |
|
|
| HF |
|
|
| SCD |
|
|
HF: heart failure; TMAO: trimethylamine-N-oxide; MI: myocardial infarction; AF: atrial fibrillation; SCD: sudden cardiac death; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells
When the coronary artery’s blood supply is blocked, MI happens [78]. Atherosclerosis, hypertension, and diabetes are among the conditions that clog the coronary arteries and cause ischemia damage to the cardiomyocytes. Studies have shown that plasma TMAO may affect the course of various cardiovascular disorders and increase the risk of MI, despite the lack of direct relationships [65, 79]. However, several studies have also shown that because of their decreased ability to repair damaged blood vessels, people with elevated TMAO levels are more likely to experience another MI and recover poorly after one. Overall, rather than directly causing MI to worsen, TMAO is more likely to be a biomarker that predicts the prognosis of individuals admitted for MI [33, 65].
According to findings, TMAO is widely thought to be a pathogenic metabolite that causes hypertension [80, 81]. Additionally, research indicates that TMAO may influence the renin-angiotensin system and amplify Ang II’s hypertensive effect [77, 82]. TMAO causes vascular dysfunction and hypertension, two important risk factors for ischemic stroke [77]. According to studies, hypertension may cause the gut-blood barrier to become more permeable, allowing more TMA to enter the bloodstream and raising the quantity of TMAO [55]. Thus, TMAO-induced inflammation raises the risk of cerebral embolism by causing endothelial damage.
TMAO and LC are linked to SCD, which is mostly due to HF, atherosclerosis, and arrhythmias. Although there are some cardioprotective benefits to LC supplementation in certain situations (such as lowering all-cause mortality after MI), the formation of TMAO that goes along with it raises questions regarding long-term cardiovascular safety, particularly for people who already have heart issues [83].
The potential connection between AF and the chemical TMAO, which is produced by gut microorganisms. It has been shown that TMAO causes an increase in electrical remodeling and inflammation of the ganglionated plexi, which speeds up the course of arrhythmias such as AF. TMAO can aggravate acute electrical remodeling in AF models and enhance atrial electrophysiological instability in normal canines by enhancing autonomic remodeling and boosting the p65 NF-κB signaling pathway [84].
Elevated plasma TMAO levels have been associated in several studies with a higher risk of cardiovascular events and death [11, 67]. Additionally, TMAO prevents cardiac mitochondria from oxidizing fatty acids and pyruvate, which may have an impact on energy metabolism [44]. Nonetheless, there is an ongoing debate over the causative relationship between TMAO and cardiovascular disease, as certain studies have found no connection. Additionally, TMAO has been connected to a higher prevalence of metabolic syndrome and T2D. Elevated levels of TMAO in the bloodstream are linked to an increased risk of hypertension and other cardiometabolic disorders [67].
The median TMAO level in healthy individuals is 3.45 μM, with a range of 0.73 μM to 126 μM [85]. A population-based investigation revealed somewhat different reference intervals for men (1.28–9.67 μM) and women (1.08–17.12 μM). Food, specifically the amount of fish and shellfish that men eat, and renal function all affect TMAO levels. Elevated TMAO levels in patients with coronary artery disease (CAD) are linked to a higher atherosclerotic load as measured by Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) scores. Liquid chromatography-tandem mass spectrometry (LC-MS/MS), which is particularly stable in plasma samples stored at –80°C for up to 5 years, can be used to precisely analyze TMAO [5, 85, 86].
Lastly, elevated malondialdehyde (MDA) and decreased glutathione (GSH) levels indicate that chronic exposure to large dosages of TMAO can induce oxidative stress and inflammation in the liver [38]. Rats treated with TMAO have been shown to increase inflammatory markers and promote early atherosclerosis modifying lipid metabolism and biomarkers. The substance has been connected to issues with the heart, the kidneys, diabetes as well as neurological disorders [5, 87]. Even though experimental and observational studies show a significant correlation between elevated levels of plasma TMAO and adverse cardiovascular events, the precise mechanism is still in place and unidentified [11]. Further investigation is required to ascertain if higher than-normal TMAO levels are the cause.
Since TMAO synthesis is predominantly regulated by gut microbiota, treatment options for managing and lowering TMAO levels center on dietary adjustments, probiotic and prebiotic therapies, antibiotics, exercise, and lifestyle changes [88].
