Abstract
Aim:
This study aimed to develop and evaluate an in-house enzyme-linked immunosorbent assay (ELISA) based on autochthonous antigens to detect immunoglobulin G (IgG) antibodies against Helicobacter pylori (H. pylori) in adult sera.
Methods:
Whole-cell antigens from three genetically characterized clinical isolates of H. pylori were mixed and used as coating antigens. This assay was validated with a panel of human sera samples of H. pylori seropositive and seronegative patients. Likewise, sera samples from patients with uninvestigated dyspepsia, who were also evaluated by invasive and noninvasive tests (i.e., histopathology, rapid urease test, and stool antigen test), blood donors and patients with confirmed viral and parasitic diseases were also collected. The IgG response against H. pylori was detected by the in-house assay using the commercial ELISA IBL (Germany), as a reference test. Statistical analysis was performed with GraphPad Prism version 5.01.
Results:
The in-house ELISA showed high repeatability and reproducibility. Sensitivity was 91.1%; 95% confidence interval (CI): 87.2–94.0, specificity was 94.8% (95% CI: 85.0–94.8), and accuracy was 91.6% (95% CI: 88.5–94.6). The in-house ELISA showed an excellent area under the curve (0.96; 95% CI: 0.93–0.98) and a better IgG detection by the inverse cumulative distribution. The frequency of seropositivity in patients with dyspepsia (76.0%) was significantly higher (P < 0.05) than in healthy individuals (57.7%) and patients with other infectious diseases resembling H. pylori infection symptoms (54.4%). The H. pylori seroprevalence was estimated to be 62.7%. A good correlation was found between IgG seropositivity and H. pylori infection diagnosed by histopathology, rapid urease test, and stool antigen test in Cuban adults with dyspepsia.
Conclusions:
The in-house ELISA demonstrated good diagnostic accuracy and potential usefulness for estimating H. pylori exposure in the adult population, henceforward, this method could be used as an alternative for H. pylori diagnosis in the Cuban setting.
Keywords
Diagnostic accuracy, Helicobacter pylori , antibody detection, ELISAIntroduction
Helicobacter pylori (H. pylori) infection is associated with progressive gastric diseases, such as gastric and duodenal ulceration, chronic active gastritis, gastric lymphomas, and gastric cancer, making diagnosis and treatment of this microorganism essential [1–3].
In Cuba, the rates of H. pylori infection in adults are between 50–59% [4, 5], similar to developing countries (50.8%) [6] comparable with prevalence found in Latin America and the Caribbean region (59.3%) [7]. Due to its high frequency, early detection of H. pylori infection is very essential to prevent gastroduodenal complications as gastric ulcer perforation, digestive bleeding, and gastric cancer. Different diagnostic tests are available to detect this microorganism using invasive [e.g., histopathology, culture, and rapid urease test (RUT) from biopsy] and noninvasive [e.g., stool antigen test (SAT), urea breath test (UBT), and serology] techniques [8]. Recent clinical guidelines support the “test and treat” strategy using SAT and UBT, for uncomplicated dyspepsia in patients younger than 50 years [9, 10]. Accordingly, the choice of a diagnostic test depends on the prevalence of H. pylori, the local incidence of age-related gastric cancer, the advantages and disadvantages of every method, the clinical condition of the patient, and the test availability [11].
Specific H. pylori antibodies [immunoglobulin G (IgG), M (IgM), and A (IgA)] produced by local and systemic immune responses in infected individuals can be detected by rapid serological assays [12, 13]. The use of H. pylori IgG levels to differentiate recent from past infection is controversial [14, 15]. However, the anti-H. pylori-IgG antibody levels correlate with increased diagnostic accuracy in a population with a high prevalence of H. pylori infection (greater than 30%) and in the absence of other noninvasive tests [16].
Enzyme-linked immunosorbent assay (ELISA) tests commercially available with sensitivity and specificity in the range of 75–95% and 79–90% respectively, are used routinely in clinical laboratories as well as for epidemiological studies [8, 12]. In accordance with evidence-based diagnostic criteria [17], validation of serological systems in each population is recommended before their use, as antigenic variations with the local H. pylori strains may affect the accuracy of ELISA system [18, 19].
