Affiliation:
1Service d’Hépatogastroentérologie, CHU Dupuytren, 87042 Limoges, France
2Unité INSERM U-1248, Pharmacologie & Transplantation, Faculté de Médecine et Pharmacie, Limoges University, 87024 Limoges, France
3FHU SUPPORT (Fédération Hospitalo-Universitaire SUrvival oPtimization in ORgan Transplantation), 87024 Limoges, France
Email: paul.carrier@chu-limoges.fr
ORCID: https://orcid.org/0000-0001-9750-2506
Affiliation:
1Service d’Hépatogastroentérologie, CHU Dupuytren, 87042 Limoges, France
2Unité INSERM U-1248, Pharmacologie & Transplantation, Faculté de Médecine et Pharmacie, Limoges University, 87024 Limoges, France
3FHU SUPPORT (Fédération Hospitalo-Universitaire SUrvival oPtimization in ORgan Transplantation), 87024 Limoges, France
ORCID: https://orcid.org/0000-0002-6951-0784
Affiliation:
1Service d’Hépatogastroentérologie, CHU Dupuytren, 87042 Limoges, France
2Unité INSERM U-1248, Pharmacologie & Transplantation, Faculté de Médecine et Pharmacie, Limoges University, 87024 Limoges, France
3FHU SUPPORT (Fédération Hospitalo-Universitaire SUrvival oPtimization in ORgan Transplantation), 87024 Limoges, France
ORCID: https://orcid.org/0000-0001-6039-1355
Affiliation:
3FHU SUPPORT (Fédération Hospitalo-Universitaire SUrvival oPtimization in ORgan Transplantation), 87024 Limoges, France
4Service d’Hépatogastroentérologie, CHU Trousseau, 37170 Tours, France
ORCID: https://orcid.org/0000-0003-2843-1710
Explor Dig Dis. 2024;3:362–381 DOI: https://doi.org/10.37349/edd.2024.00056
Received: March 19, 2024 Accepted: June 21, 2024 Published: August 26, 2024
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero-Universitaria di Modena, Italy
The article belongs to the special issue Cirrhosis and Its Complications
Ascites is a frequent complication in patients with cirrhosis, associated with a bad prognosis. Ascites is associated with severe complications, such as spontaneous bacterial peritonitis and kidney dysfunction, which must be diagnosed and managed rapidly. First-line management is based on diuretics use. Beta-blockers role remains debated but an early administration could probably decrease complications associated with portal hypertension. Albumin infusion is validated in large volume paracenteses, spontaneous bacterial peritonitis, or kidney dysfunction, but is debated in other situations. Technical progresses allow the worldwide use of TIPS (transjugular intrahepatic portosystemic shunt), but patient selection must be rigorous because of potential severe complications. An alternative treatment, automated low-flow ascites pump, can be offered in patients without TIPS possibility: It is a recent technique, whose patients’ selection and installation conditions were improved, with interesting results. Liver transplantation remains the gold standard, but the lack of grafts, and specific side effects, lead to prefer other methods. In case of acute kidney injury due to hepatorenal syndrome, terlipressin remains the standard of care; continuous infusion is associated with fewer side effects.
Ascites is a peritoneal effusion. The main cause is cirrhosis in 80% to 90% of patients, and intricate causes, including cirrhosis can be associated and must be screened [1, 2]. Ascites is the most common complication in patients with cirrhosis and impacts significantly quality of life and, overall, life expectancy. That’s why specific and rapid management is necessary. It is associated with specific and severe complications, such as acute kidney injury (AKI) due to hepatorenal syndrome (HRS), spontaneous bacterial peritonitis, hyponatremia, or hepatic hydrothorax.
Ascites is diagnosed at clinical examination, based on the definitions of the consensus conference of the International Ascites Club [3], which remains the standard (Table 1).
