Affiliation:
1Internal and Metabolic Medicine, Department of Internal Medicine and Post-Acute Care, University of Modena and Reggio Emilia, 41124 Modena, Italy
Email: cristinafelicani@gmail.com
ORCID: https://orcid.org/0000-0002-3842-4701
Affiliation:
1Internal and Metabolic Medicine, Department of Internal Medicine and Post-Acute Care, University of Modena and Reggio Emilia, 41124 Modena, Italy
Affiliation:
1Internal and Metabolic Medicine, Department of Internal Medicine and Post-Acute Care, University of Modena and Reggio Emilia, 41124 Modena, Italy
ORCID: https://orcid.org/0000-0001-6217-2914
Affiliation:
1Internal and Metabolic Medicine, Department of Internal Medicine and Post-Acute Care, University of Modena and Reggio Emilia, 41124 Modena, Italy
Affiliation:
1Internal and Metabolic Medicine, Department of Internal Medicine and Post-Acute Care, University of Modena and Reggio Emilia, 41124 Modena, Italy
Affiliation:
2Department of Organ Deficiencies and Transplants, Anesthesia and Intensive Care Unit, S. Orsola-Malpighi Hospital, University of Bologna, 40138 Bologna, Italy
ORCID: https://orcid.org/0000-0002-4509-0586
Affiliation:
3IBD Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, S. Orsola Hospital, 40138 Bologna, Italy
Affiliation:
1Internal and Metabolic Medicine, Department of Internal Medicine and Post-Acute Care, University of Modena and Reggio Emilia, 41124 Modena, Italy
ORCID: https://orcid.org/0000-0002-4794-9809
Explor Dig Dis. 2024;3:241–261 DOI: https://doi.org/10.37349/edd.2024.00050
Received: January 29, 2024 Accepted: May 23, 2024 Published: July 05, 2024
Academic Editor: Agustín Albillos, University of Alcalá, Spain; Jose Carlos Fernandez-Checa, Institute of Biomedical Research of Barcelona (IIBB), CSIC, Spain
The article belongs to the special issue Advances in Hepato-gastroenterology: Diagnosis, Prognostication, and Disease Stratification
Transabdominal ultrasound is a valuable diagnostic approach for evaluating the gastrointestinal tract and related disorders. This dynamic examination provides real-time visualization of the digestive tube and surrounding structures, assessment of peristaltic movements, estimation of compressibility of intestinal loops, and recognition of painful spots requiring specific attention. Since ultrasound imaging is non-invasive, painless, reproducible, inexpensive and requires no special preparation, it is used as a major diagnostic tool in emergency settings and in outpatient follow-up of several disorders. Costs, encompassing both accessibility and actual procedural expenses, are lower than those associated with other diagnostic techniques. However, the incorporation of gastro-intestinal ultrasound (GIUS) in clinical practice has not been widely used on a global scale. The purpose of this paper is to provide an overview of the execution techniques as well as the main areas of application for GIUS. Through illustrative iconographic representation, emphasis was placed on its potential within the diagnostic and therapeutic pathway of various acute and chronic gastrointestinal disorders.
Gastro-Intestinal UltraSound (GIUS) has witnessed a significant expansion over the past decade. Continuous technological innovations, which have allowed for the acquisition of increasingly detailed images in a straightforward manner, have undoubtedly facilitated its dissemination beyond the restricted realms of dedicated, highly specialized diagnostic centers. Presently GIUS accompanies clinicians at the bedside of patients in numerous healthcare settings.
Although not consistently achieving the sensitivity and specificity of alternative diagnostic techniques, ultrasound (US) possesses distinctive attributes that are pivotal in evaluating intestinal pathology. This dynamic examination offers a real-time opportunity to visualize the characteristics of the digestive tube and surrounding structures. Moreover, it allows clinicians to observe peristaltic movements, assess the compressibility of intestinal loops, and identify any tender regions warranting focused assessment. These characteristics, in conjunction with the established merits intrinsic to US imaging in terms of non-invasiveness, repeatability, tolerability, and cost-effectiveness, have positioned GIUS as a diagnostic tool at the primary level, both in urgent scenarios [1–3] and in outpatient follow-up of different pathologies [4].
Abundant literature attests to the usefulness of GIUS in clinical practice. Accordingly, GIUS has been integrated into numerous international guidelines and endorsed by leading US scientific societies [2–7]. Nonetheless, despite its increased dissemination, comprehensive proficiency in this field remains predominantly confined to a few expert centers, as the examination is highly operator-dependent, requiring both specific training and in-depth knowledge of gastrointestinal pathology [8–10].
The aim of this paper is to present a perspective on execution techniques and principal applications of GIUS. Emphasis is placed, through illustrative iconographic representation, on its potential within the diagnostic and therapeutic scenarios of various acute and chronic gastrointestinal pathologies.
Evaluation of quality and effectiveness of a methodology involves assessing multiple parameters, including diagnostic accuracy, safety, tolerability, availability, and costs [11]. One of the main advantages of GIUS over traditional endoscopy is its ability to assess transmural alterations of the intestinal wall and adjacent structures, similar to Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) [12]. Moreover, as a dynamic and real-time examination, GIUS offers significant insights in evaluating intestinal motility compared to CT, enabling the observation of motility under physiological conditions without the need of administering any oral contrast agent. The use of US, which does not involve any ionizing radiation, ensures safe examination in fertile or pregnant women and children. Additionally, it can be repeated at short intervals [13, 14].
This non-invasive and painless procedure requires no specific preparation, other than 6–8 hours fasting and, if needed, modest bladder filling to study distal portions of the rectum [15]. The costs of GIUS, encompassing both accessibility (lower equipment costs) and actual procedure expenses are lower than competing techniques [16]. For instance, in northern Italy, in the Emilia Romagna region, a complete abdominal US costs 60.45€, versus 115.15€ of CT, rising to 175.60€ with contrast medium. An upper abdominal MRI costs 160.10€ and 249.45€ with contrast medium, while endoscopic examinations’ costs range from 350€ for colonoscopy to 850€ for video capsule endoscopy [17].
However, despite its clear advantages in diagnostic assessment and planning of subsequent diagnostic-therapeutic steps, the integration of GIUS into clinical practice remains suboptimal internationally. Conventional radiological imaging remains favored in many countries due to standardized protocols and the ability to retrieve and reevaluate archived images, which can be reviewed by multiple operators. A notable limitation of US is its operator-dependency for both image acquisition and interpretation. Nonetheless, it is important to recognize that operator-dependency is a feature to a variable extent of all diagnostic tests [18]. In the case of GIUS, operator-dependency is further compounded by patient-specific variables. Typically, the diagnostic accuracy of US markedly diminishes in obese patients due to increased depth and density of tissue that US waves must penetrate, often resulting in suboptimal image quality. Such variability introduces significant challenges, as the quality of the outcomes relies heavily on the operator’s expertise as well as on the physical attributes of the patient, thereby influencing the overall reliability and utility of US in clinical settings. What is certain is that acquiring expertise requires extensive dedicated training, starting from those proficient in standard abdominal US [14, 15].
