Affiliation:
Liver Institute PLLC, Tucson, AZ 85712, USA
ORCID: https://orcid.org/0009-0007-7297-951X
Affiliation:
Liver Institute PLLC, Tucson, AZ 85712, USA
Email: shabib@liverinstitutepllc.org
ORCID: https://orcid.org/0000-0002-4264-714X
Explor Dig Dis. 2024;3:262–274 DOI: https://doi.org/10.37349/edd.2024.00051
Received: April 05, 2024 Accepted: May 27, 2024 Published: July 23, 2024
Academic Editor: Jean Francois D. Cadranel, GHPSO, France
The article belongs to the special issue Cirrhosis and Its Complications
With the rising prevalence of chronic liver disease worldwide, the incidence and prevalence of acute-on-chronic liver failure (ACLF) are increasing and attribute to higher morbidity, mortality, and healthcare costs. Many of such patients die without being considered for the lifesaving treatment option of liver transplantation. The underutilization of liver transplantation as a therapeutic option in the setting of ACLF, is due to multiple reasons; with the heterogeneity of ACLF and the lack of universal definition being the key players. Liver transplantation listing and allocation are based on MELD score. As of now, we do not know where MELD score stands in regard to defining ACLF and the prognostication of such patients. This insight is very important for the efficient identification of potential liver transplantation candidates in the setting of ACLF. This review paper investigates the role of liver transplantation in the setting of ACLF. In light of recent evidence, MELD score is not the perfect model in the setting of ACLF either. The safety of liver transplantation, either deceased donor or living donor, among ACLF patients has been debated. The short-term mortality rate of ACLF patients has created a need for a standard liver transplant selection criterion for these patients. Based on published literature, we find that three commonly used ACLF definitions may be used in combination to define the sensitivity, specificity, and futility of ACLF and we propose an algorithm to best identify patients for urgent liver transplantation in the setting of ACLF. Moreover, we discuss the data on the safety of liver transplantation in the setting of ACLF. Future validation of this multifaceted approach could bridge the gap between ACLF patients and appropriately guided medical intervention.
Acute-on-chronic liver failure (ACLF) and the criteria surrounding its diagnosis has been a topic of debate since its literary introduction by Ohnishi et al. in 1995 [1]. ACLF can tersely be described as the rapid progression of chronic liver disease (CLD) due to an exacerbating event with a high probability of accelerated mortality [2]. In contrast to the progressive manifestation of clinical hepatic decompensation events in patients with compensated to decompensated cirrhosis, ACLF patients experience rapid hepatic and extrahepatic failure due to an acute precipitating event [3]. Such precipitating events of ACLF involve extrahepatic and hepatic factors. Extrahepatic factors include gastrointestinal (GI) hemorrhage, procedures, and acute infection [4]. Hepatic factors include viral hepatitis, alcohol intake, drug-induced liver injury (DILI), and autoimmune-related factors [4]. From a macro-perspective, bacterial infections, alcohol overindulgence, and hepatitis B virus (HBV) reactivation are the leading precipitating events for ACLF in the world [5]. Moreau et al. in 2013 [6] published the results of European Association for the Study of the Liver-Chronic Liver Failure Consortium (EASL-CLIF) Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) study conducted at multiple centers in Europe. It was designed to establish the definition of ACLF in a prospective cohort. CANONIC study discovered a greater than 15% mortality rate at 28 days in ACLF patients [6]. ACLF’s pathophysiology has not been definitively established but oxidative stress and extreme systemic inflammation are thought to play a pivotal role in its development [7, 8]. The induced cytokine storm by systemic inflammation is thought to cause portal hypertension, disruption of organ function, and ultimately acute organ failure [9]. Treatment of ACLF includes identifying and eliminating the precipitant, attending to failing organs, and possibly liver transplantation (LT) [10].
The importance of ACLF understanding and characterization lies in the unpredictability of its manifestation at any CLD stage paired with its associated high short-term mortality rate.