Several commercial ELISA tests are available [14–16]; however, they are not produced locally in Cuba and must be purchased from other countries. Thus, the routine use of ELISA tests for the diagnosis of H. pylori is not feasible due to high costs and long waiting times. Therefore, this study evaluated the diagnostic accuracy and clinical relevance of a novel ELISA system (H. pylori autochthonous antigen ELISA) with the aim of providing a diagnostic tool to detect serological responses against H. pylori infection in Cuban patients.
Materials and methods
Design and size study
This is a cross-sectional diagnostic study [20] conducted for one year (July 2016 to June 2017) for assessing H. pylori-induced antibody responses using serum samples from Cuban adults following the main methodological steps.
First, the development of an indirect ELISA system using whole-cell sonicated from Cuban H. pylori strains as coating antigen [21]. The test was validated using a panel of human sera samples of seropositive H. pylori-infected patients (cases, n = 23) and seronegative H. pylori-noninfected patients (controls, n = 23) obtained during a study carried out on Cuban adults with dyspeptic symptoms, whose H. pylori status was already established using culture, histopathology and RUT [22] (Table S1).
Next, this immunoassay was evaluated with serum samples collected from three groups: 1) patients with dyspepsia exposed at the same time point to the H. pylori detection by the three standards diagnostic tests (i.e., histopathology, RUT, and SAT); 2) blood donor healthy individuals; and 3) patients with confirmed viral and parasitic diseases resembling H. pylori infection symptoms. A commercial ELISA kit for detection of IgG antibodies against H. pylori previously validated [23] was used as reference test. A minimum sample size of 302 participants was deemed sufficient for this estimation based on national and regional published expected prevalence of 59% [4, 7, 24] with a precision of 5% and a type I error of 5%.
This study is in agreement with the update version of STROBE Statement Guidelines for reporting observational studies [25]. The STROBE checklist for cross-sectional studies is included in Table S2.
Ethics approvals
The study was performed in compliance with ethical principles outlined in the Declaration of Helsinki and was consistent with Good Clinical Practice guidelines. The patients and healthy individuals were enrolled in the study after informed written consent was obtained. Biological samples from each participant were obtained and handled following the protocol approved by Ethical Committee of the “Pedro Kourí” Institute of Tropical Medicine (IPK) (Ethic number: 2015/41).
Patients, healthy individuals, and setting
Patients with uninvestigated dyspepsia, aged 18 to 80 years attending at Primary Health Institution Eduardo Díaz, Artemisa were considered potentially eligible. Those with upper abdominal symptoms and an indication for routine upper gastroscopy were included. Non-eligible criteria comprised those patients that used antibiotics, proton pump inhibitors, non-steroidal anti-inflammatory drugs, and bismuth salts within 3–4 weeks before enrollment or who were suffering from malignant diseases. All eligible patients were investigated at the same time point to the H. pylori detection standards tests: histopathology, RUT, and SAT.
During the investigation period, healthy individuals were selected from volunteer blood donors at the Artemisa’s Blood Donation Bank with interest in participating in the study.
Collection and processing of specimens
Venous blood (5 mL) was collected from patients and blood donor individuals participating in the study. The serum was separated by centrifugation (3,000 rpm for 15 min) using a refrigerated centrifuge model Jouan KR 22i (Jouan, France), aliquoted, and immediately stored at –20°C until analyzed for serological tests. Poor-quality samples were excluded from this study.
Serum samples (57) donated by the Department of Microbiology, Center for Research, Diagnosis and Reference of IPK were also included to evaluate the in-house ELISA cross-reactivity. These samples were obtained from seropositive patients with viruses or parasites-infection (i.e., cytomegalovirus, hepatitis A and E, toxoplasmosis, and toxocariasis).
Four biopsy samples (areas around the pyloric antrum and gastric corpus) were taken of each eligible patient using an Olympus fibroendoscopy [4]. The procedure under topical anesthesia after a minimum of eight hours of fasting was conducted by experienced gastroenterologists. Two biopsy samples were taken for histopathology and two additional biopsy samples were taken for RUT. Additionally, a fresh stool sample was also collected and stored at −20°C until analyzed. Frozen samples were immediately defrosted and tested to avoid possible degradation of proteins.