Unlike parenchymal organs, interpreting US images of the gastrointestinal tract presents more challenges, even under normal conditions, due to thin walls, tortuous course, and gaseous content. Furthermore, image quality is compromised in obese patients, limiting the use of high-frequency transducers [10].
In essence, every US examination should be guided by clinical suspicion, and a systematic approach is essential to study the entire intestine [19]. To establish a proper clinical context, it is recommended that the examination follows a comprehensive US study of other abdominal organs. The evaluation is initiated with the use of a low-frequency convex probe (1–8 MHz), which provides a panoramic view, facilitating the clinician’s orientation to abdominal anatomy and enabling the identification of major alterations that might not be immediately apparent. This initial overview is crucial, particularly when delineating the relationship between various abdominal organs and the intestinal loops. The comprehensive nature of this exploration ensures that no potential pathological entity is overlooked. Following this general examination, attention should be directed toward a meticulous, high-resolution examination utilizing a high-frequency linear probe (5–10 MHz). This probe is indispensable for investigating superficial structures in greater detail. Its application is particularly useful for evaluating bowel wall, where precision in measurement is paramount. Wall thickness, which is a critical parameter, should invariably be measured with the linear probe due to its superior resolution, which ensures accuracy and reliability. Such precision is less attainable with a convex probe, given its broader focus and lower resolution [18, 19].
In addition to providing measurements of wall thickness, the linear probe excels in delineating the bowel wall’s layers, thus enabling the detection of any stratification loss, which is a hallmark of various pathologies [18]. The use of Doppler features alongside the linear probe can further characterize the blood flow within the bowel wall, providing insight into the state of perfusion and contributing to the differential diagnosis [15]. The high-frequency linear probe also plays a vital role in evaluating the mesenteric fat, providing insights into edema or inflammation, and the identification of mesenteric lymphadenopathies. The characteristics of mesenteric lymph nodes, including size, shape, echogenicity, and vascular patterns, are best appraised using the high frequency linear probe, which can detect even subtle variations in these parameters [18].
For ultrasonographic assessment of deeper intestinal tracts, such as the rectum and in obese patients, a convex probe is often favored due to its ability to penetrate deep tissue. This enables a comprehensive evaluation of areas that might be less accessible to high-frequency probes, although with a lesser resolution of detail [14, 15, 18].
Longitudinal, transverse, and oblique scans are employed and optimized based on the specific gastrointestinal section under examination. To maintain a systematic approach, it is convenient to start at the cecum, usually located in the right iliac fossa. Cecum is recognizable by its typical content of air and fecal material as well as its relationship with the narrower ileal loop. This ileal loop usually contains more fluid and particulate content and exhibits noticeable peristalsis. Progressing onwards, longitudinal and transverse scans must be performed to span the entire colonic frame, tracing its path to the rectum, which is better visualized with partial distension of the bladder. For the exploration of jejunal and ileal loops, parallel transverse scans are recommended, moving across the abdomen from one side to the other in a craniocaudal direction, encompassing the entire abdominal area (Video 1) [18–20].
Video 1 can be viewed at the following link:
https://drive.google.com/file/d/1iKBsNxo9U6yvobG3VksFOPSsdvETgSPM/view?usp=sharing
Starting from cecum, the terminal ileum serves as a convenient point of origin for visualizing the remainder of the ileum and jejunum with parallel transverse scans in a craniocaudal direction, covering all abdominal quadrants [14, 15]. By employing right and left subcostal scans, along with longitudinal and transverse scans in the epigastric region, it is possible to explore the gastric antrum and duodenum. Shifting focus to the patient’s left side allows for the exploration of the entire stomach, while left intercostal scans reveal the gastric antrum and splenic flexure of the colon [14, 19, 21]. The terminal portion of the esophagus, located at the esophageal hiatus, can be explored using upward-angled longitudinal scans in the epigastric region. Notably, the proximal esophagus, situated behind the left thyroid lobe, can also be examined through longitudinal and lateral cervical scans [19]. When it comes to studying the terminal part of the rectum, transperineally scans come into play [18].
A vital element in scanning intestinal loops involves applying gradual compression with the transducer, mimicking the pressure exerted during abdominal palpation. This compression helps displace endoluminal gas, assess the compressibility of the examined loop, and identify tender points that might disclose underlying pathology [18].
Several elements need to be systematically evaluated during a GIUS examination:
Wall thickness must be measured perpendicularly through all layers, from the lumen/mucosa interface to the serosa. Normal values are up to 3 mm in the small intestine and 4 mm in the colon. The stomach can measure up to 6 mm, and the rectum up to 7 mm [14, 18, 19, 22]. While wall thickness is a quantifiable parameter and a primary pathological criterion in many studies, it is nonspecific, thickening being present in various inflammatory, infectious, ischemic, or neoplastic conditions [23]. Markedly asymmetric thickening is often observed in intestinal neoplasms and lymphomas [14, 24]. The intestinal wall consists of five layers, each with a distinct US pattern more discernible with slight parietal thickening rather than in physiological conditions (Figure 1) [25]. Though US layer definition does not precisely match anatomopathological classification due to the underlying physical principles of US imaging [26], but the loss of this stratification is a significant diagnostic feature [14].
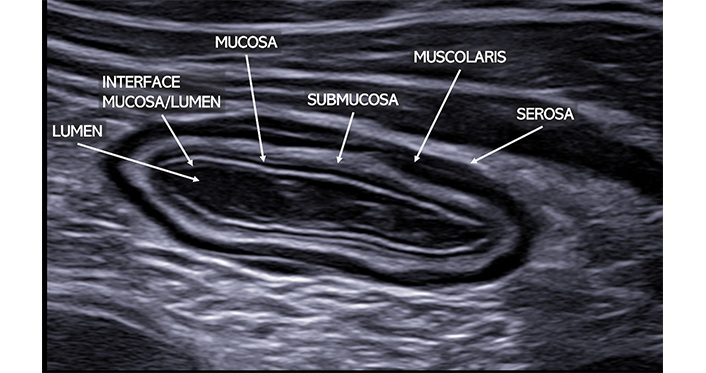
Normal ileal loop visualized in transverse scan with a high-frequency linear probe. The typical bowel stratification is clearly detectable. Starting from the inside, it is possible to clearly identify: the lumen (anechoic), the interface between the mucosa and submucosa (hyperechoic), the muscularis (hypoechoic), and the serosa (hyperechoic)
Caliper measurements within the normal range are up to 2–2.5 cm for small intestinal loops and up to 5 cm for the colon. Obstructive or paralytic processes result in increased caliper of intestinal loops [15]. Intraluminal content varies depending on the distance and type of meal ingested: Generally, there’s more fluid/particle-rich content in the small intestine, transitioning to a greater presence of gas artifacts in the colon (Figure 2) [15].