Dietary treatments remain a cornerstone of TMAO lowering. According to studies, plant-based diets, such as the Mediterranean diet, reduce TMAO levels by encouraging a microbiota composition that suppresses TMA-producing bacteria. High-fiber diets are also useful because they promote the proliferation of good gut bacteria while decreasing the abundance of TMA-producing species such as Escherichia coli and Clostridium sporogenes. Another option for lowering TMAO levels is to consume less red meat, which is high in LC and choline [88–90].
Probiotics, or live microorganisms that provide health advantages when taken, and prebiotics, nondigestible carbohydrates that encourage beneficial bacteria, are increasingly being employed to modify TMAO synthesis. Probiotic strains such Lactobacillus reuteri, Lactobacillus plantarum, and Bifidobacterium longum have showed potential in lowering TMAO levels by suppressing TMA-producing bacteria and increasing beneficial microbial populations. Prebiotics such as inulin and fructooligosaccharides also aid by promoting the growth of bacteria that do not produce TMA. These therapies can help to restore gut microbial balance and lessen the hazards associated with TMAO [69, 91–95].
Antibiotics including rifaximin and metronidazole have been investigated for their capacity to inhibit TMAO formation by targeting gut bacteria that create TMA. While antibiotics are effective, long-term use is discouraged due to concerns about antibiotic resistance and the potential disruption of healthy gut flora. Short-term treatment may provide targeted relief, however, combining antibiotics with probiotics can help reduce the harmful impact on the microbiota, while caution is suggested due to potential inflammatory responses [96–102].
Regular physical activity, particularly endurance and aerobic workouts, is another excellent way to lower TMAO levels. Exercise has been demonstrated to increase microbial diversity, reducing TMA-producing bacteria while promoting the growth of TMA-independent species such as Lactobacillus and Bifidobacterium. Moderate-to-high-intensity aerobic exercises, such as cycling or running, have been shown to reduce TMAO levels while also enhancing cardiovascular health. To improve cardiovascular health and regulate TMAO levels, guidelines recommend at least 150 min of moderate-intensity aerobic exercise per week. The Physical Activity Guidelines for Americans (2023) encourage at least 150 min of moderate-intensity aerobic exercise per week, with a goal of 300 min per week for maximum health benefits. These guidelines recommend a combination of aerobic activities, such as walking, cycling, or swimming, with resistance training. This regimen promotes positive changes in gut microbiota composition, which improves metabolic health, cardiovascular function, and may lower TMAO levels. Regular physical exercise is critical for managing comorbidities and lowering systemic inflammation, which has been associated to increased TMAO production [103–109]. Readers are kindly referred to the Table 4 for the summary of current interventions to reduce the levels of TMAO.
Summary of interventions to reduce the levels of TMAO
| Intervention | Efficacy | Population |
|---|---|---|
| Plant-based diets (e.g., Mediterranean) | Reduces TMAO by promoting beneficial gut bacteria | General population, those with cardiovascular/metabolic issues |
| High-fiber diet | Reduces TMA-producing bacteria | General population, those with metabolic/gut health issues |
| Reduced red meat consumption | Lowers TMAO by reducing LC and choline | Those with cardiovascular/metabolic diseases |
| Seafood | Increases TMAO levels; should be avoided for TMAO reduction | General population, those looking to lower TMAO |
| Legumes, soy, quinoa, fermented foods | Decreases TMAO by promoting beneficial bacteria | General population, those with metabolic/cardiovascular concerns |
| Probiotics | Suppresses TMA-producing bacteria (e.g., Lactobacillus, Bifidobacterium) | Those with high TMAO or gut dysbiosis |
| Prebiotics | Promotes growth of TMA-independent bacteria | Those with gut health issues |
| Exercise (endurance/aerobic) | Increases microbial diversity, reduces TMA-producing bacteria | General population, those with cardiovascular/metabolic issues |
| Antibiotics (rifaximin & metronidazole) | Reduces TMAO by targeting TMA-producing bacteria | Short-term use for those with high TMAO levels |
| Omega-3 supplements | Can help reduce TMAO levels | Those aiming to lower TMAO or improve metabolic health |
| Choline/LC-lowering drugs | May reduce TMAO levels | Those with elevated TMAO due to diet or metabolism |
LC: L-carnitine; TMA: trimethylamine; TMAO: trimethylamine-N-oxide
TMAO is primarily generated from TMA, and meals high in choline, LC, and betaine are key dietary sources of its production. As a result, TMAO is especially abundant in certain food types, such as meat and dairy products, according to several studies conducted over the years [89, 110]. As a result, eating seafood that is low in long-chain N3 may raise TMAO levels. Some examples of these fish include prawns/shrimps, squid, and white flesh fish. Furthermore, research has shown that eating red meat significantly raises TMAO levels. Conversely, fish that live in shallow waters lower the levels of TMAO. Additionally, consuming a plant-based diet containing soy, legumes, and quinoa, dietary supplements rich in omega-3 and fermented foods significantly decreases TMAO levels, according to other studies [77, 82]. Findings have also shown that some food types such as the Western diet have higher quantities of TMA precursor levels consequently altering TMAO levels in the long term [111]. Several investigations also found that changing one’s lifestyle to include exercise can significantly lower TMAO levels [112]. Moreover, consuming probiotics and prebiotics linked to certain genes that inhibit TMA-producing bacteria has also been shown in some studies to help control the generation of TMAO [112, 113]. Finally, research also showed that taking some drugs in addition to antibiotics may lower TMAO levels [111].
