The liver operates an array of vital digestive, metabolic, immunological, and purificatory physiological functions including synthesis of albumin, regulation of glucose and lipid homeostasis, detoxification of ammonia and xenobiotics, choleresis and production of hepatokines and hormones [1]. However, in healthy states, it is not involved in the storage of fatty substrates and, whenever the normal liver threshold is exceeded, accumulated triglycerides will impair whole-body insulin sensitivity, trigger sterile low-grade hepatic metaflammation, and promote the progression of the fibrosing process that will eventually conduce to cirrhosis in a subset of individuals [2, 3].
While the histopathological cascade described above was first recognized in the 1800s by distinguished liver pathologists, such as Addison, Rokitansky, Pepper, and Bartolow, until the years 1958–1971 the debate, with the contributions by Zelman and Thaler, mainly focused on such hepato-histopathological changes being indistinguishable irrespective of the inciting triggers: alcohol, obesity, and diabetes [4]. From the perspective of clinical nosography, this finding eventually led to coining the innovative definitions of “nonalcoholic steatohepatitis (NASH)” and “nonalcoholic fatty liver disease (NAFLD)” in the 1980s. “Nonalcoholic” is a composite word that, at least in the spelling used by Ludwig et al., and by Schaffner and Thaler; does not require any hyphens [4] at variance with the use that is sometimes encountered in contemporary literature (“non-alcoholic”).
To convert a “negative” definition (i.e., nonalcoholic) into a positive diagnosis highlighting the pathogenic (dysmetabolic) origin, while also avoiding stigmatizing alcohol consumption, a panel of international experts have proposed renaming NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD). Although endorsed to an unprecedented extent [5], MAFLD retains some elements of ambiguity and amplifies NAFLD’s heterogeneity, therefore creating major implications for research arena and clinical practice [6, 7].
Compared to the history of NAFLD briefly summarized above, the notions that NAFLD and NAFLD-related metabolic conditions could also be a risk factor for the development of primary liver cancer (PLC) in a limited proportion of individuals are more recent (Table 1) [8–13].
Earliest case reports and systematic published studies supporting the notions that “cryptogenic cirrhosis” equals “NAFLD-cirrhosis” in most cases; and that PLCs, hepatocellular carcinoma (HCC), and cholangiocarcinoma (CC) may indeed occur as a complication of NAFLD and related metabolic disorders
| Author, year [Ref] | Method | Findings | Conclusion |
|---|---|---|---|
| Caldwell et al. [8], 1999 | Findings from 70 consecutive CryptoCir probands reassessed for alcohol consumption, evaluated by the IAH score and for risks of viral hepatitis and NASH were compared to 50 consecutive NASH patients, 39 nonalcoholic patients with HCV cirrhosis, and 33 with cirrhosis owing to PBC | The CryptoCir group, that comprised 70% of women in their 60s, had a prevalence of T2D and obesity significantly higher than those with cirrhosis owing to either PBC or HCV. Conversely, the prevalence of obesity and T2D was similar to the NASH patients who were, on average, 10 years younger | Data suggest that NASH is an under-recognized cause in many CryptoCir patients, most of whom are older ladies with T2D and obesity |
| Zen et al. [9], 2001 | A 58-year-old lady who did not drink alcohol and was negative for all serological markers of HBV and HCV infection received a diagnosis of T2D, treated with insulin therapy. Four years later, liver biopsy (performed to investigate altered liver tests) was compatible with NASH | In the follow-up, the patient was re-biopsied for multifocal hepatopathy and 3 out of the 4 liver nodules were moderately differentiated HCC (10 years after the diagnosis of NASH), well-differentiated HCC (11 years later) and dysplastic nodule (11 years later) | This case study is the first proof-of-concept published anecdotal evidence that HCC may develop as a late NASH complication |