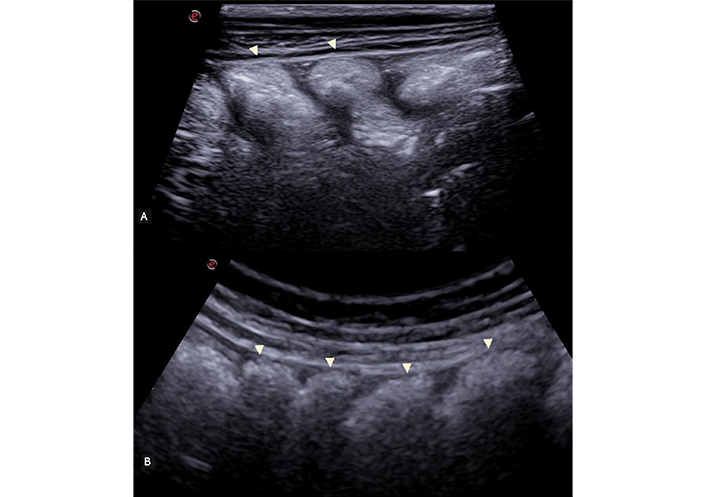
Longitudinal scan of the ascending colon, visualized with a high-frequency linear probe (A) and with a convex probe (B). The characteristic haustra of the colon is clearly visible (arrowhead)
US is unique in allowing the assessment of intestinal peristalsis, although operator dependency remains an issue. Hyperdynamic kinetics can be observed in conditions such as infectious colitis, malabsorption-related enteropathies, and the initial stages of mechanical ileus. However, in advanced diseases or paralytic ileus, peristaltic movements are reduced or absent [27].
Under physiological conditions, typically Doppler evaluation will not reveal any vascular signal in a nonticketed wall. Increased vascularization may be visible in case of wall thickening, suggesting an inflammatory or neoplastic condition. Conversely, the complete absence of blood flow raises suspicion of ischemic etiology [14]. In addition, contrast-enhanced US (CEUS), where available, could provide enhanced visualization of blood flow that traditional US might not capture. Currently available contrast agents, composed of gas microbubbles, upon their injection into the bloodstream enhance ultrasonographic imaging by providing superior visualization of tissue perfusion and microcirculation, offering a distinct contrast with adjacent tissues. Despite optimal adjustments, color Doppler fails to match the detailed assessment of tissue perfusion achievable with CEUS, as it does not allow for differentiation between non-vascularized structures and those with slow or minimal vascular flow. Moreover, US contrast agents boast a remarkable flexibility in administration, as they are suitable for use irrespective of organ function—this includes scenarios such as renal failure. They are also safe for children or pregnant women, and they have an excellent safety profile, with a low incidence of allergic reactions and adverse events. Preparing, administering the contrast agent, and interpreting CEUS results extend the examination by 5–20 minutes [28]. In specific cases, which will be discussed later in this article, CEUS represents a supplementary diagnostic resource [15, 23].
Several acute and chronic intestinal pathologies cause changes in the structures surrounding the affected loops, which are crucial for diagnosis. Inflammatory collections (abscesses or phlegmons), fluid accumulation, mesenteric hypertrophy, mesenteric lymphadenopathies, or pneumoperitoneum can be observed and are diagnostically significant [15, 21, 23, 27].
GIUS is recommended as the initial diagnostic method in patients presenting with an acute abdomen [1–3]. In expert hands, this technique has excellent diagnostic accuracy, providing a radiation-free complement to physical examination. It enables physicians to integrate clinical data, signs, symptoms, and images directly at the patient’s bedside [1] and has virtually infinite repeatability without any side effect, which is particularly valuable in cases involving women of reproductive age and children [13, 20]. Notably, when performed during the initial clinical evaluation, it can be a decisive diagnostic tool in patients with acute abdominal pain, helping to identify signs of acute appendicitis, acute diverticulitis, small bowel obstruction, and intestinal ischemia [2, 3].
The EFSUMB guidelines advocate US as the preferred initial approach in all suspected cases of acute appendicitis (“Ultrasound first and always”), which is applicable to both adults and children [3, 5]. This strategy has demonstrated the ability to reduce unnecessary surgical interventions by 50% without compromising patient care [29]. In this context, US sensitivity, specificity, and accuracy have proven to be comparable, although slightly lower in most cases, to CT or MRI [30].
On the other hand, the American College of Radiology’s (ACR) Appropriateness Criteria provides guidance on the most suitable imaging techniques for diagnosing acute appendicitis, particularly in the context of right lower quadrant pain. According to these guidelines, abdominal and pelvic CT with intravenous contrast is typically the most appropriate initial imaging choice. US, however, may also be considered appropriate and is advised as the preferred imaging method in pregnant women, in conjunction with MRI of the abdomen and pelvis without intravenous contrast [31]. The difference between these approaches may be attributed to differences in patient characteristics and physical constitution, and partly to differences in health policy, a discussion of which is beyond the scope of this paper. Despite these differences, a point of care US, performed as a complement to clinical evaluation, can help to guide subsequent diagnostic pathways more precisely, facilitating an optimization of the use of radiological investigations without increasing costs. For instance, point-of-care ultrasonography is increasingly performed by emergency physicians to diagnose acute appendicitis but adequate equipment and training are mandatory [3]. Clearly, when US results are inconclusive or the diagnosis proves challenging—such as in cases involving obese patients, a retrocecal appendix, or focal inflammation—resorting to cross-sectional imaging is mandatory [3].
The objective of US examination is threefold: rule out alternative abdominal pathologies, confirm a typical presentation of appendicitis, or, conversely, exclude it when the appendix exhibits normal characteristics throughout its length. Additionally, US examination can raise the suspicion of complicated appendicitis (gangrenous or perforated), necessitating further diagnostic exploration and surgical assessment. Careful attention should be paid to the point of maximum tenderness, typically in the right iliac fossa. US findings for acute appendicitis include wall thickening > 3 mm in the longitudinal section, target sign appearance with anteroposterior diameter > 6 mm in the longitudinal section, non-compressibility, and tenderness upon gradual probe pressure (ultrasound McBurney’s sign). Abundant parietal vascular signals on color Doppler are indicative of acute inflammation, while peri-appendiceal fluid accumulation, mesenteric hypertrophy, and mesenteric lymphadenopathy are also relevant markers (Figure 3) [3, 21].