Immunoassays
In-house ELISA development
Three isolates representative of H. pylori species colonizing Cuban patients, classified according to genotypes in 139A (vacAs1m2/cagA-/iceA2), 227A (vacAs1m2/cagA+/iceA1) and 146 (vacAs1m1/cagA-/iceA2) [26] were used to obtain the H. pylori whole-cell sonicated antigens (coating antigen).
In summary, pure bacterial cells were centrifugated (3,000 rpm for 30 min at 4°C) and the precipitate washed with phosphate buffer saline (PBS) pH = 7.3, was resuspended in a PBS solution containing 50 μg/mL DNase and a protease inhibitor cocktail for bacterial cell extracts (1:200 dilution) (Thermo Fisher Scientific Inc., Illinois, USA). The antigen extract was incubated (4°C for 10 min) and lysozyme (0.2 mg/mL) was then added. Later, the lysate solution was submitted to ultrasonication (10 cycles/24 Hz, 10 s “on” with an interval of 10 s “off”) using Ultrasonic Disintegrator Soniprep 150 instrument (MSE, London, UK) and the cellular detritus was eliminated using Optima™ L-90K ultracentrifuge (Beckman Coulter Inc., California, USA) at 13,000 rpm, 4°C for 20 min. Supernatants were collected and conserved at −20°C until later use. Protein concentration was determined by standard BCA Protein Assay Kit (ThermoFisher Scientific Inc., Illinois, USA) with bovine serum albumin (BSA) as standard. Whole-cell sonicated antigens were verified using 10% polyacrylamide gel with sodium dodecyl sulphate (SDS-PAGE) [21].
In-house ELISA optimization
The coating antigen was an equimolar mixture of H. pylori whole-cell sonicated antigens. The system was optimized using different antigen concentrations (1−20 µg/mL), primary antibody dilution (1:200, 1:400, 1:800, 1:1,000), and conjugated secondary antibody dilution (1:5,000, 1:10,000, 1:20,000). A 96-well Nunc Maxisorp plate (Invitrogen, Thermo Fisher Scientific Inc., MA, USA) was coated (100 μL) with optimal concentration of antigen in coating buffer pH 9.5 (50 mmol Na2CO3-NaHCO3) overnight at 4°C and washed three times with PBS containing 0.05% Tween 20 (PBS-Tween 20). After blocking ELISA plates with 1% BSA in PBS (150 μL) for 1 h at 37°C with gentle shaking, the plates were washed with PBS-Tween 20, and optimal dilution of human serum samples in PBS containing 2% fetal bovine serum (PBS-FBS) were reacted for 1 h at 37°C. The best dilution of goat anti-human IgG antibody (whole molecule) conjugated with peroxidase (Sigma-Aldrich, Germany) in PBS-FBS was added for 1 h at 37°C to each reacting well after washing the plates with PBS-Tween 20. The final detection was performed with ortho-phenylenediamine dihydrochloride substrate (10 mg) (Nacalai Tesque, Inc., Japan) and 2% H2O2 in 0.1 mol McIlvaine buffer pH 5.0. The plate was incubated at 37°C in the dark for 10 min and the enzymatic reaction was stopped using 12.5% H2SO4. The absorbance (OD) reading was carried out using the ELISA Microplate Reader model MRX Revelation (Dynex Technologies, Inc., Virginia, USA) at a wavelength of 490 nm, using 630 nm as a reference.
Cut-off value calculation
The cut-off index value (CO) was calculated using a negative serum control samples set and the following formula: CO = mean OD490 + two standard deviations. The positive result was considered if the relation OD490 sample/CO ≥ 2.0 and negative if OD490 sample/CO < 2.0.
Reference ELISA test
A commercial sandwich immunoassay for the qualitative and quantitative determination of IgG antibodies against in human serum, H. pylori IgG ELISA (IBL International, Hamburg, Germany), was performed according to manufacturer’s instructions. The optical density was determined using a microplate reader at 450 nm. CO values of > 1.2 were considered positive, values < 0.8 were considered negative, and values from 0.8 to 1.2 were considered indeterminate.