Grading of ascites according to the International Ascites Club [3]
| Grade | Amount of fluid in the abdominal cavity | |
|---|---|---|
| 1 | Mild ascites | Only detectable by ultrasound examination |
| 2 | Moderate ascites | Moderate distension of abdomen |
| 3 | Large or gross ascites | Marked abdominal distension |
If ascites is generally controlled thanks to a treatment based on diuretics, in some cases, frequent paracentesis may be required. International Ascites Club gives specific definitions that remain widely used [4]. When ascites recurs and three or more paracenteses are performed within one year, diagnosis of recurrent ascites is retained. Refractory ascites is defined as “ascites that cannot be mobilized or the early recurrence of which (i.e., after large volume paracentesis) cannot be satisfactorily prevented by medical therapy” [4].
Ascites analysis is always necessary to assess its origin and to check for potential ascites infection. The main parameters of ascites associated to cirrhosis are SAAG (serum-ascites albumin gradient) superior to 11 g/L [5], low protein concentration under 15 g/L, neutrophil count under 250/mm3 in absence of infection, and absence of abnormal cell in cytology. In some difficult cases, notably if a malignant cause is suspected, ascites cholesterol concentration can also be discriminant.
In patients with compensated cirrhosis, the incidence of ascites is 5% to 10 % of patients per year. Occurrence of ascites is generally considered as a turning point in patients’ natural history and leads to a mortality risk of 40% at 1 year and 50% at 2 years [6]. Factors associated with high mortality rates include hyponatremia, altered kidney function, sarcopenia, hepatic encephalopathy, and low urinary sodium excretion [7]. Recurrent ascites, and overall refractory ascites, are associated with a lower survival, respectively 50% and less than 50% at 1 year [8, 9]. Presence of ascites is associated with other complications, such as abdominal hernias, respiratory restrictive insufficiency, or sarcopenia [10]. Impact on quality of life is important, notably on working and social life. Hospitalizations are also frequent, with an economic impact [11].
Ascites results from an imbalance between liquid production and absorption in the peritoneum. Cirrhosis decompensation leads to a systemic disease [12]. In case of portal hypertension, it implies a splanchnic arterial vasodilatation, inducing an arteriolar underfilling and an increased pressure in the splanchnic capillaries [13]. Sodium retention mechanisms, including renin-angiotensin-aldosterone system, sympathetic nervous system, and arginine-vasopressin secretion, are induced. Concurrently, increased pressure in splanchnic vessels and capillary leakage lead to an increased lymph production in ascites, exceeding reabsorption capacities.
This situation can favour complications:
In case of advanced splanchnic vasodilatation, effective volaemia is imbalanced, leading to peripheral organ hypoperfusion, particularly in kidneys. When renin-angiotensin-aldosterone system, sympathetic nervous system, is exceeded, renal afferent arteries are constricted with a HRS risk [14].
Similarly, arginine-vasopressin secretion can be altered, inducing a major risk of hyponatremia [15].
A specific condition, cirrhotic cardiomyopathy, can aggravate portal hypertension [16].
Cirrhosis is associated to a chronic inflammation, probably caused by bacteria and bacterial products, called pathogen associated molecular patterns (PAMPs). This situation is favoured by bacterial translocation and microbiota disturbance. Patients are exposed to an increased infection risk, notably ascites infection [17].
Pathophysiology is summarised in Figure 1.