The alarmingly high risk of death in a short period of time perpetuated by ACLF has prompted researchers to discern universally accepted identification factors for its diagnosis. As a result, three main variations of ACLF definitions have evolved from the EASL-CLIF, Asian Pacific Association for the Study of the Liver (APASL), and the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) (refer to Table 1) [11]. The EASL-CLIF definition applies to patients with acutely decompensated cirrhosis that experience extrahepatic organ failure, not excluding an extrahepatic precipitation event, and high short-term mortality [12, 13]. The manifestations of acute hepatic decompensation include coagulopathy, hepatic encephalopathy, ascites, GI hemorrhage, and bacterial infections [13]. The use of Sequential Organ Failure Assessment (SOFA) score allows researchers using this definition to confirm organ failure by investigating the function of the brain, kidney, liver, coagulation, circulation, and respiration [12]. Applied to the CANONIC study, which utilized European populations, the EASL-CLIF criteria revealed 30% prevalence of ACLF manifestation in hospitalized cirrhosis patients [5]. Additionally, it was found that decompensated cirrhosis patients with ACLF had a 32.8% mortality rate at 28 days [14]. NACSELD defines ACLF as 2 or more extrahepatic organ failures including grade III and IV hepatic encephalopathy, mechanical ventilation, renal replacement therapy, and shock (circulatory failure) in the presence of acutely decompensated cirrhosis [13]. The utilization of this criteria in a North American population revealed a 24% prevalence of ACLF in admitted cirrhosis patients [5]. In the United States, it was reported that patients with infected decompensated cirrhosis with one to four organ failures had a 30-day mortality rate ranging from 27% to 77% respectively [14, 15]. Ultimately, the ACLF definitions of NACSELD and EASL-CLIF are predominantly used in North America and Europe [12]. Identifying irregularities of organ function, rather than organ failure, in ACLF assessment may lower complications upon ACLF identification and future intervention (such as organ stabilization or facilitated ACLF reversibility). The APASL definition regards ACLF as an acute hepatic injury evident by jaundice and coagulopathy, the presence of ascites and/or hepatic encephalopathy within a month in a patient with CLD or cirrhosis (identified or unidentified), and a 28-day mortality [13, 16]. A retrospective cohort with a sample size of 565 patients found that 41% of patients who underwent a liver transplant in Shanghai, China could be classified as having ACLF when evaluated with APASL criteria [17]. Patients with or without cirrhosis and with no prior decompensation are considered for ACLF evaluation based on the APASL definition in contrast with the EASL-CLIF and NACSELD criteria [18]. While EASL-CLIF includes extrahepatic organ failure in assessment criteria, the APASL definition does not. The use of the APASL definition is beneficial because it can potentially identify patients that are in the early stages of ACLF [9]. In the West, sepsis and bacterial infections, extrahepatic precipitations, and alcoholic hepatitis are among the main acute precipitating events [18, 19]. In contrast, active HBV infection/reactivation was among the most prevalent etiologies of ACLF in the Asia-Pacific region [20]. Events are only classified as acute insults, according to the APASL definition, if they cause acute liver decompensation events such as jaundice, ascites or hepatic encephalopathy, and coagulopathy [12, 18]. Common precipitants, like bacterial infection, in the West would not be classified as acute insults by the APASL definition but rather as complications of the syndrome [10]. The variation in ACLF criteria among the APASL, EASL-CLIF, and NACSELD definitions makes it difficult to accurately quantify the number of patients in different parts of the world that suffer from this syndrome and to predict uniform short-term mortality rates.