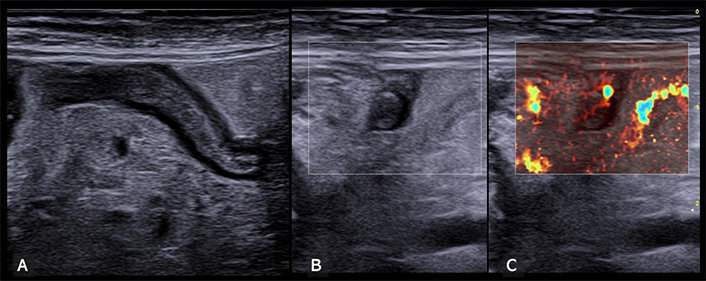
Inflamed appendix. (A) Longitudinal view of an inflamed appendix, characterized by a noticeable thickening of both the appendix wall and its caliper. Additionally, there is a marked hyperechoic thickening of the surrounding mesentery. (B) Transverse ultrasound view further demonstrates the inflamed appendix, consistently highlighting thickening of the wall and mesentery across different scanning planes. (C) This image, corresponding to the transverse view in panel B, distinctly shows enhanced vascularization in the mesentery at microvascular imaging, indicative of a typical inflammatory response in appendiceal inflammation
The diagnosis of acute appendicitis is made easier in clinical practice when multiple US signs are present, specifically the observation of a thick-walled appendix with hyperechoic peri-appendiceal tissue in the area of greatest pain. Conversely, mesenteric lymphadenopathy, modest fluid accumulation, and mesentery thickening are nonspecific signs observed also in several other conditions [32].
GIUS has high diagnostic accuracy, with sensitivity and positive predictive values of approximately 90%. It often visualizes diverticula even in the absence of inflammation, helping clinicians in risk stratification and identify complications such as abscesses, phlegmons, perforations, or strictures. However, CT remains the gold standard for managing acute diverticulitis and its complications, particularly in obese patients and distal sigmoid diverticula [32]. In several European countries, a “step-up” diagnostic approach has been proposed, where GIUS serves as the primary tool followed by CT in cases of inconclusive or challenging US results, complex localizations, and complicated diverticulitis where CT offers superior delineation beneficial for potential surgical intervention [3, 32–35].
Diverticula can be visualized even in the absence of inflammation as protrusions of the wall containing echogenic material (air, feces, or fecaliths) with a posterior acoustic shadow (Figure 4A) [36, 37]. Diagnostic US criteria for acute diverticulitis encompass segmental wall thickening (> 5 mm) of a short intestinal segment with loss of compressibility, presence of an inflamed diverticulum in the thickened area (with varying echogenic appearances: hypo- or hyperechoic, with or without air artifacts in the lumen), and peri-loop mesenteric hyper-echogenicity due to inflammation (Figure 4B) [3, 21, 38]. Moreover, US can visualize potential complications such as abscesses, fistulas, free air, or abdominal fluid accumulation [36]. Grayscale US frequently struggles to differentiate between phlegmons and abscesses, as both conditions can manifest as heterogeneous hypoechoic masses. This similarity in visual presentation on US complicates the diagnosis, especially when no vascular signals are detectable on color Doppler. In such cases, phlegmons, which are typically managed conservatively, show diffuse enhancement on CEUS, while collections of pus remain completely avascular and are delineable after contrast administration. This is particularly important because abscesses, depending on their dimensions, often require intervention through percutaneous or surgical drainage to effectively manage the condition. Moreover, US and CEUS are pivotal in-patient follow-ups, enabling serial, non-invasive imaging with dynamic monitoring and timely adjustments of treatment strategies (Figure 5) [21, 39].
Diverticula. (A) Non-complicated diverticulum on the sigmoid colon. This image shows a small, air-filled sac (diverticulum, between calipers) protruding from the wall of the sigmoid colon in the mesentery. The walls of the diverticulum and the adjacent bowel wall are not thickened, and the entire adjacent mesentery is also not thickened. (B) This image shows a diverticulum with markedly thickened and hypoechoic walls (arrowhead). A diverticulum, filled with air, also is visible (arrow). The adjacent bowel walls are thickened and hypoechoic (between calipers), while the mesentery is thickened and hyperechoic. These are clear signs of acute inflammation
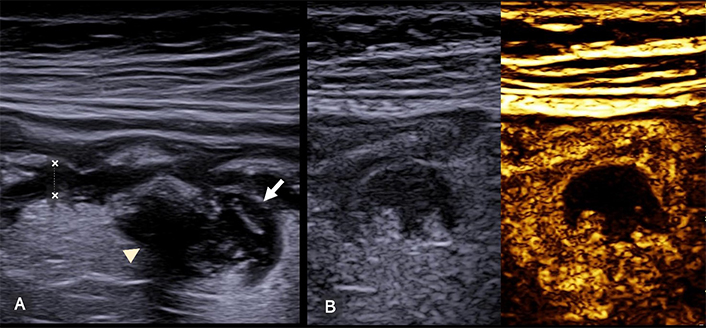
Complicated diverticulitis. (A) The sigmoid colon (longitudinal scan) has thickened walls (between calipers) and a diverticular outpouching (arrow). The mesentery is hyperechoic and thickened and a hypoechoic inflamed area surrounds the diverticula (arrowhead). (B) On CEUS the hypoechoic area within the mesentery appears completely avascular, confirming the presence of a small peridiverticular abscess
Small bowel obstruction is a common cause of acute abdomen and is characterized by varied and nonspecific clinical manifestations such as vomiting, acute pain, and abdominal distension. Early evaluation is imperative, and GIUS helps to confirm or rule out intestinal obstruction, offering insights into its location and potential causes, including adhesions, Crohn’s disease, tumors, hernias, and volvulus [40]. Moreover, GIUS assesses the risk of critical intestinal ischemia associated with obstruction, aiding in further diagnostic exploration and surgical assessment [1, 2].
In this context, the diagnostic accuracy of GIUS is comparable to, if not superior to CT. It exhibits greater sensitivity (91% vs. 87%) and specificity (96% vs. 81%), notably surpassing X-rays, which stand at approximately 75% sensitivity and 66% specificity [41]. However, CT remains the preferred diagnostic modality for assessing obstructive causes and complicated cases necessitating urgent surgical intervention, as well as for inconclusive US findings [2, 40]. Therefore, the EFSUMB guidelines advocate US as the primary approach in managing acute abdomen cases suspected of small intestinal obstruction, particularly in young patients and during pregnancy. Combining GIUS with CT remains suitable, as CT retains its diagnostic relevance in defining the obstruction location, organic causes, and severity [2].