Immunoblot
Divergences in the serological results were elucidated using immunoblot technique according to reported by Feliciano-Sarmiento et al., 2018 [21]. In brief, SDS-PAGEs were transferred onto membranes (Protran® BA 83, Schleicher & Schleicher, Germany) using 50 V at 4°C overnight. Membranes were blocked with 0.5% skim milk powder in 0.02% PBS-Tween 20 (antibody diluent) and incubated at room temperature for 1 h. Next, human sera diluted with antibody diluent (1:25) was added and the membranes were incubated at 4°C overnight with soft agitation using a MX-RL-E Analog Rotator instrument (Scilogex, Connecticut, USA). Blots were washed and incubated with peroxidase-conjugated secondary anti-human IgG antibodies, dilution 1:6,000 (Sigma-Aldrich, Germany) at room temperature for 1 h. Finally, the immunoreactive bands were revealed using 3,3’ diaminobenzidine tetrahydrochloride as a substrate-staining chromogen (DAB, Sigma Aldrich, MO, USA) diluted with 0.03% H2O2 in PBS. A molecular weight protein marker (10–260 kDa Color BurstTM, Sigma Aldrich, MO, USA) was used in each run. The membranes were scanned using the GS-800TM Calibrated Imaging Densitometer (BioRad Laboratories Inc., USA). The intensity of molecular weight and the visualized bands was calculated by the informatics program Quantity One® version 4.6.7 (BioRad Laboratories Inc., USA).
Standard tests for H. pylori status definition
The patients were classified as H. pylori infected patients if at least two of the tests (histopathology, RUT, and SAT) were positives, otherwise H. pylori status was considered as negative.
Histopathology analyses were performed with biopsies fixed in 10% formalin solution, cut into slides, and stained with specific stain hematoxylin and eosin and Giemsa [8]. Two pathologists examined each glass slide blindly for the presence/absence of H. pylori. Two biopsy specimens were inoculated into a vial containing 0.3 mL of urease test broth (Stuart’s transport medium, BBL, Cockeysville, MD, USA) at room temperature. A positive test was considered by the color change from the original yellowish to fuchsia [27]. The sandwich ELISA for H. pylori detection in stool (Standard Diagnostics, Inc., Korea) was performed according to manufacturer’s instructions [28].
Statistical analysis
All the experimental data were entered into Microsoft Excel spreadsheets (Microsoft Corp., Redmond, WA, USA, 2010) and analyzed using GraphPad Prism software version 5.01 for Windows, GraphPad Software, San Diego, California, USA. Absolute and relative frequencies, mean, and standard deviations were determined for each assay performed. ELISA’s coefficient of variation (CV) was calculated by repeated analysis for reproducibility intra-assay (duplicate within a single run of a plate) and inter-assay (quadruplicate on three different days by two analysts) using H. pylori-seropositive and seronegative samples set (Table S3). The % CV values less than 15 for inter-assay and less than 10 for intra-assay are generally acceptable.
Quantitative data was analyzed by the Mann-Whitney U sum rank for groups (patients vs. controls; patients vs. healthy individuals) and the differences between variables were compared by the 2way ANOVA with Bonferroni posttest. A P value ≤ 0.05 was considered significative.
The DeLong’s test was used to evaluate the area under the curve (AUC) of the in-house ELISA’s ROC curve. The accuracy of the H. pylori autochthonous-ELISA was evaluated by determining the sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, diagnostic accuracy with 95% confidence interval (95% CI), and the Youden index using EpiDat Software version 3.1. The agreement between index and reference tests was determined by Cohen’s kappa (κ). These values were interpreted as follows: into slight (0.0–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and almost perfect (0.80–1.0) agreements [29].
Results
Optimization and accuracy of H. pylori autochthonous-ELISA
The standardization of the in-house ELISA with human serum samples panel resulted in optimal concentration for the coating antigen (10 μg/mL) and dilution rates for the primary antibody (dilution 1:1,000) and secondary antibody conjugated with horse-radish peroxidase (1:10,000). The CV value for intra-assay was 9.4% and for inter-assay 11.2%, showing high reproducibility and precision. No significant difference in CVs obtained was noted by analysts at repeated tests (days 1–3; P = 0.6110).