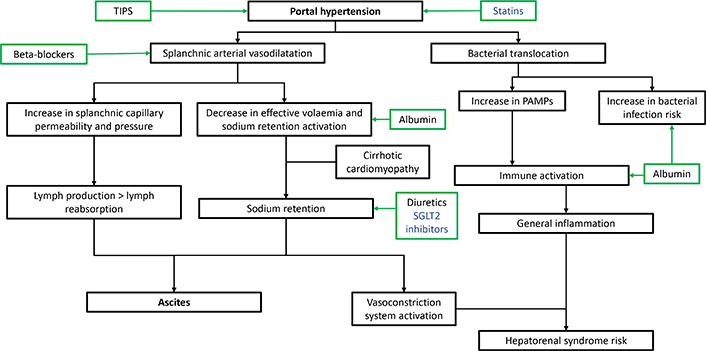
Ascites pathophysiology and mechanisms of action of available treatments. TIPS: transjugular intrahepatic portosystemic shunt; PAMPs: pathogen associated molecular patterns; SGLT2: sodium glucose co-transporter 2
In case of large volume paracentesis, i.e., above 5 litres, the main risk is post paracentesis circulatory dysfunction (PPCD) [18, 19]. PPCD leads to pathophysiological changes, with higher plasmatic renin concentration, higher plasmatic noradrenaline, and higher porto-cave gradient pressure. It enhances hyponatremia, AKI, specifically HRS, early ascites recurrence and mortality [20]. Prevention is based on plasma expansion during the paracentesis, when more than 3 litres of liquid are removed [21]. Even if the studies comparing different plasma expanders show no differences, some concerns remain regarding their use, so albumin is preferred in this situation [2]. In large volume paracentesis, human albumin is the most efficient at 6–8 g of 20% albumin for each litre retrieved [3]. Most recommendations give no limit to the maximum amount of fluid that can be removed during paracentesis [22, 23]. Nevertheless, the limit of 8 litres should not be exceeded, because of a higher PPCD risk, estimated at 40% at 2 years [24]. PPCD risk seems to be higher in case hyponatremia (< 130 mmol/L), hemodynamic instability (systolic blood pressure under 90 mmHg) or AKI and probably needs a more important albumin compensation [25].
It is held that a paracentesis is associated with a low risk of bleeding (3.3% of cases, including 1% of major bleeding), even in case of coagulopathy (INR > 1.5, platelets count < 50,000/mm3) [26].
In absence of specific data, treatment is generally reserved to grade-2 and grade-3 ascites, because of the absence of impact on grade-1 ascites [2]. Also, the paradigm of cirrhosis management tends to change, with the aim to prevent complications as soon as possible [27]. Treating the underlying cause of ascites may allow recompensation of the cirrhosis, notably with alcohol withdrawal, hepatitis B or C treatment [28, 29].
Even if the upright position can reduce diuretics effect and enhance sodium reabsorption, there is no data concerning the effect of prolonged decubitus [30]. Dietary sodium restriction can lead to ascites resolution in 10% of ascites [31]. Because of a risk of kidney injury or hyponatremia in case of excessive sodium restriction, it is recommended a moderate sodium restriction (80–120 mmol per day, corresponding to 4.6–6.9 g) [32].
Due to a higher risk of kidney failure, weight loss should not exceed 0.5 kg in absence of oedema and 1 kg in case of oedema [33]. Urine sodium excretion is a good marker to adapt the diuretics dosage. In 24 hours, excretion must exceed intake (at least, 80 mmol/24 hours) or Na/K ratio must be above 1 [34]. It is considered that in case of resistance to treatment or difficulty to treat, diuretics must be stopped when urine sodium excretion is below 30 mmol/24 hours [2]. Anti-mineralocorticoid is the cornerstone [35]. The initial recommended dose of spironolactone is 100 mg per day, with a maximum of 400 mg. Furosemide is frequently associated. It is introduced at 40 mg per day, with a maximum at 160 mg. The choice of treatment is based on the risk of side effects: generally, short-term anti-aldosterone seems to be more efficient [36] whereas association with loop diuretics is preferred in a long-term management [37]. Adverse effects occur in around 40% of patients. The most frequent are gynecomastia, hepatic encephalopathy, hyponatremia, hypokalemia or hyperkaliemia, and muscle cramps [2]. Sometimes, notably when unbearable symptoms or hyponatremia occur, treatment must be changed: amiloride or eplerenone are an alternative to spironolactone (100 mg of spironolactone corresponds to 10 mg of amiloride or 50 mg of eplerenone.), whereas bumetanide or torsemide can be offered instead of furosemide (furosemide 40 mg corresponds to torsemide 7.5 mg or bumetanide 1 mg) [22].