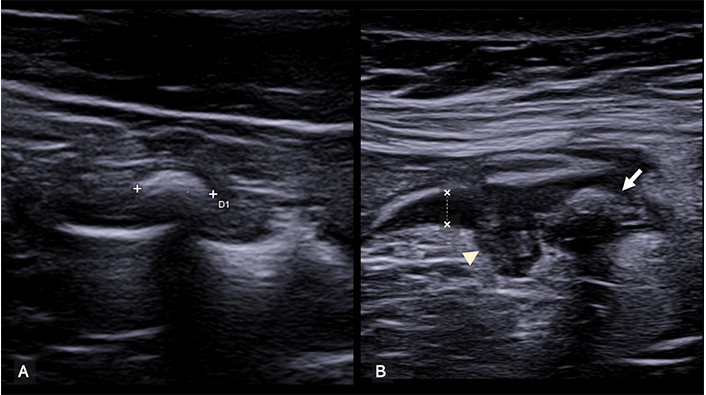
US signs of uncomplicated intestinal obstruction include identification of dilated intestinal loops (> 2.5 cm) containing fluid and particulate material, along with altered peristalsis. It also provides a dynamic assessment of peristaltic movements, aiding the differentiation between dynamic and mechanical ileus. In dynamic ileus, physiological peristalsis diminishes or disappears, whereas mechanical ileus initially shows increased peristaltic movements that gradually decrease in the advanced stages. In the initial phases, thickening of the intestinal wall (> 3 mm) with hypertrophy of the valvulae conniventes (“keyboard sign”) may be visible, whereas over time, the wall tends to thin out and the valvulae to flatten (Figure 6). The site of obstruction can be determined by identifying the point at which dilated proximal loops transition to nondilated distal loops. This is particularly crucial for identifying specific pathologies causing obstruction, such as hernias, intussusceptions (Figure 7), neoplasms, or Crohn’s disease. Color Doppler imaging enables the assessment of parietal vascularization, allowing early identification of signs of ischemic distress [1, 2, 21].
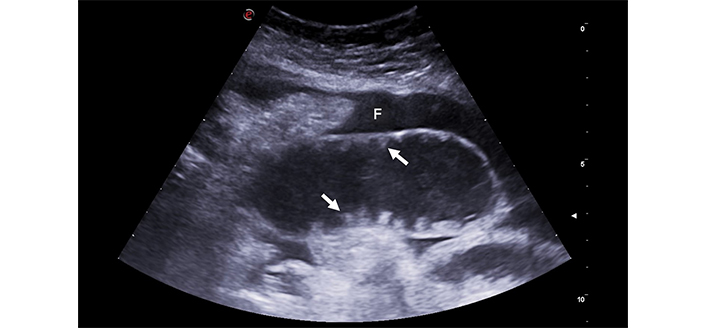
Intestinal obstruction. The image depicts a significantly distended small bowel loop, filled with fluid and having notably thin walls. The valvulae conniventes, (arrows), are flattened, indicating chronic obstruction. Fluid effusion (F), a sign of distress, surrounds intestinal loops
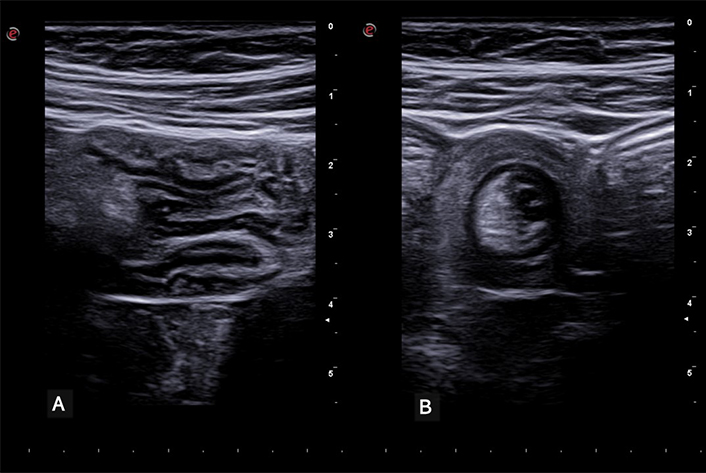
Intussusception. (A) Longitudinal scan showing a segment of the intestine invaginated into itself. The characteristic ‘trident’ or ‘telescopic’ appearance of the bowel layers is evident, indicative of intussusception. (B) Transverse view of the same intestinal segment. The invaginated intestinal segment is visible, displaying the classic ‘target’ or ‘donut’ sign. This cross-sectional view provides further confirmation of intussusception, characterized by the concentric rings formed by the intussuscepted bowel
US signs indicating worsening obstruction include intraperitoneal fluid or free air, wall thickening > 4 mm, decreased or absent peristalsis in a previously hyperdynamic loop, and absence of wall vascularization on color Doppler [2, 42, 43].
For strongly suspected acute mesenteric artery ischemia cases presenting with abdominal pain, hemodynamic instability, and multi-organ insufficiency, a prompt angio CT is recommended as gold standard examination [44]. Yet, as the clinical presentation is often nonspecific, abdominal US and GIUS can serve as a preliminary panoramic investigation. This aids in guiding patients displaying US signs of ischemia toward immediate CT evaluation [2].
Distinct US patterns emerge based on the underlying etiology. In acute arterial ischemia asymmetric hypoechoic thickening of the intestinal wall, with reduced or absent peristalsis, may be visible. Furthermore, in this scenario CEUS is exceptionally sensitive in detecting reduced or absent parietal perfusion, a hallmark of ischemic segments, thus facilitating a prompt and accurate differential diagnosis from inflamed tracts, which typically exhibit increased vascularization often visible with color Doppler. CEUS is largely credited for its safety, with a low risk of adverse reactions. The US study during clinical assessment and a potential CEUS, which in expert hands extends the evaluation by 5–15 minutes may be crucial for preserving intestinal function and preventing further complications in ambiguous cases with a less suggestive clinical picture (Figure 8) [45, 46].
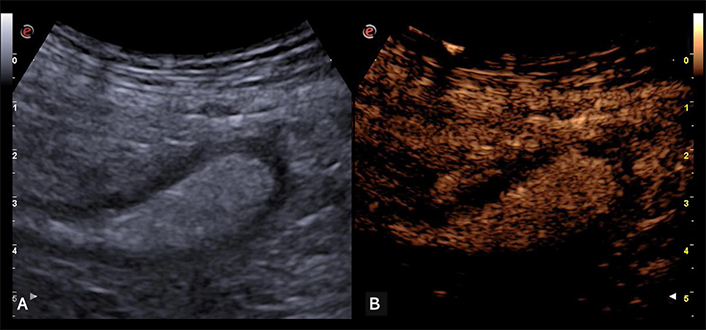
Intestinal ischemia. (A) The descending colon is moderately distended with speckled content and thickened and hypoechoic walls. (B) On CEUS the walls appear completely avascular, indicating intestinal ischemia
In acute venous ischemia, a uniform hypoechoic and homogeneous thickening of the intestinal wall due to mucosal edema, along with reduced peristalsis, intraluminal secretions, and perienteric fluid accumulation can be observed [2].