Three common acute-on-chronic liver failure definitions
| Definition | The European Association for the Study of the Liver-Chronic Liver Failure Consortium (EASL-CLIF) | Asian Pacific Association for the Study of the Liver (APASL) | North American Consortium for the Study of End-Stage Liver Disease (NACSELD) |
|---|---|---|---|
| Criteria |
|
|
CLIF-SOFA: Chronic Liver Failure Consortium-Sequential Organ Failure Assessment
The establishment of accurate screening and futility criteria in the setting of ACLF would be an advantageous decision-making process for ACLF patients to guide management. In the context of ACLF patients admitted to and undergoing supportive care in the ICU, such criteria would allow the accurate assessment of prolonged ICU stay and urgent LT consideration. Bajaj et al. [13] have proposed the usage of NACSELD and EASL-CLIF ACLF definitions to best estimate ACLF futility and prognosis, respectively. This suggestion was curated using data collected by Cao et al. [21] in which 137 patients of 468 patients were diagnosed with ACLF using the EASL-CLIF ACLF definition (29.3%) and NACSELD ACLF definition (7.4%). Using NACSELD ACLF and EASL-CLIF ACLF criteria to assess 90-day mortality, it was found that NACSELD ACLF criteria had a lower negative predictive value (85.10%) and lower sensitivity (36.08%) when compared to EASL-CLIF ACLF criteria negative predictive value (95.24%) and sensitivity (85.00%) [21]. In terms of 7-day mortality, NACSELD ACLF criteria yielded higher accuracy (98.50%), higher specificity (99.53%), and a higher positive predictive value (94.29%) compared to EASL-CLIF ACLF criteria accuracy (76.62%), specificity (75.40%), and positive predictive value (17.56%) [21]. Transplant-free survival rate was assessed on days 7, 28, and 90 after admission. The EASL-CLIF ACLF transplant-free survival rate at days 7, 28, and 90 were reported to be 89%, 58.4%, and 32.2% respectively [21]. The NACSELD ACLF transplant-free survival rate at days 7, 28, and 90 were 71.4%, 37.1%, and 5.7% respectively [21]. Based on this data, Bajaj et al. [13] concluded that EASL-CLIF ACLF criteria could be used to discern LT consideration and priority for ACLF patients while NACSELD ACLF criteria could be best used to estimate LT futility. Subsequently, Habib et al. [22] validated that NACSELD is best to define futility. In essence, NACSELD ACLF criteria could aid in rapid ICU admission, subsequent organ support management, and for urgent LT consideration or palliative care referral. Categorization based on EASL-CLIF ACLF criteria allows for informed risk assessment and possible urgent liver transplant consideration. A decision-making algorithm that strategically uses ACLF definitions and parameters would best benefit healthcare workers and ACLF patients alike.
A formula for evaluating 90-day mortality in cirrhosis patients known as the Model for End-Stage Liver Disease (MELD) score has been implicated in defining ACLF [22]. The genesis of the MELD score can be traced back to the prognostication of transjugular intrahepatic portosystemic shunt (TIPS) procedure survival assessment in patients with portal hypertension model in 2001 [23]. This score incorporates creatinine level, serum bilirubin, international normalized ratio (INR), and the etiology of cirrhosis into the following formula: “3.8 [loge serum bilirubin (mg/dL)] + 11.2 [loge INR] + 9.6 [loge serum creatinine (mg/dL)] + 6.4” [23]. Later it was modified to MELD-Na and, subsequently, it was modified to MELD 3.0 in which MELD score is adjusted to age, gender, and albumin level [24]. In essence, MELD score reinforces the idea that the sicker patient is prioritized during LT consideration with the lower limit and higher limit score being 6 and 40 [25, 26]. The manifestation of extrahepatic organ failures and sudden hepatic decompensation in ACLF patients may not always be reflected in their calculated MELD score. In a large cohort of veteran affair (VA) data, MELD-Na underestimated short-term mortality compared to expected mortality. Moreover, only < 1% of ACLF cohort was considered and listed for transplantation. Compared to the expected death rate based on MELD-Na, mortality risk was higher for patients with ACLF, and discrepancy increased as the grade of ACLF rose [27]. Patients with severe ACLF are disadvantaged by MELD-based organ allocation policy [28]. This is particularly alarming when considering the role of MELD score in LT allocation. When compared to higher priority patients on the LT waitlist, termed 1a, patients with ACLF involving three or more organ failures had higher 14-day waitlist mortality of 27.7% regardless of MELD-Na scores [29]. At 28 days, ACLF patients with a MELD score of less than 25 had a 43.8% mortality rate [29]. A new scoring approach incorporating ACLF grade, a multiorgan failure assessment, and MELD score in a 90-day mortality assessment has found that ACLF grade has a greater impact at lower MELD scores [30]. Although this method has potentially reconciled the disproportionate consideration of ACLF patients for LT, more studies must be done with larger populations to validate its findings. One in four patients living in the United States die either on the LT waitlist or are not physically capable of surviving LT [31].
In summary, MELD-Na score underestimates the short-term mortality risk, especially among patients with multiorgan organ failure who have relatively low MELD scores. Such patients will be disadvantaged and possibly deprived of the lifesaving treatment option of LT. Standard criteria for ACLF diagnosis must be established and incorporated into LT consideration to strengthen the practice of precision medicine in ACLF patients.