Since the publication of a study by Sersté et al. [38] in 2010, that suggested a deleterious effects of beta-blockers in patients with refractory ascites, the role of beta-blockers remains unclear. Some studies emphasize a negative role, notably in spontaneous bacterial peritoneal, increasing circulatory dysfunction, whereas others do not show any impact, including in case of ACLF. A recent meta-analysis showed no negative effects, but it was based on 8 studies only [39, 40]. Prospective studies are needed to evaluate the beta-blockers’ role. Baveno VII conference recommends avoiding beta-blockers in case of blood pressure under 90 mmHg, serum creatinine above 1.5 mg/dL or natremia under 130 mmol/L.
The class of alpha-beta-blockers is emerging in portal hypertension. Classical non-heart-selective beta-blockers such as propranolol or nadolol target β1 receptors, which decreases heart rate, and β2 receptors, which impacts α-adrenergic tone on splanchnic circulation. Prazosin, an alpha-blocker, was promising in portal hypertension reduction, in a Spanish study [41] involving 46 patients with portal hypertension. It showed a greater effect of portal pressure lowering in patients treated with propranolol and prazosin, compared to patients treated with propranolol and isosorbide-5-mononitrate (–24.2% ± 11% vs. –16.1% ± 11%; p < 0.01). Carvedilol, an alpha-beta-blocker, progressively replaced classical beta-blockers in portal hypertension. All these treatments were classically used in variceal bleeding primary or secondary prevention. PREDESCI multicentre study assessed the role of carvedilol or propranolol in portal hypertension-associated events, including ascites, in 201 patients with compensated ascites. The results showed that the treatment effectively delayed ascites formation in 9% of patients treated with beta-blockers vs. 20% with placebo, during a median 37-month follow-up (p = 0.03) [42]. Despite some criticisms, notably concerning material and methods (mostly hepatitis C patients included, porto-cave gradient measurements…), and previous studies that showed no differences [43, 44], in 2022, Baveno VII conference recommended introducing carvedilol in case of compensated cirrhosis, even with only a high liver stiffness result [45]. The same year, a meta-analysis (with a 51% weight of PREDESCI cohort) also suggested a benefit of carvedilol in patients with compensated cirrhosis, associated to clinically significant portal hypertension [46].
Sodium glucose co-transporter 2 (SGLT2) inhibitors, a recent class developed in cardiac diseases and diabetes, block glucose and sodium reabsorption in the renal proximal convoluted tube. They logically increase sodium excretion and reduce renin secretion and renin activity at the juxtaglomerular apparatus. Studies were carried out in heart failure, diabetes, and kidney injury essentially [43]. Some case reports suggest a potential benefit on ascites however [47–49]. Studies concerning SGLT2 inhibitors and ascites were registered in ClinicalTrials.gov: NCT05999773, NCT05014594, NCT05013502, but no results have been published yet. The studies characteristics are reported in Table 2.