| Bugianesi et al. [10], 2002 | Twenty-three out of forty-four CryptoCir patients retrospectively identified among 641 cirrhosis-associated HCCs were actively followed up and compared to viral- and alcohol-associated HCC | The prevalence of obesity and T2D was significantly higher in patients with CC, who also had higher glucose, cholesterol, triglyceridemia, and IR; aminotransferase levels were lower. Iron status and prevalence of mutations in the HFE gene did not differ. At LRA hypertriglyceridemia, T2D, and normal aminotransferases were independently associated with HCC arising in CryptoCir | Characteristics compatible with NASH are more common in HCC arising in patients with CryptoCir than in age- and sex-matched HCC cases owing to viral or alcoholic etiology suggesting that HCC may occur as a late complication of NASH-cirrhosis |
| Marrero et al. [11], 2002 | Among 105 consecutive HCC patients, the most common etiologies of CLD were HCV and CC (51% and 29%, respectively). Half of the CryptoCir patients exhibited either histologic or clinical features associated with NAFLD. In 50% of cases, HCC was diagnosed during surveillance (group I); in the remaining patients, HCC was symptomatic (group II) | Group I patients had smaller cancers (P = 0.01), were more likely to be eligible for surgical treatment (P = 0.005) and had higher survival rates than group II patients (P = 0.001). CC patients were less likely to have been submitted to surveillance for HCC and, accordingly, were diagnosed larger tumor burdens | HCV and CryptoCir were the most common etiologies of HCC. NAFLD accounted for at least 13% of the cases |
| Michelini et al. [12], 2007 | The authors speculated that IR might be a risk factor also for CC, like other cancer types | To illustrate their speculation, these authors reported on 3 ICC cases that exhibited clinical manifestations of IR such as obesity, dyslipidemia, and NAFLD as the common grounds predisposing to CC | This case study is the first proof-of-concept published anecdotal evidence that conditions belonging to the domain of MetS may be the only biologically plausible risk factor in a fraction of CC cases |
| Welzel et al. [13], 2007 | The SEERM database was utilized to evaluate comorbid conditions of 535 ICC patients, 549 ECC patients, and 102,782 cancer-free controls. Data were analyzed with LRA | Further to established risk factors, several endocrine and metabolic comorbidities were strongly associated with both ECC and ICC: cholelithiasis (P < 0.001), diabetes (P < 0.001), thyrotoxicosis (ECC, P = 0.006; ICC, P = 0.04). Conditions associated with ICC alone included obesity (P = 0.01) and NAFLD (P = 0.02) | This pioneering study identified several novel metabolic risk factors for ECC and ICC |
CLD: chronic liver disease; CryptoCir: cryptogenic cirrhosis; ECC: extrahepatic cholangiocarcinoma; HBV: hepatitis B virus; HCV: hepatitis C virus; IAH: International Autoimmune Hepatitis; ICC: intrahepatic cholangiocarcinoma; IR: insulin resistance; LRA: logistic regression analysis; MetS: metabolic syndrome; PBC: primary biliary cholangitis; SEERM: Surveillance, Epidemiology and End Results-Medicare; T2D: type 2 diabetes
While it is logical and somewhat foreseeable that PLC may eventually develop in some individuals with NAFLD, more recent observations substantiate the probably unexpected theory that, further to PLCs, NAFLD might also be a precursor of some extra-hepatic cancer types. This invited editorial will shortly summarize history, mechanisms, and implications of this intriguing association.
In 2014, evidence was published that NAFLD patients (particularly those with NASH) were exposed to a strong risk of developing colorectal neoplasms, in addition to other extra-hepatic outcomes (such as cardiovascular disease, T2D, and chronic kidney disease) [14, 15]. Five years later, a seminal study by Allen et al. [16], pointed out that this risk was NAFLD-related and independent of obesity. This is noteworthy given that obesity had historically been deemed responsible for increased mortality rates owing to a gamut of cancers of potentially metabolic origin, notably including cancers of the colon and rectum, esophagus, liver, gallbladder, pancreas, kidney; non-Hodgkin’s lymphoma and multiple myeloma (in either sex); stomach and prostate (in men); genital tract (in women) [17].