The severity of ACLF as demonstrated by its high short-term mortality rate and sudden manifestation emphasizes the importance of LT in prolonging the lifespan of an afflicted patient. There are 58 federally approved Donation Service Areas in the United States governed by the “Share 15 Regional” policy that mandates a 15 MELD score cutoff [32]. LT can be performed from a living donor (LDLT) or a deceased donor (DDLT) [19]. The number of LTs in the United States has increased over five years to 9,400 total per year with only 4.3% of procedures being LDLTs in 2020 [31]. In 2021, of 9,234 adult LT recipients, 93.8 % underwent DDLT and 6.2 % LDLT [33]. Programs in North America and Europe have modified deceased donor criteria out of increasing organ demand for LTs [34]. The elevated risk of perioperative complications and donor death during LDLT is believed to be the reason for its decreased use in the West [35]. Though the exact number of recipient MELD scores cannot be described, 15.7% of candidates had a MELD score of 25–34 and 4.9% of candidates had a MELD score of 35–39 [31]. The increase in mean MELD score in many regions across the United States has shed light on the beneficial nature of LDLT in that transplantation can be performed when patient health hasn’t significantly deteriorated [32]. The number of liver transplant centers that perform LDLT has increased over 9 years from 28 to 43 in 2019 [36]. Refer to Figure 1 for data comparing LDLTs and DDLTs in the United States during 2022. Data on LDLT in the United States is readily available but variation in ACLF characterization has made it difficult to discern the exact number of ACLF patients that undergo LDLT.
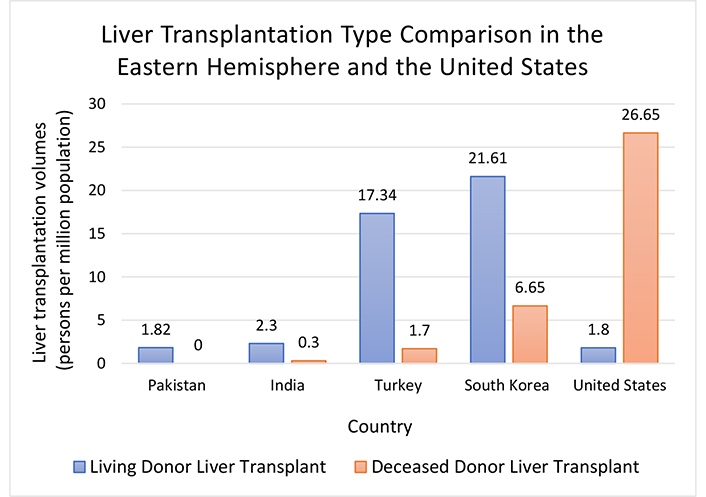
Liver transplantation type comparison in the Eastern hemisphere and the United States: living donor liver transplant vs. deceased donor liver transplant. Graph was constructed using data provided by International Registry in Organ Donation and Transplantation [Internet]. c2024 [cited 2024 Mar 9]. Available from: https://www.irodat.org/
The predominance of LDLT in the Eastern hemisphere (roughly 90% of all LTs), as opposed to DDLT in the United States, can be attributed to differences in culture, religion, and politics [37]. More specifically, a common belief among Southeast Asians regarding the necessity to preserve the human body in its entirety after death has stigmatized the practice of DDLT [38]. The inability to acquire substantial deceased donor parts in the Eastern hemisphere serves as an additional deterrent from DDLT in ACLF patient treatment [19]. In 2022 LT volumes were compared quantitatively by persons per million population, Pakistan recorded 1.82 LDLT to 0 DDLT, India recorded 2.30 LDLT to 0.3 DDLT, Turkey recorded 17.34 LDLT to 1.7 DDLT and South Korea recorded 21.61 LDLT to 6.65 DDLT (refer to Figure 1) [39]. From 1991 to 2013, it was documented that 78 out of 155 European LT centers performed 6,224 LDLTs [40, 41]. Of these LDLT cases, it is unknown how many were performed on ACLF patients. In India, there are approximately 90 to 100 LT centers where in 85% of cases LDLT is performed [42]. A small sample of 218 ACLF patients was observed by Choudhary et al. [42] in which 80% of ACLF grade 1, 72.7% of ACLF grade 2, and 35% of ACLF grade 3 groups underwent LDLT. In Korea, 56 centers performed LTs with 65.1% of LTs being LDLT and 45.3% of LTs being DDLT at five major LT centers in 2020 [43]. As of 2018, there are over 45 LT centers in Turkey with 57% of LT cases being LDLT between 1994 and 2017 [44]. ACLF patients undergoing LDLT in such cases are unknown.