Tabular comparison if ascites infusion protocols in patients with decompensated cirrhosis
| Name | Year | Country | Study population | Design | Dose | Patients number | Duration | Results |
|---|---|---|---|---|---|---|---|---|
| ANSWER [54] | 2018 | Italy | Uncomplicated ascites with diuretics | Randomized, multicentric, placebo-controlled, open-label | Standard medical treatment (SMT) or SMT plus HA (40 g twice weekly for 2 weeks, and then 40 g weekly) | Albumin: 218SMT: 213 | 18 months | Survival at EOT:Albumin = 77% Control = 66% p = 0.028 |
| MACHT [55] | 2018 | Spain | Patients with ascites on liver transplantation waiting list | Randomized, multicentric, placebo-controlled, open-label | Albumin (40 g every 2 weeks) + midodrine (15–30 mg/day) vs. placebo | Albumin: 87Placebo: 86 | 12 months | No difference in survival or side effects |
| ATTIRE [57] | 2021 | UK | Hospitalized patients with decompensated cirrhosis who had a serum albumin level of less than 30 g per liter at enrollment | Randomized, multicenter, open-label, parallel-group trial | Targeted albumin solution (for serum albumin > 30 g/L) for up to 14 days or until discharge, vs. SMT | Albumin: 380SMT: 397 | 15 days | No difference in a composite criterion (infection, survival, kidney dysfunction) but more side effects in albumin arm |
| Di Pascoli et al. [56] | 2019 | Italy | Patients with refractory cirrhosis | Single-centre, non-randomized open trial | Albumin 40 g per weak vs. SMT | Albumin: 45SMT: 25 | 24 months | Reduction in mortality (p = 0.032), in hospitalization (p = 0.008) |
| PRECIOSA study (NCT03451292) | In progress | Worldwide | Patients with decompensated cirrhosis | Randomized, multicentric, placebo-controlled, open-label | Albumin 1.5 g/kg for 10 days vs. SMT | 410 patients | 1 year | Active, not recruiting |
EOT: end of treatment
Statins are interesting in liver cirrhosis, due to several potential mechanisms of protection in decompensated cirrhosis, particularly reduction in portal pressure, improved liver sinusoidal endothelial dysfunction, and prevention in cirrhosis progression [50]. Despite a probable interest here, clinical impact in ascites is not well known [51].
Albumin role is essential, due to its oncotic, antioxidants, and anti-inflammatory qualities. Cirrhosis induces modifications leading to a decreased production and structure modifications, that alter its functions [52, 53]. Recently, studies investigated the potential long-term benefit of albumin infusions. The ANSWER multicentre study followed 440 patients with uncomplicated ascites, receiving high-dose diuretics [54]. The treatment including 40 g albumin twice a week for two weeks, followed by 40 g weekly was compared to standard medical treatment: it showed a higher overall 18-month survival (77% vs. 66%; p = 0.028). The MACHT study included Spanish patients waiting for liver transplantation [55]. One hundred and ninety-six patients with cirrhosis and ascites were distributed between two arms, one with midodrine and albumin (40 g every 2 weeks) and one with placebo: There was no benefit in survival or complications risk. Another Italian study, with a protocol close to that of ANSWER (albumin: 20 g twice per week) showed the following: cumulative incidence of 24-month mortality was significantly lower in patients treated with albumin than in patients treated with standard of care (SOC) (41.6% vs. 65.5%; p = 0.032) [56]. Hospitalizations for encephalopathy, ascites and infections were less frequent. The ATTIRE study aimed to evaluate the effect of albumin infusion versus SOC treatment in 777 UK patients with cirrhosis [57]. There was no difference in survival between the two arms, but side effects, sometimes severe, like pulmonary oedema were observed. Nevertheless, even though ascites was not an inclusion criterion, the reason for admission was new onset or worsening ascites in 62.1% of patients in albumin group versus 70.8% in SOC group. The ongoing international open-label PRECIOSA study (NCT03451292), comparing an original 1.5 g/kg for 10 days albumin infusion protocol to SOC in patients with decompensated cirrhosis and ascites during one year will probably help in current practice decisions. Furthermore, socio-economic data would probably be warranted in these managements.
Transjugular intrahepatic portosystemic shunt (TIPS) is now a well-developed treatment that allows a rapid decrease in portal hypertension [58]. It was notably developed in patients with recurrent and refractory ascites [59].
Technical progresses were obtained since TIPS development and, nowadays, a polytetrafluoroethylene self-expandable covered 8 mm stent is classically recommended [60, 61]. Seven RCTs [9, 59, 62–66], including 452 patients in total, and 8 meta-analyses [67–74] were published. Despite heterogeneity in material depending on the times (The first TIPS were not covered, and their diameter was higher.), and in patients characteristics (refractory ascites versus recurrent, disease stage), the main results were:
Ascites control is around 60% to 80%.
Global survival is improved, compared to repetition of paracenteses.