Mean OD values of the IgG antibodies from H. pylori positive patients obtained by the in-house ELISA test, were significantly higher than those of the H. pylori negative patients (P < 0.001) (Figure 1).
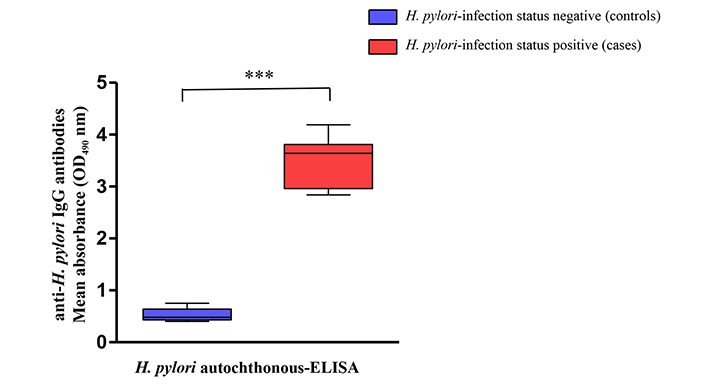
Recognition of anti-H. pylori (immunoglobulin G) IgG antibodies level using a panel of samples from H. pylori-negative controls (boxes in blue) and H. pylori-positive cases (boxes in red). *** The mean absorbance values (OD490) obtained with H. pylori autochthonous-enzyme-linked immunosorbent assay (ELISA) showed difference statistically significant (P < 0.001) in the anti-H. pylori IgG levels between cases and control groups
The precision parameters for H. pylori autochthonous ELISA demonstrated a sensitivity, specificity, and, accuracy of 91.1%, 95% CI: 87.2–94.0; 94.8%, 95% CI: 85.0–94.8; and 91.6%, 95% CI: 88.5–94.6, respectively, with a positive predictive value of 97.2%, 95% CI: 94.4–98.7 and a negative predictive value of 84.1%, 95% CI: 77.4–89.1. A Youden index was 0.9 and the values of the positive likelihood ratio (17.45, 95% CI: 8.47–35.92) and negative likelihood ratio (0.09, 95% CI: 0.06–0.14). The agreement between both ELISA systems according to Cohen’s kappa calculation (κ = 0.83, 95% CI: 0.78–0.89) was considered near perfect. The optimal AUC level obtained with the in-house ELISA (0.96, 95% CI: 0.93–0.98) was as good as the AUC for H. pylori reference-ELISA (0.97, 95% CI: 0.96–0.99). No statistical differences were found between the AUC values (P = 0.2377).
Diagnostic performance of autochthonous antigen-based ELISA for the detection of H. pylori
In this study, a total of 405 sera samples were obtained from 121 patients with dyspeptic symptoms and suspect of H. pylori-infection, 57 from seropositive patients to other digestive infections caused by virus or parasites and 227 from healthy individuals.
IgG detection by both tests, the autochthonous antigen ELISA and the commercial reference ELISA, was positive in 68.5% (122/178) of the patients and 55.1% (125/227) of the healthy controls. In 28.7% (51/178) of the patients and 33.5% (76/227) of the healthy individuals, the IgG test was negative. The analysis of divergent data (31 patients) showed a better agreement (0.63, 95% CI: 0.36 to 0.91) between in-house ELISA and immunoblot for the detection of H. pylori seropositive (7/12) and H. pylori seronegative (24/19) subjects. Poor agreement (0.31, 95% CI: 0.09 to 0.53) was found between H. pylori reference ELISA and immunoblot for seropositive (24/12) and seronegative (7/19) subjects.
IgG antibody response showed higher seropositivity rates in patients with dyspepsia suspected of H. pylori infection than in healthy individuals and patients infected with viral and parasitic diseases (Table 1). In addition, significant differences in antibody responses were found between the patients with dyspepsia and healthy individuals (P < 0.001), as well as this group concerning symptomatic patients with other unrelated infections (P < 0.05) for both ELISA tests. No differences were found among patients confirmed with viruses and parasites and the healthy adult population (P = 0.7615). The seroprevalence was 62.7% (95% CI: 58.01–67.43) using the in-house ELISA.