The underutilization of LDLT in the West and DDLT in the East has compelled researchers to investigate the successfulness of both in terms of varying survival rates. A study by Kwak et al. [45] analyzed survival rates in 1,000 LTs, 81.9% LDLTs and 18.1% DDLTs, from 1993 to 2017. One to ten years follow-up observance found that there was a minimal difference in survival rates when comparing LDLT and DDLT transplant groups, with post-10-year survival rates of 69.7% and 62.1% for post-LDLT and DDLT patients respectively. Furthermore, the 1-year survival rates for patients with a high MELD score (greater than or equal to 30) were investigated by Yim et al. [46] in patients undergoing LDLT and DDLT. While the LDLT and DDLT groups had similar rates of rejection at 1 year, the LDLT patients had a lower in-hospital mortality rate of 17.5% compared to 27.1% in DDLT patients. It is clarified that the post-LT mortality for LDLT patients is like that of DDLT patients [46]. In essence, Yim et al. [46] attempted to prove the beneficial nature of LDLT for high MELD score patients compared to awaiting DDLT patients. Moon et al. [47] conducted a retrospective study in which 327 high MELD score patients were divided into non-ACLF and ACLF groups. The ACLF group was determined using the World Congress of Gastroenterology 2014 criteria and divided into three grade groups depending on the number of concurrent organ failures at the time of operation. The graft survival post-LDLT in high MELD score ACLF patients at 1, 3, and 5 years was noticeably worse than non-ACLF patients with 5-year graft survival rates being 70.5% and 81.0% respectively [47]. Regardless, the patient survival rates at each time point were comparable. Moon et al. [47] advocate for the use of LDLT in ACLF patients based on these results but urge LDLT to be conducted hastily in high MELD score patients before ACLF manifestation. Kulkarni et al. [48] found LDLT in APASL-declared ACLF patients from 2019 to 2021 yielded a 73% survival rate after 1 year post operation. In contrast, a study by Singh et al. [49] compared the 1-year post mortality rate of 704 CLD patients and 103 ACLF patients, based on EASL-CLIF criteria, that underwent LDLT. It was discovered that the 1-year post-LT mortality rate in CLD patients was 9.80%, 31.06% in ACLF patients, and 38.89% in ACLF grade 3 patients [49]. The discrepancy in mortality rates was attributed to variability in donor age and respiratory failure before LDLT initiation in ACLF patients [49]. It is difficult to discern graft survival rates in ACLF patients compared to non-ACLF patients in LDLT and DDLT procedures. Graft survival rate is still a useful metric, nonetheless. A study observing graft survival rate after LT in ACLF, defined by EASL-CLIF criteria, and non-ACLF patients was performed by Agbim et al. [50]. Graft survival rate post-LT for non-ACLF patients at 90 and 360 days was 95% and 90% respectively whereas the graft survival rate for ACLF patients at the same time points was 87% and 78% respectively [50]. Though graft survival rates in ACLF patients after 90 days and 360 days were lower than that of non-ACLF patients they were still relatively acceptable. Graft function in ACLF, indicated by EASL-CLIF criteria, and non-ACLF patients post-LT was investigated further by Goosmann et al. [51] by retrospective analysis. Graft function of ACLF and non-ACLF patients were comparable between both groups after assessment of alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, and INR during the follow-up period [51]. Increased availability of LDLT would shorten median waitlist time while decreasing mortality among patients [52]. Still, the use of LDLT generally in the United States is lacking compared to DDLT. A significant increase in LDLT implementation in the United States could circumvent waitlist mortality for ACLF patients and other patients alike.