In chronic arterial ischemia asymmetry between wall thickness can be observed but it’s usually less pronounced, as collateral circulation often compensates for perfusion [2, 47].
GIUS exhibits high positive predictive value (90%) in identifying ischemic colitis in elderly patients with sudden abdominal pain, diarrhea, or rectal bleeding. It demonstrates segmental thickening (extension > 10 cm) of the descending colon wall, showing no vascular signals on color Doppler [2, 48].
When intestinal perforation is suspected, GIUS is recommended as a first line investigation, followed by CT in case of negative or inconclusive findings. This approach is justified by CT’s superior sensitivity in revealing pneumoperitoneum or pneumoretroperitoneum [49]. Clearly, the traditional radiological abdominal scan approach is losing significance, given its lower sensitivity (55–85% compared to 92% for US and 95% for CT) [50–52]. This approach does not offer any insights into the site and cause of perforation and fails to diagnose most other causes of acute abdomen [53].
While the presence of air poses a known challenge in performing US examinations, the focus should be on detecting gas collections and small air bubbles in upper positions, particularly between the anterior liver surface and the abdominal wall. These manifestations appear as hyperechoic lines or foci accompanied by reverberation artifacts. When free air obscures the left hepatic lobe and adjacent abdominal structures, a slight compression may displace the gas, revealing the liver in an alternating pattern [54]. Additionally, observing the movement of air artifacts in response to changes in patient position strongly suggests pneumoperitoneum [55].
In the realm of diagnosing inflammatory bowel diseases, colonoscopy remains the gold-standard examination due to its capacity for biopsies and histological diagnosis [56]. However, for assessing proximal extension and potential complications, recent consensus guidelines by the European Crohn’s and Colitis Organization (ECCO) and the European Society of Gastrointestinal Radiology (ESGAR) recommend GIUS, CT, or MRI as imaging techniques for identifying Crohn’s disease at its initial presentation, as well as for evaluating its location, activity, and potential complications with comparable diagnostic accuracy [6, 12]. Consequently, GIUS is endorsed as the primary choice for both the initial diagnostic phase and disease follow-up, primarily due to its favorable aspects of tolerance, safety, repeatability, and accessibility [57].
GIUS can reveal wall thickening with loss of stratification and reduced or absent peristalsis within the affected segments, thereby identifying the extent of the involved intestinal tract. Additionally, mesentery hypertrophy is often observed, appearing as hyperechoic with lymphadenopathies present, along with heightened vascularity evident in both the intestinal wall and mesentery, as seen on color Doppler imaging [3, 21]. When considering complications, strictures manifest as intestinal segments with thickened walls, accompanied by luminal narrowing and dilation of the upstream intestinal tract, often accompanied by accentuated peristalsis (Figure 9) [57, 58]. Fistulas appear as hypoechoic tracts, sometimes containing hyperechoic spots within, linking adjacent intestinal loops or an intestinal loop with other structures such as the bladder, muscles, or skin (Figure 10) [38, 58]. Wall abscess formations can be visualized as hypo-anechoic lesions, frequently displaying internal echoes due to air or debris presence, and thickened walls. The administration of contrast agents enables the differentiation between abscesses and phlegmons, as mentioned previously, and its virtually unlimited repeatability is extremely useful for short-term follow-up [21, 39, 46].
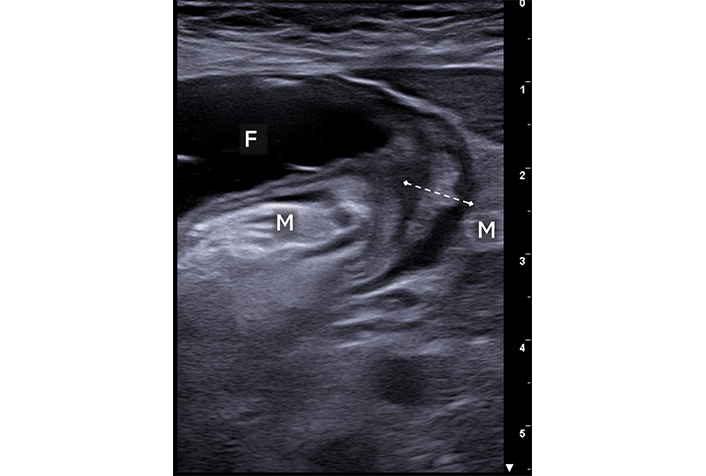
Crohn’s disease. A short intestinal segment is shown with thickened walls (between calipers) and a significantly narrowed lumen. Upstream segment dilation with fluid content (F) is noted. The surrounding mesentery of the thickened loop is also thickened and hyperechoic (M)
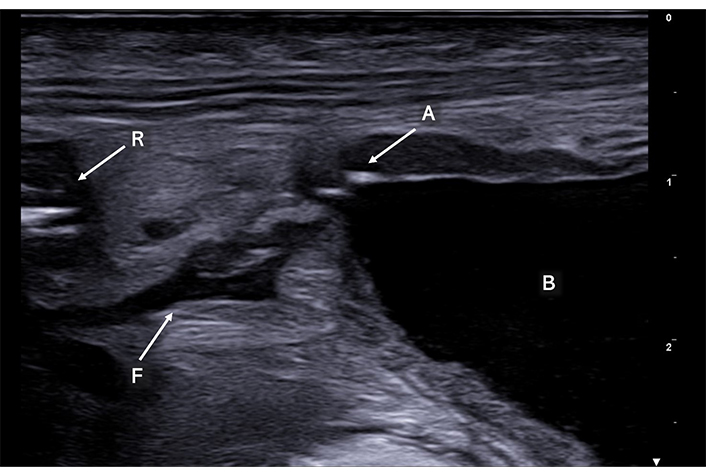
Enteric fistula (F) appears as a serpiginous, hypoechoic tract, delving into the thickened mesentery, establishing a pathological communication between the rectum (R) and the bladder (B). Notably, punctiform air artifacts (A) are observed inside the bladder adjacent to the anterior wall
Due to its primary impact on the mucosa and submucosal layers, endoscopy stands as the gold-standard examination for diagnosing ulcerative colitis [59]. However, GIUS has been proven to be an exceptional tool for non-invasive evaluation of disease extent, activity, and response to pharmacological interventions [4, 6, 60, 61].
Typically, moderate thickening of the intestinal wall (generally ranging between 4 and 9 mm) is observed, with preserved stratification in mild forms of the disease [62]. As ulcerative colitis advances to moderate and severe stages, stratification becomes less discernible and eventually disappears, causing the colon to appear contracted and linearized, thus losing its characteristic haustration (Figure 11) [4, 21, 61–63]. The intensity of wall vascularity observed in color Doppler imaging is directly correlated with clinical and endoscopic disease activity [64].