Seroprevalence of IgG antibody positive and negative–responses in patients with uninvestigated dyspepsia, blood donor healthy individuals, and patients confirmed with viral and parasitic diseases using H. pylori autochthonous-ELISA in comparison with H. pylori reference-ELISA
| Subjects | Serology result by ELISA n (%) | κ (95% CI) | |||
|---|---|---|---|---|---|
| H. pylori autochthonous | H. pylori reference | ||||
| IgG+ | IgG– | IgG+ | IgG– | ||
| Patients with uninvestigated dyspepsia (n = 121) | 92 (76.0) | 29 (24.0) | 93 (76.9) | 28 (23.1) | 0.93(0.81–1.01) |
| Healthy individuals (n = 227) | 131 (57.7) | 96 (42.3) | 145 (63.9) | 82 (36.1) | 0.76(0.67–0.85) |
| Patients confirmed with viruses and parasites (n = 57) | 31 (54.4) | 26 (45.6) | 33 (57.9) | 24 (42.1) | 0.93(0.83–1.03) |
| Total subjects (n = 405) | 254 (62.7) | 151 (37.3) | 271 (66.9) | 134 (33.1) | - |
-: not applicable
The results obtained by autochthonous antigen ELISA showed a good correlation with the reference ELISA (r = 0.97 for all samples). However, when the Cuban population samples were tested, the inverse cumulative frequency distribution curves showed a difference in the frequency patterns derived from the OD values. These values were significantly higher (P < 0.001) for the in-house ELISA compared to the commercial ELISA (Figure 2A). This assay also showed significant differences in IgG OD values from patient sera compared to those from healthy individuals (P < 0.001; Figure 2B).
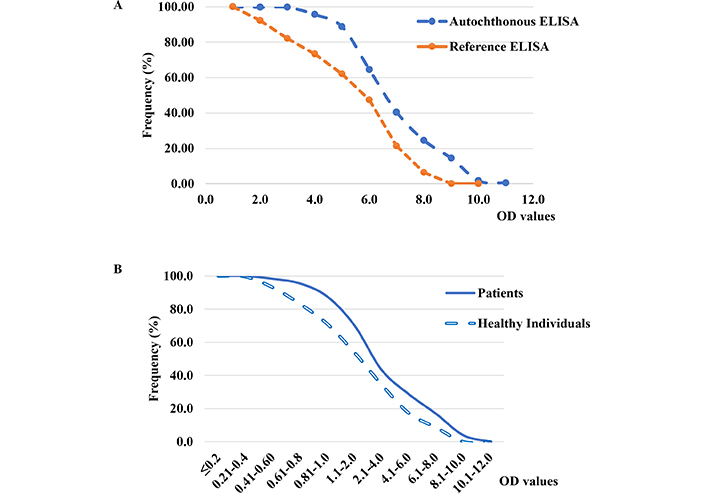
Inverse cumulative frequency distribution curves of absorbance (OD) values of anti-H. pylori immunoglobulin G (IgG) antibodies obtained from A) sera of Cuban adult population (n = 405) evaluated by autochthonous ELISA using reference commercial enzyme-linked immunosorbent assay (ELISA), and B) sera of dyspeptic patients (n = 121) and healthy individuals (n = 227) in autochthonous ELISA. The difference between the frequency distribution of IgG OD values was statistically significant (P = < 0.001)
Patients with dyspepsia were classified as H. pylori infected (76) and H. pylori noninfected (45) on the basis of case status as defined by standard tests. The rates of seropositivity and seronegativity for these patients were analyzed in the Figure 3. Significant differences between positive and negative serological responses in patients infected with H. pylori (P < 0.01) were found. The seropositivity response was slightly higher in patients SAT-positive (98.6%), followed by those having RUT positive (95.9%) and patients with histopathology positive (85.9%) to H. pylori. We observed differences in serological response (P < 0.05) in non-infected patients, who also exhibits a negative result for histopathology, RUT, and SAT tests. The kappa index was considered fair when serology data was cross-checked with histopathology (0.30), while RUT and SAT tests had moderate and substantial values (0.54 and 0.61), respectively. The better correlation was found between in-house ELISA and SAT tests.