TIPS seems to be more efficient in recurrent ascites that in refractory ascites. In refractory ascites, global survival is 93% versus 52%. For this reason, it is suggested to propose TIPS early in disease history.
The main side effects are hepatic encephalopathy, heart insufficiency, and prosthesis infection or dysfunction.
An increase of muscular mass has a positive impact on survival.
Patient selection must be rigorous. A clinical hepatic encephalopathy must be screened as well as related important risk factors, such as large port-systemic shunts, kidney insufficiency, sarcopenia, hyponatremia, elderly.
In case of large shunts, above 6 mm, radiologic occlusion is possible, and limits the risk of hepatic encephalopathy [75].
According to meta-analyses, age above 70 years is associated with elevated hepatic encephalopathy and mortality rates [76]. A prospective model was proposed to determine the risk in patients over 70 years old [76, 77].
Hepatic encephalopathy must be screened before programming TIPS insertion, including minimal encephalopathy evaluation [78, 79]. It is considered as a contraindication to TIPS [80]. In fact, the risk is estimated between 22% and 40% [58] and is decreased with recent 8-mm covered stents [81]. Prevention of encephalopathy is based on rifaximin, independently of TIPS indication [82]. If hepatic encephalopathy cannot be controlled, treatment can be increased wit disaccharides, stent reduction or occlusion, or liver transplantation.
It is important to screen the nutrition status: sarcopenia is associated to complications post-TIPS [67], even if mortality is not increased [83]. TIPS may improve sarcopenia [84].
Hepatic function is a prognostic factor: a model for end-stage liver disease (MELD) score above 18 and Child Pugh C score are often considered as contraindications [79]. Bureau et al. [85] previously showed that platelets count under 75,000/mm3 and a bilirubin concentration above 50 μmol/L are associated with a poorer survival.
Heart evaluation is necessary based on an electrocardiogram, brain natriuretic peptide (BNP) concentration and a transthoracic cardiac ultrasound. Using tissue Doppler of the mitral annulus, the main parameters, to be measured for the diastolic dysfunction are the e’ wave (representing the speed of elongation of the myocardial fibres in the longitudinal plane) and the E/e’ ratio, and measurement of indexed left atrial volume, measurement of systolic pulmonary artery pressure estimated by the peak velocity of tricuspid regurgitation. A screening of valvopathy is recommended, as well as an evaluation of the ventricular systolic function [81, 86, 87]. The Toulouse algorithm, built in 2019, suggested that BNP < 40 pg/mL and NT-proBNP < 125 pg/mL are associated with absence of post-TIPS heart dysfunction [88].
Radiological investigation is recommended for portal thrombosis, liver tumor or vascular malformation screening. In case of portal thrombosis, radiological expertise is often necessary [89].
Other complications, including stent thrombosis on TIPS are rare, but must be known by clinicians [90].
This recent technique aims to resolve ascites thanks to an implantable class III medical device that pumps ascites from the peritoneal space to the urinary bladder. The alfapump® set up requires a surgical procedure, that does not exceed 30 minutes [91]. A pumping system (FlowControlTM software) allows to estimate daily retrieved volume of ascites, based on volume and frequency of recent previous paracenteses [92]. The alfapump® classical indication is refractory ascites with contraindications to TIPS insertion. Also, it can be a waiting treatment, in case of a project of liver transplantation [82]. The alfapump® is contraindicated in case of loculated ascites, untreatable obstructive uropathy, severe life-threatening comorbidities, contraindication to general anesthesia, recent bladder cancer, and ongoing abdominal or urinary tract infection [93]. Available studies include one RCT, 5 observational prospective studies, and 2 retrospective studies. In 2019, a meta-analysis reported a decrease in paracenteses numbers and quantity of fluid removed [pooled estimate rates were 0.62 (95% CI = 0.49–0.74)] for the absence of required large volume paracentesis, in patients receiving automated low-flow ascites pump. However, 30% of these patients experienced an AKI, 27% an ascites infection and 20% a urinary infection, despite the heterogeneities of the studies [94]. In addition, in a real-world patients’ registry, including 106 patients during a 24-month follow-up, the median survival was 10.1 months. One hundred and eight surgical interventions were necessary in 72 patients, including 48 pump explants [95]. It is important to note that seven AKI occurred, not always early, within a median of 160 days (12 to 605 days), with two related deaths. Although it remains an uncommon technique, experience obtained by certain teams and literature data resulted in a recent consensus [93]. Studies did not include patients with a MELD score above 21, the most seen threshold is 15 [96, 97]. The main complications are local infections (pain, local inflammation signs), pump dysfunction and AKI. In this last situation, albumin and eventually terlipressin can be required, with a temporary reduction of retrieved volume. Also, it remains an expensive which is only available in a few centres. Carefully selecting the patients is recommended before implantation, notably concerning the risk of infection and AKI, and a close kidney function follow-up is necessary.