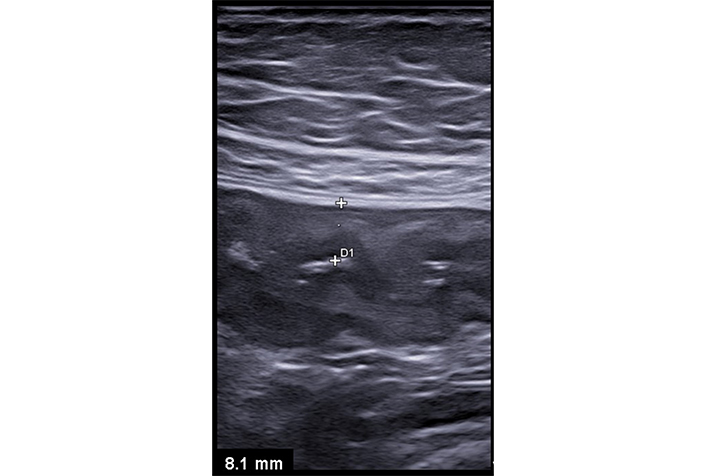
Ulcerative colitis. The descending colon appears, contracted with thickened and hypoechoic walls (between calipers) hallmarks of inflammatory activity. While ultrasound effectively highlights these inflammatory changes, it is through endoscopic examination that a definitive diagnosis of ulcerative colitis is established
Given the increasing use of US as a primary diagnostic tool in acute conditions and/or for monitoring patients with inflammatory bowel disease, cases of toxic megacolon might be encountered. This condition is more likely to manifest as dilation of the transverse colon (often exceeding 6 cm), accompanied by thin, hypoechoic intestinal walls, and liquid or gaseous content [63].
It is crucial to emphasize that the differential diagnosis among various causes of diarrhea necessitates, alongside GIUS examination, integration with clinical data, laboratory assessments, and radiological or endoscopic inquiries. In instances of infectious colitis, fluid-filled intestinal loops, symmetric wall thickening with retained stratification, a hyperechoic appearance, and heightened vascular signals may be observed in color Doppler imaging. While these findings could be accompanied by evident mesenteric lymphadenopathy, they generally do not involve substantial thickening of adjacent mesenteric fat tissue [36].
A definite diagnosis of celiac disease with GIUS remains elusive. However, while individual US indicators of the disease lack specificity, taken collectively they support the diagnostic suspicion of intestinal malabsorption [65]. These indicators encompass fluid-filled dilation of loops, accentuated valvulae conniventes exhibiting a brain-like appearance, increased peristalsis, free peritoneal fluid, and inflammatory mesenteric lymphadenopathy (Figure 12). These US signs wane and vanish following adherence to a gluten-free diet, while their persistence could signal resistance to the dietary regimen [21].
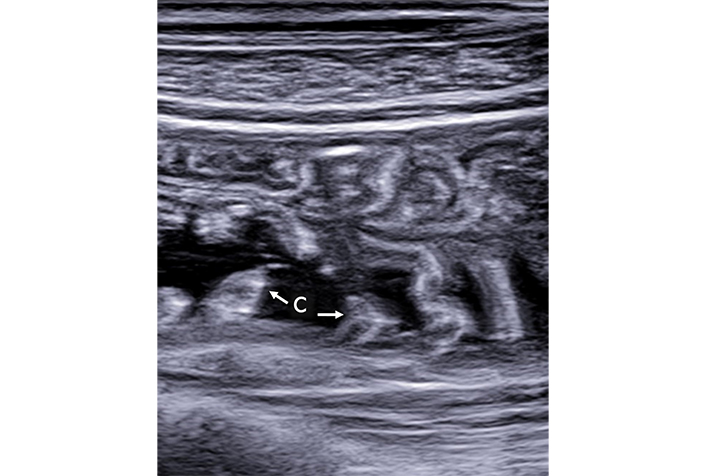
Malabsorption. In this depiction, the valvulae conniventes (C) are hypertrophic and are distinctly visible due to the presence of anechoic fluid content
Although GIUS is not the primary method for detecting or staging intestinal neoplasms, it may guide further diagnostic exploration. Malignancies causing hypoechoic or heterogeneous, frequently asymmetrical, wall thickening leading to diminished lumen diameter with subsequent dilation of the upstream loops due to substantial stenosis can be observed (Figure 13). Polypoid formations protruding into the lumen may also be visible in some cases, typically appearing as hypoechoic nodules/masses. GIUS aids in identifying small-sized intestinal lesions that might otherwise elude endoscopic examination, thus guiding subsequent diagnostic and therapeutic approaches. Such lesions could include intestinal lymphomas, gastrointestinal stromal tumors (GISTs), neuroendocrine tumors, and, albeit rare, carcinomas (Figure 14) [65, 66].
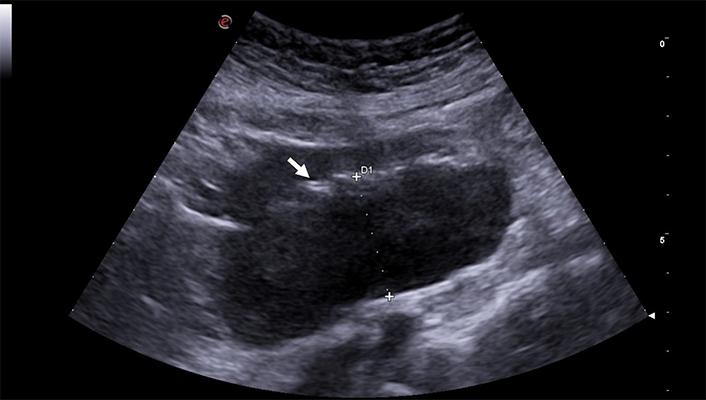
Intestinal lymphoma shows a typical asymmetric thickening of the walls of a small intestine loop. The posterior wall (between calipers) is significantly thicker than the anterior wall, and the intestinal lumen (arrow) is displaced in an eccentric position. The thickening begins and ends abruptly and appears markedly hypoechoic
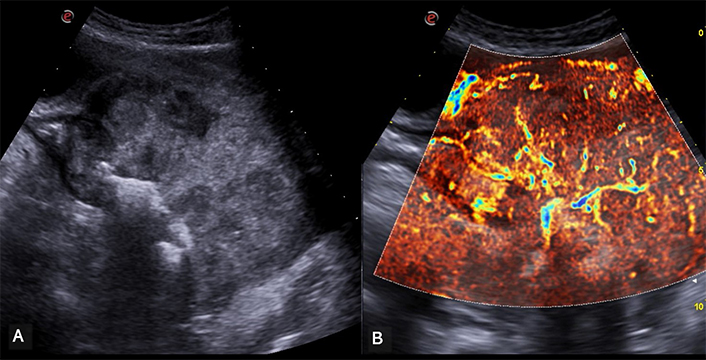
Rectal Adenocarcinoma. (A) This image illustrates a pronounced asymmetric thickening of the rectal walls with the lumen in an eccentric position. The thickening has a markedly heterogeneous echostructure. (B) This image focuses on the same area of thickening, revealing the presence of intralesional vascular signals, clearly delineated using microflow imaging
A comprehensive overview of the main advantages and disadvantages of GIUS is summarized in Table 1.