LC plays an important role in fatty acid metabolism and energy production, offering benefits such as enhanced muscle performance, cognitive support, and metabolic efficiency. However, its metabolic conversion by gut microbiota into TMAO raises significant concerns due to the association of elevated TMAO levels with cardiovascular diseases like atherosclerosis, MI, stroke, and HF. While short-term LC supplementation has demonstrated positive effects, including increased endurance, improved hepatic fat metabolism, and myocardial stability, evidence indicates that the prolonged supplementation may pose risks, particularly for individuals with pre-existing cardiovascular or renal conditions. Based on this current clinical evidence, long term usage of LC should be strict avoided and one must not exceed the daily allowance to avoid the short term side effects as well as the risk of long term complications due to prolonged elevated TMAO levels.
Emerging evidence also highlights the importance of dietary modifications to mitigate these risks. A shift toward plant-based diets, combined with the use of probiotics especially strains like Lactobacillus and Bifidobacterium and prebiotics, may reduce TMAO levels and associated cardiovascular threats. Additionally, lifestyle interventions such as regular physical activity of minimum 150 min per week and pharmacological approaches as discussed in this review could further alleviate TMAO-related complications. Future investigations should focus on understanding population-specific responses and genetic predispositions, influencing TMAO synthesis. Strategies to optimize the therapeutic potential of LC while minimizing its cardiovascular risks are imperative to guide clinical practice and public health recommendations. This balanced approach could ensure safer and more effective utilization of LC in healthcare.
ACS: acute coronary syndrome
AF: atrial fibrillation
ALC: acetyl-L-carnitine
ASCVD: atherosclerotic cardiovascular disease
ATP: adenosine triphosphate
BCAAs: branched-chain amino acids
CAD: coronary artery disease
CKD: chronic kidney disease
CPT: carnitine palmitoyltransferase
DRIs: Dietary Reference Intakes
ER: endoplasmic reticulum
F/B: Firmicutes-to-Bacteroidetes
FMOs: flavin monooxygenases
FNB: Food and Nutrition Board
GPLC: glycine propionyl-L-carnitine
GSH: glutathione
HF: heart failure
IL-6: interleukin 6
LC: L-carnitine
LC-MS/MS: liquid chromatography-tandem mass spectrometry
MACE: major adverse cardiac events
MAFLD: metabolic-dysfunction-associated fatty liver disease
MDA: malondialdehyde
MI: myocardial infarction
NAFLD: non-alcoholic fatty liver disease
NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells
PH: pulmonary hypertension
SCD: sudden cardiac death
SCFAs: short-chain fatty acids
SYNTAX: Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery
T2D: type 2 diabetes
T2DM: type 2 diabetes mellitus
TC: total carnitine
TCA cycle: tricarboxylic acid cycle
TMA: trimethylamine
TMAO: trimethylamine-N-oxide
HSW: Conceptualization, Writing—original draft, Writing—review & editing, Supervision, Validation. FR: Writing—original draft, Writing—review & editing. AWI: Writing—original draft, Writing—review & editing. JAK: Writing—original draft, Writing—review & editing. AAS: Writing—original draft.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