Over time, a consistent body of accumulating epidemiological studies have fully supported the notion that, collectively, the “metabolic fatty liver syndromes (MFLS)”, namely both NAFLD and MAFLD, are indeed associated with a variety of extra-hepatic cancers. For example, in 2021, Mantovani et al. [18] conducted a meta-analytical review of 10 published observational studies globally comprising 182,202 adults, approximately one quarter of whom had NAFLD, diagnosed with either imaging techniques or International Classification of Diseases codes. During a 5.8-year median follow-up, 8,485 incident extrahepatic cancers were registered, conferring to NAFLD an excess risk ranging from 1.5-fold to 2-fold of developing gastro-esophageal, pancreatic, and colorectal cancers and a slightly lower excess risk (ranging from 1.2-fold to 1.5-fold) of incident cancers of lung, breast, female genital tract and the urinary system. Interestingly, all the estimates of risks were independent of potential confounding factors (such as age, sex, smoking, obesity, and diabetes). Among the strengths of this study, the overall heterogeneity of pooled analyses was low. Moreover, findings were unaffected by sensitivity analyses and no significant publication bias was revealed by funnel plot analysis. Among the weaknesses of the published studies included in this metanalysis, there was no biopsy-proven diagnosis of NAFLD in any of them. A subsequent meta-analytic study published by Thomas et al. [19] by comparing 64 studies for analysis of incident HCC (625,984 patients) and extrahepatic cancer (41,027), found that extra-hepatic cancers were over 8-fold more common than HCC in NAFLD and were not associated with the stage of advanced hepatic fibrosis or cirrhosis. This is in striking contrast with HCC where the most advanced stages of liver fibrosis are associated with an increasing risk of disease [20, 21]. Finally, an umbrella meta-analysis by Yi et al. [22] based on the scrutiny of 39 published meta-analyses results found that individuals with NAFLD exhibited an increased risk of the following extra-hepatic cancers: thyroid, extra-hepatic and intra-hepatic cholangiocarcinoma, pancreatic, esophago-gastric, urinary tract, breast, and lung. The entity of this risk, however, ranged, based on various cancer types, from hazard ratio (HR) 1.25 for lung cancer to HR 2.63 for thyroid cancer. No significant association was found linking NAFLD with the following cancer types: blood, female genital tract, and prostate. Compared to the well-consolidated body of published studies demonstrating the association of NAFLD with extra-hepatic cancer, the association of MAFLD with extra-hepatic cancer appears to be at its dawn. However, as awaited, some recent studies have documented an increased risk of colon cancer [23] and excess mortality of MAFLD owing to malignancy [24].
While it is readily intuitive to associate those profound cellular, vascular, histological, and immunological perturbations as well as the metabolic remodeling that collectively occur in NAFLD, and particularly in fibrosing NASH and NASH-cirrhosis, with the development of NASH-HCC also in non-cirrhotic livers [25–27] how can NAFLD be postulated to mechanistically predispose to incident extra-hepatic cancers? In this regard, it is important to remember that NAFLD is a systemic disorder [28] and even more so is, by definition, MAFLD [24]. On these grounds, one can hypothesize that either the MFLS and extra-hepatic cancer share a common ancestor, for example, a pro-inflammatory systemic milieu associated with metabolic derangements; or that the MFLS are directly involved in the various phases of initiation, development, and progression of cancer at various anatomic sites. The anatomical location of such cancer types, the gastrointestinal tract being more exposed than the genito-urinary tract, may probably offer a clue to understanding these poorly defined pathomechanisms. Excellent reviews have specifically been devoted to exploring this fascinating topic [29, 30]. Additionally, it is conceivable that NAFLD heterogeneity accounts for seemingly conflicting findings published by different investigators. For example, “lean NAFLD” compared to “obese NAFLD” and MAFLD probably exhibits different patho-mechanisms given that expanded adipose tissue per se acts as an endocrine organ favoring the development and progression of hormone-sensitive cancers. Other major modifiers of extra-hepatic cancer risk likely include the stage of hepatic fibrosis [31] and the estimated cardiovascular risk [32]. The Figure 1 illustrates the best-characterized modulators of the risk of extra-hepatic cancers in those with the MFLS.