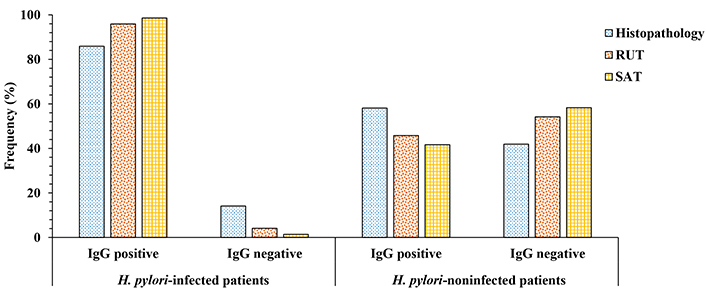
Comparison of positive and negative serological responses (in %) obtained in infected and noninfected patients with H. pylori using the autochthonous antigen ELISA. H. pylori status was defined by histopathology, rapid urease test (RUT) and stool antigen test (SAT). The difference of anti-H. pylori IgG response between H. pylori-infected patients was statistically significant (P < 0.01). A significant difference was also found in non-infected patients (P < 0.05). IgG: immunoglobulin G
Discussion
During an H. pylori infection, IgG antibodies may appear about three weeks after the onset of infection, persist throughout the infection and, return to normal values in about a year. Taking it into account, different commercial serological tests for H. pylori IgG antibody detection are available and routinely used in clinical laboratories. The IgG method is limited by its inability to discriminate between current and past exposure [8]. Therefore, to maintain the international consensus goal of testing and treating strategy for H. pylori infection, a positive serologic result requires subsequent verification by a test that can identify active infection [9, 10]. However, IgG detection has inestimable value in developing countries with high prevalence of infection, limited access to diagnostic tools and where there is genetic diversity among circulating H. pylori strains [30, 31].
ELISA technique is widely recognized for its ease of use, affordability, and high sensitivity and specificity in detecting antibodies [13–16, 22]. Recently, a serological study conducted at primary health care facilities in Cuba identified a commercial ELISA that was previously validated as an effective screening tool for diagnosing H. pylori infection in the community [32]. In addition, some studies suggest the use of a coating antigen obtained from local circulating strains based on the genetics and ethnicity of the population to increase the accuracy of H. pylori IgG detection by ELISA. [18, 19].
In the current study, the autochthonous antigen ELISA system exhibits a good performance for IgG antibodies detection. The results endorsed a good sensitivity and accuracy (91.1% and 91.6%, respectively), as well as positive and negative predictive values (97.2% and 84.1%, respectively), which are within the ranges reported in the international literature [19, 33]. The use of a coating antigen based on the local antigen mix covering different genotypes of Cuban strains may explain the high specificity in this assay. The AUC value indicates the predictive ability of the in-house ELISA to discriminate serological response between healthy individuals and H. pylori infected patients.
Other studies describe the development and optimization of the in-house ELISA systems based on local strains for screening purposes. A study conducted in Vietnam evaluating an autochthonous ELISA based on sonicated H. pylori antigen, previously absorbed with Campylobacter jejuni antigen to remove cross-reacting antibodies shows a specificity and sensitivity of 96.4% and 91%, respectively [18], similar to the current study.
In Pakistani patients, a whole-cell surface antigen ELISA (wsELISA) had sensitivity, specificity and accuracy of 93%, 100%, and 94%, respectively [34]. Another in-house ELISA (HpAfr-ELISA) using whole-cell antigens prepared from strains belonging to the main African genetic H. pylori populations [35], exhibited a good diagnostic accuracy (90.5% specificity; 97.6% sensitivity). The performance of the HpAfr-ELISA confirms its usefulness as a tool for the assessment of H. pylori infection in the African setting. Finally, in Brazil, an in-house ELISA using whole cell lysates of H. pylori as antigen (wlELISA) and a monoclonal conjugate has been evaluated in Brazilian adult sera [36], showing a specificity and sensitivity of 84.4% and 86.9%, respectively. Similar to our study, the coating antigen is prepared based on a pool of several genetically verified strains, however, the autochthonous ELISA shows values of sensitivity (91.1%), and specificity (94.3%) higher than the wlELISA. Diverse factors can affect the accuracy of serological tests, including the severity and staging of gastroduodenal diseases, different genetic backgrounds of H. pylori strains, and the gold standard methods used to compare ELISA tests [16, 18].