Though DDLT and LDLT procedures demonstrate increased survival rates compared to patients that do not proceed with either, post-LT ACLF patient quality of life (QoL) must be considered. ACLF QoL post LT was also investigated by Goosmann et al. [51] by using PHQ4, EQ-5D-3L, and WHO-QOL-BREF surveys to assess health status and anxiety and depression prevalence. The ACLF post-LT group had lower scores regarding self-care and unassisted action capability while one out of four patients showed signs of anxiety and depression [51]. With that in mind, the low response rate of both ACLF and non-ACLF post-LT groups decreases the external validity of the researchers’ findings. There is limited information on the QoL assessments of ACLF post LT, especially in terms of DDLT and LDLT.
Considering the high short-term mortality rate of ACLF patients, LT can be regarded as a beneficial and definitive treatment. Chen et al. [53] collected clinical data of 401 transplanted patients, 29 patients having ACLF grade 2–3, and found that 90-day and 1-year survival rates, 89.7% and 87.0% respectively, of transplanted ACLF patients were greater than nontransplant controls. Another study involving 73 patients demonstrated 83.9% 1-year survival in ACLF grade 3 patients, those with 3 or more organ failures, post-LT compared to 7.9% 1-year survival in non-transplanted patients with multiple organ failure [54]. Lastly, a cohort of 200 patients with ACLF was observed at the Paul Brousse Hospital with 25% of the sample receiving a LT [55]. The ACLF grade 3 patients had an 89% 1-year survival rate compared to the 5% 1-year survival rate of nontransplant ACLF patients [55]. When observing the discrepancy in 1-year survival rates between ACLF post-LT patients and nontransplant patients, it is evident that LT leads to an improvement in prognosis.
The optimal timing of LT has been debated among scholars. While early transplantation seems like the most effective route of care, the correction of organ dysfunction before LT in ACLF patients has been argued for. A study of 3,636 ACLF grade 3 patients from the United Network for Organ Sharing database that underwent LT revealed a greater one-year survival percentage, 88.2%, of ACLF patients that improved in ACLF grade before the LT compared to those that did not, 82.0% [56]. However, time allocated to the correction of organ dysfunction, thus prolonging the LT, could potentially risk the worsening of the ACLF grade. It is argued that the fragility of ACLF patients, proven by posttransplant hospitalization frequency and secondary afflictions such as bacterial infection may result in suboptimal recovery post-LT and thus decreased optimization of the transplanted organ use [57]. A precipitant that causes ACLF in a patient may be a contraindication for LT. Uncontrolled infections can worsen with immunosuppressant use, which is needed after a LT, and could result in a lower QoL or survival post-LT [58]. Respiratory failure is also considered as a contraindication by some experts [58]. A study conducted by Artzner et al. [59] between 2007 and 2017 identified the following risk factors that relate to 1-year morality in post LT ACLF grade 3 patients: the individual is 53 years old or older, possesses arterial lactate level greater than or equal to 4 mmol/L, requires mechanical ventilation with a PaO2/FiO2 ratio under 200, and has a leukocyte level under 10 G/L. The overall health of the patient, fitness for surgery, and possible contraindications must be analyzed before proceeding with LT in ACLF patients to circumvent futility of the operation and the worsening of patient health.