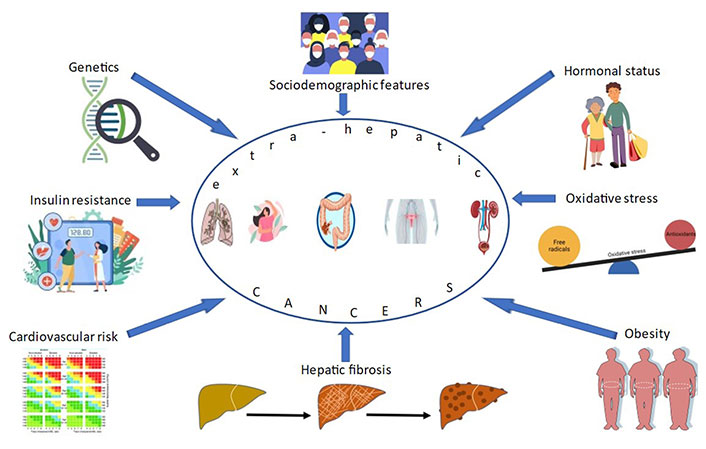
Principal modifiers and putative pathomechanisms involved in the association of NAFLD and MAFLD with extra-hepatic cancers. This illustration, based on published studies [18, 19, 22–24, 29–32], highlights the knowns of this topic. However, our understanding of this complex scenario is far from complete
Supplementary disease cofactors [33] and particularly lifestyle habits may also participate in fine-tuning the odds of extra-hepatic cancers among those with MFLS. These risk modifiers include epigenetics, alcohol consumption, eating habits (scarcity of fiber and an excess of saturated fats), and sedentary behavior which closely interact with viral infections, drugs, and immunological factors in predisposing to/protecting from extra-hepatic cancers.
A detailed analysis of molecular mechanisms that can lead to extra-hepatic cancers is out of the scope of this editorial and has been covered elsewhere [29, 30]. In short, while the chief mechanisms may vary according to the variable extra-hepatic cancer site, they can generally be categorized into four effects. A) Insulin resistance that will induce proliferative and anti-apoptotic effects via increased insulin growth factor-1 factor axis; B) dysfunctional adipose tissue which, via nuclear factor kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), mitogen-activated protein kinase (MAPK) decreased adiponectin, increased leptin and resistin, carries out anti-apoptotic effects, enhanced proliferation and angiogenesis, invasiveness, and increased motility; C) inflammation, which facilitates cell proliferation, neo-angiogenesis, de-differentiation, and metastasis development via interleukin-6 (IL-6)/Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) and IL-6/MAPK TNF-α/Wnt/β-catenin; and D) gut dysbiosis which further triggers inflammation and exerts anti-apoptotic effects via microorganism-associated molecular patterns (MAMPs)/ toll-like receptors (TLRs) inflammasome-derived IL-18 [34, 35].
However, given that the picture is incomplete, additional prospective studies will have to better define the pathomechanics of this scenario. Such as extensively discussed elsewhere [36–38], an improved understanding of this will lead to personalized medicine approaches in prevention, diagnosis, management, prognostication, and individualized follow-up protocols.
Abbreviations
| HCC: | hepatocellular carcinoma |
| IL-6: | interleukin-6 |
| MAFLD: | metabolic dysfunction-associated fatty liver disease |
| MFLS: | metabolic fatty liver syndromes |
| NAFLD: | nonalcoholic fatty liver disease |
| NASH: | nonalcoholic steatohepatitis |
| PLC: | primary liver cancer |
| T2D: | type 2 diabetes |
Declarations
Author contributions
The author contributed solely to the work.
Conflicts of interest
The author declares that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.