In the present study, the use of H. pylori whole-cell sonicated proteins as antigens allowed the identification of remarkable differences between seropositive and seronegative IgG-response in the evaluated populations. The results support that higher rates of IgG anti-H. pylori were found in symptomatic patients compared to healthy individuals and patients with other digestive diseases. However, the study had a number of limitations, that may explain the seropositivity that was found in these two population groups. These included the lack of information on symptoms associated with dyspepsia, whether there was a history of infection, and the lack of confirmation of H. pylori status with standard tests. Interestingly, serum anti-H. pylori IgG levels were higher in H. pylori-positive patients compared to H. pylori-negative patients. This suggests the potential usefulness of autochthonous antigen ELISA for the diagnosis of H. pylori infection in adults with dyspepsia and H. pylori-suspicion. Although there is controversy among physicians and researchers about the use of antibody levels against H. pylori as a marker of infection [8, 10, 11], we have found that the in-house ELISA method, based on antigens from local H. pylori strains, can increase the test accuracy in different populations when appropriate adjustments are made [18, 34]. In addition, the use of bacterial antigens from different genetic backgrounds makes the ELISA test more reliable for use in different populations. Therefore, IgG detected by autochthonous ELISA may be a suitable initial screening method for H. pylori infection in adults with uninvestigated dyspepsia. Furthermore, this diagnostic test may prove advantageous for the detection of H. pylori positive patients in scenarios where endoscopy is inaccessible or not justified and when the patient has specific clinical conditions such as complicated peptic ulcer, atrophic gastritis, stomach mucosa-assisted lymphoid tissue (MALT) lymphoma, and gastric carcinoma [12, 32, 33].
This assay is simple, sensitive and specific, provide rapid results, and use basic supplies and instruments that do not require high levels of automation to develop and use in national labs and other resource-limited settings.
In conclusion, the autochthonous antigen ELISA has promising potential for use in primary care and hospital settings as an adjunct to the algorithm for diagnosis of H. pylori in Cuban adults. In addition, it may also be useful in seroepidemiological surveys in order to improve the disease control.
Abbreviations
| AUC: | area under the curve |
| CI: | confidence interval |
| CO: | cut-off index value |
| CV: | coefficient of variation |
| ELISA: | enzyme-linked immunosorbent assay |
| H. pylori: | Helicobacter pylori |
| OD: | absorbance |
| PBS: | phosphate buffer saline |
| RUT: | rapid urease test |
| SAT: | stool antigen test |
Supplementary materials
The supplementary materials for this article are available at: https://www.explorationpub.com/uploads/Article/file/100547_sup_1.pdf
Declarations
Acknowledgments
We would like to acknowledge our technical assistants Tatiana Almaguer and Cecia Torres for their excellent technical support during the conduct of the study. The authors are grateful for the contribution of Mr. Mercedes Díaz and Mr. Ana Gloria Arteaga of the Polyclinic Eduardo Díaz Ortega endoscopy service for their demonstrative support during the study.
Author contributions
RC: Conceptualization, Investigation, Validation, Data curation, Writing—original draft. RF: Conceptualization, Methodology, Formal analysis, Writing—original draft, Writing—review & editing. SV: Methodology, Formal analysis, Supervision, Writing—review & editing. OF: Conceptualization, Formal analysis, Writing—review & editing. RM: Investigation, Validation, Writing—review & editing. AD and OG: Methodology, Formal analyses, Writing—review & editing. RL: Conceptualization, Methodology, Formal analysis, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The project was approved from the Ethical Review Committee of the “Pedro Kourí” Institute of Tropical Medicine (IPK), Cuba.
Consent to participate
Informed consent was obtained from all participants and institutions involved in the study.
Consent to publication
Not applicable.
Availability of data and materials
The data presented in this study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2024.