In the United States, 12% of candidates die on the LT waitlist and 13% are too sick for the LT procedure [31]. Currently, the Organ Procurement and Transplant Network (OPTN) oversees LT waitlists, transplantation, organ donation, and matching [31]. In the case of LTs, the highest short-term mortality, assessed by MELD 3.0 and MELD-Na score and other criteria, is given priority [31]. As mentioned earlier, criteria for LT candidacy can negatively impact ACLF patients given their higher waitlist mortality. For this reason, a selection process that accounts for ACLF manifestations would sufficiently increase ACLF patient prioritization. The CLIF-SOFA score is a scoring system that has been proven to be accurate in predicting short-term mortality in ACLF patients and thus a better predictor than MELD of mortality in ACLF patients [60, 61]. Thus, consideration of CLIF-SOFA score and MELD score in LT deliberation could potentially benefit ACLF patients in the future. LT candidacy is currently determined by the definitive irreversibility of the liver disease that is deemed fatal in the absence of a liver transplant, high probability of patient survival during the operative and perioperative period, and adequate improvement in patient QoL and survival [62]. Universally accepted contraindications include active alcohol or illicit substance abuse, brain death, uncontrolled sepsis, extrahepatic malignancy, cardiopulmonary disease, anatomic barriers, and acquired immune deficiency syndrome (AIDS) [62]. Contraindications that vary by transplant center include advanced age (typically over 65 years old), portal vein thrombosis (PVT), and risk of noncompliance during post transplantation care [62]. A study conducted by Sacleux et al. [55] found that the main contraindications identified in their study of LT criteria in ACLF patients were active alcohol consumption (73%), severe malnutrition (12.5%), and uncontrolled infections (12.5%). After data analysis, they argued infection should not be considered a contraindication because patients with ACLF can experience systemic inflammatory response syndrome that manifests similarly to infections like sepsis [55]. Cardiovascular health screening for LT consideration assessing coronary arterial disease (CAD) through stress testing has the potential to negatively impact already very ill patients with ACLF, especially those in the ICU [60]. For this reason, coronary angiography has been used to assess CAD in ACLF patients and has proven to be successful [60]. Other methods to sufficiently evaluate ACLF patients, especially those in the ICU, would expedite LT qualification. Organ shortages have made it increasingly formidable to assess LT futility for ACLF patients suffering from multiple organ failures [55]. In Europe and North America, ACLF qualification for LT candidacy is primarily derived from the presence of multiple organ failures, presence of acutely decompensated cirrhosis, and 28-day mortality [19]. The timing of LT is an important and debated topic in the setting of ACLF. Assessing the ability for ACLF patients to recover successfully by means of alternative medical intervention is crucial as LT is advised against in such cases [63]. This decision may be disadvantageous due to the unpredictable nature of ACLF progression. It has been argued that early identification and treatment of ACLF could preserve the remaining hepatocytes of the patient and thus allow for successful liver regeneration before the LT [19]. In this case, LT would be deferred. Short-term mortality rate is shown to increase with higher ACLF grade [19]. Urgent LT is considered for patients with higher ACLF grades, such as those presenting with multiple organ failures. LT futility for severely ill ACLF patients must be evaluated in these cases.
Currently, there is a lack of consensus among LT centers regarding ACLF patient selection criteria based on resources and expertise. Habib et al. [22] aimed to assess the 28-day mortality rate of ACLF patients using a multifaceted approach based on APASL, NACSELD, EASL-CLIF, and MELD criteria to create a formula for identifying and treating potential LT candidates in the setting of ACLF. ACLF classification, ACLF futility, and LT eligibility criteria must be evaluated concurrently to accurately determine the need for LT in ACLF patients. After an exploratory analysis of 623 patients, it was found that a MELD score of 21 yielded the most accurate MELD score prediction cutoff for a 28-day mortality rate of greater than or equal to 15% [22]. Utilizing this finding, 28% of patients in the study met the basic criteria for ACLF diagnosis [22]. According to the data collected by Habib et al. [22], MELD-21 criteria are optimal for identifying potential LT candidates in the setting of ACLF. Patients in the study who met EASL-CLIF and NACSELD ACLF criteria were 31.8% and 22.2% of the sample, respectively [22]. EASL-CLIF and MELD-21 criteria performed equally well at predicting 28-day and 90-day mortality risk [22]. For this reason, when used together, these criteria aid the identification of ACLF patients who could be potential candidates for urgent LT [22] (refer to Figure 2). NACSELD ultimately was declared the most accurate indicator of 28-day morality with specificity and sensitivity being 86% and 88.8%, respectively [22]. EASL-CLIF criteria, like NACSELD, yielded a specificity of 86% [22]. Based on previously published findings, the futility of ACLF and the advantageousness of urgent LT in the setting of ACLF is best estimated using NACSELD criteria [22]. It is suggested that palliative care would be the best option regarding patient care to avoid inevitable resource depletion and prolongment of suffering for ACLF patients who do not meet liver transplant candidacy criteria but meet NACSELD criteria [22]. ACLF patient classification indicated by a high 28-day mortality risk according to MELD-21 criteria would be advantageous in that the need for beneficial, urgent LTs in ACLF patients could be better determined, especially when considering other ACLF definition criteria.