This table outlines the advantages and disadvantages of using GIUS for various gastrointestinal conditions, highlighting its diagnostic strengths and limitations compared to other imaging methods
| Condition | Advantages of GIUS | Disadvantages of GIUS |
|---|---|---|
| Acute appendicitis | Highly effective in children and thin adultsCan visualize the inflamed appendix, assess for free fluid and local fat inflammationCan rule out other causes of right lower quadrant pain | May miss appendix if retrocecalLess sensitive than CT |
| Acute diverticulitis | Rapid assessment tool that helps identify inflamed diverticula and complications such as abscesses or perforationsUseful in settings where immediate CT is unavailable | Less detailed than CT, particularly in obese patients or in visualizing smaller abscesses |
| Small bowel obstruction | Can quickly identify dilated bowel loops and the presence of fluid, potentially pinpointing the location of the obstructionReal-time evaluation of bowel peristalsis can help differentiate between mechanical ileus and paralytic ileusIdeal for serial examinations due to lack of radiation | Limited in differentiating between simple and strangulated obstructions; less detailed than CT in identifying the exact cause or level of obstruction |
| Intestinal ischemia | Can rapidly detect signs of ischemia such as bowel wall thickening and free fluid; useful for initial bedside evaluationCEUS can demonstrate the absence of vascularization in the thickened intestinal tract, providing critical information on the extent of ischemia | May not identify the cause of ischemia, such as arterial emboli or venous thrombosisLess sensitive and specific than CT |
| Intestinal perforation | Quick to perform and can identify free intra-abdominal air and fluid indicating perforation, guiding urgent surgical interventionCan also adeptly detect small air bubbles anterior to the liver | Less sensitive in detecting localized perforations compared to CTLess effective in pinpointing the exact location of the perforation compared to CT |
| Crohn’s disease | Useful in detecting and monitoring bowel wall thickening, abscesses, and other complicationsNon-radiative and can be repeated frequently for follow-ups | Less effective than MRI in visualizing deep structures and assessing fistulae or the full extent of intestinal involvement |
| Ulcerative colitis | Can assess bowel wall thickness and vascularization during flares and can be useful for quick evaluations during symptomatic periods | Does not provide detailed mucosal imaging as endoscopy and has limited utility in assessing deep ulcerations |
| Infectious enteritis | Quick, non-invasive assessment tool to evaluate bowel wall thickening and to differentiate from other causes of acute abdominal pain | Not specific for identifying pathogensLimited use in mild or early disease |
| Malabsorption and celiac disease | Helps identify complications such as bowel thickening, intussusception, or lymphoma in advanced cases | Not diagnostic for celiac disease or other specific malabsorption disorders |
| Masses and neoplastic lesions | Can quickly detect the presence and location of massesUseful for initial assessment and guiding further diagnostic procedures | Limited in characterizing the nature of lesions compared to CT, MRI and endoscopy, particularly with deep or small lesions |
| Common to all conditions | No radiation exposure (particularly beneficial for pediatric populations)Can be performed bedside during the clinical evaluationCan be frequently repeated for necessary follow-ups | Limited effectiveness in obese patientsHighly operator-dependent |
CEUS: contrast-enhanced ultrasound; GIUS: gastro-intestinal ultrasound
In the realm of abdominal US, various clinical scenarios warrant distinct diagnostic and evaluation approaches. In modern medical practice, GIUS is acknowledged as a valuable tool in the evaluation of a wide range of abdominal conditions, including both chronic diseases and acute abdominal emergencies.
GIUS offers critical insights that facilitate accurate and timely diagnoses, particularly valuable in the management of inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis. Through noninvasive techniques, it provides a detailed assessment of disease progression, activity, and response to treatment.
In acute abdominal conditions, such as appendicitis and intestinal obstructions, GIUS serves as a powerful diagnostic tool that can quickly guide clinical decisions. However, it is important to contextualize its use: GIUS is part of an integrated diagnostic process and should be viewed as a complement to other diagnostic methods. It enriches the overall diagnostic strategy rather than replacing traditional imaging modalities like CT or MRI, particularly where these provide more definitive information or are more suited to the clinical context.
Furthermore, GIUS is invaluable in assessing patients with non-specific abdominal symptoms and altered bowel habits, enhancing the clinical assessment with its ability to swiftly visualize and evaluate abdominal structures. This capability makes it an excellent initial tool that can potentially reduce unnecessary exposure to ionizing radiation and reduce healthcare costs by directing the use of more invasive or expensive diagnostic procedures when necessary.
In our experience, where internal medicine specialists with over 10 years of experience in general US, and specifically in GIUS, perform the examinations, the integration of GIUS into clinical practice, though demanding, yields significant benefits. This extensive expertise ensures that the diagnostic process is not only technically proficient but also enriched by a nuanced understanding of the clinical implications. The integration of GIUS into clinical practice indeed demands focused training for healthcare providers. Mastery of this technique is crucial for maximizing its diagnostic benefits while minimizing potential for misinterpretation. Institutions should prioritize structured training programs that equip medical professionals with the skills needed to utilize GIUS effectively.
By reinforcing GIUS training and judiciously integrating it into the diagnostic repertoire, healthcare providers can leverage its full potential responsibly and effectively, ensuring it acts as a supportive tool for effective medical decision-making.
CEUS: contrast-enhanced ultrasound
CT: Computed Tomography
GIUS: gastro-intestinal ultrasound
MRI: Magnetic Resonance Imaging
US: ultrasound
During the preparation of this work, the authors used ChatGPT to generate the reference anatomical image for Video 1, which was then modified by the authors and included in Video 1. After using the tool/service, authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
CF: Conceptualization, Project administration, Supervision, Visualization, Writing—original draft, Writing—review & editing. AT: Conceptualization, Writing—original draft. EF: Visualization, Writing—review & editing. FZ, FV, AB, and EM: Writing—review & editing. PA: Supervision, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
All the images in the article were collected anonymously and there are no identifiable personal data, following local privacy policy (GDPR 679/2016) therefore the Local Ethical Committee (AVEN) exempted the ethical approval of this manuscript.
Not applicable.
Not applicable.
All datasets for this study are included in the manuscript.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Amedeo Lonardo
Amar Tebaibia ... Nadia Oumnia
Rudy El Asmar ... Samer AlMasri
Ralf Weiskirchen
Vincenzo Giorgio Mirante