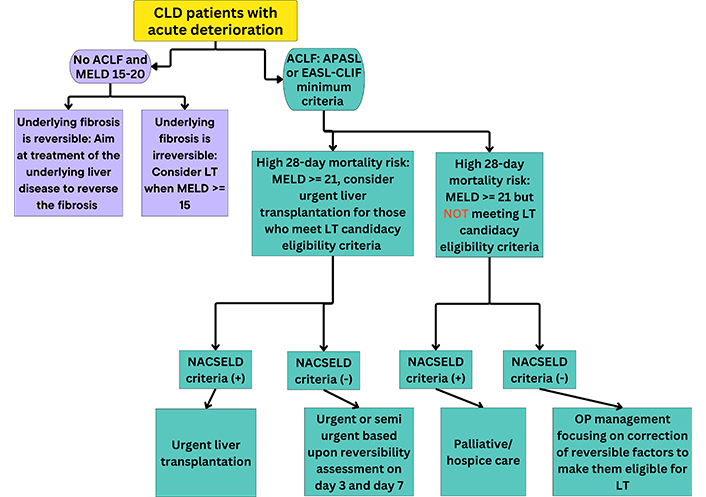
Proposed model for urgent liver transplantation (LT) consideration in the setting of ACLF. This model summarizes urgent LT treatment consideration for ACLF and non-ACLF patients in the context of EASL-CLIF, MELD-21, and NASCELD criteria. CLD: chronic liver disease; ACLF: acute-on-chronic liver failure; MELD: Model for End-Stage Liver Disease; APASL: Asian Pacific Association for the Study of the Liver; EASL-CLIF: European Association for the Study of the Liver-Chronic Liver Failure Consortium; NACSELD: North American Consortium for the Study of End-Stage Liver Disease; OP: outpatient
In summary, LT candidacy must be considered in all patients presenting with ACLF meeting minimum criteria (total bilirubin ≥ 5 mg/dL and INR ≥ 1.5). ACLF grade 3 is an indication for LT. Patients deemed eligible (without contraindications) may be considered for urgent LT if futility criteria are met. Also, patients with estimated high short-term mortality with MELD ≥ 21 without expected reversibility may be considered for urgent LT. We propose an algorithm to identify potential ACLF patients for urgent LT consideration (Figure 2).
In essence, it is important to reconcile the qualification of ACLF criteria, LT eligibility, and futility criteria so the correct patient treatment can be initiated.
ACLF is a complex syndrome with underlying diverse etiopathogenic processes with high short-term mortality rates. There is an unmet need for a simple universal definition of ACLF. LT is the only life-saving and curative option to end patient suffering and fear of death. The majority of ACLF patients are disadvantaged and die without being considered for this life-saving treatment option. The safety and efficacy of both DDLT and LDLT are established even among patients with ACLF grade 3. Additionally, the comparability between LDLT and DDLT success in ACLF patients supports its increased implementation in the West. Current LT listing and allocation are based on MELD score which is the imperfect model to define mortality risk in the setting of ACLF. This creates a disconnect between the current LT listing and organ allocation system and clinically used definitions of ACLF, suggesting an urgent need to update the system. Based on published literature, the authors propose an algorithm (Figure 2) to improve the identification of potential LT candidates sooner for urgent LT. QoL measurements are lacking for ACLF patients, and the creation of a worldwide database could reveal more about the long-term effectiveness of LDLT, DDLT, and LT in general, for this population. As more ACLF-focused research is conducted in the future, the possibility of reconciling the problems mentioned above becomes more promising.
ACLF: acute-on-chronic liver failure
APASL: Asian Pacific Association for the Study of the Liver
CLD: chronic liver disease
DDLT: deceased donor liver transplant
EASL-CLIF: European Association for the Study of the Liver-Chronic Liver Failure Consortium
INR: international normalized ratio
LDLT: living donor liver transplant
LT: liver transplantation
MELD: Model for End-Stage Liver Disease
NACSELD: North American Consortium for the Study of End-Stage Liver Disease
QoL: quality of life
SOFA: Sequential Organ Failure Assessment
AJ: Writing—original draft, Writing—review & editing. SH: Writing—review & editing, Supervision. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Guillermo Alejandro Costaguta, Fernando Álvarez
Thierry Thevenot ... Hilary M. DuBrock
Paul Carrier ... Laure Elkrief
Balasubramaniyan Vairappan ... Mukta Wyawahare