Transplantation is indicated in case of ascites associated with severe cirrhosis, hepatic failure or hepatocellular carcinoma, without possibility or response to classical therapies, including TIPS or automated low-flow ascites pump [2, 22]. Generally, it concerns patients with a MELD score above 18, with impaired kidney function, severe hyponatremia or altered nutritional status. In case of refractory ascites, because of a specific bad prognosis, transplantation waiting list scores are implemented in some countries to reduce waiting time [98]. Also, kidney function must specifically be screened, because of difficulties for a correct renal function evaluation, and an increased kidney impairment [99–101].
Classically, ascites is also observed in the first weeks after liver transplantation in patients with refractory ascites. Low sodium regimen and diuretics can help the management during this period [22].
The traditional definition remains a neutrophil polynuclear count over 250/mm3 in ascites. Clinicians must know that, in case of bloody ascites (red blood cells above 10,000/mm3). For bloody ascites (see definition later), the neutrophil polynuclear count can be corrected by subtracting 1 for every 250 red blood cells in ascites fluid [102]. Furthermore, in this case, the count can decrease to negative numbers, due to cells deterioration. To note, the use of urine dipsticks in the rapid diagnosis of ascites puncture has been widely studied before being abandoned. Clinical signs are usually diarrhoea, abdominal pain, tenderness, and fever, but presentation might be often asymptomatic. The risk of HRS is important, and must be prevented with albumin infusion, 1.5 g/kg at day 1 and 1 g/kg at day 3 [103]. As alternative, an albumin infusion protocol, 1 g/kg at day 1 and 0.5 g/kg at day 3 is possible, but further publications are necessary for its validation [104]. Patients with lower risk of renal impairment, i.e., with serum creatinine below 88 mol/L and with serum bilirubin concentration below 68 mol/L, can be exempted from prevention [105]. Also, spontaneous bacterial peritonitis is an emergency: Kim et al. [106] suggested in 2014 that every late hour increases mortality rate by 3%. Paracentesis control is recommended at 48 hours, and efficiency was defined by a 25% decrease in white cell count. A particular situation is bacterascites, defined by positive cultures without high cells count: It is most often associated to contamination or self-resolution, antibiotics are recommended only in presence of infection signs and a paracentesis control should be performed [107].
In case of infection, a first-line treatment with 3rd generation cephalosporin is recommended. Cefotaxime is generally prescribed at 3 g per day, in two or three administrations, and ceftriaxone is probably more efficient at 2 g per day instead of 1 g, because of a worse distribution in ascites [108, 109]. In case of healthcare-associated or nosocomial spontaneous bacterial peritonitis, the risk of resistance to antibiotics is increased and piperacillin-tazobactam is often the first choice, in case of low sepsis or in area with low prevalence of multidrug resistance. Otherwise, carbapenem alone or combined with daptomycin, vancomycin or linezolid [2] can be used. These recommendations are enforced by recent modifications in ascites bacterial ecology: Cocci Gram positive bacteria prevalence is now superior to Gram negative bacteria prevalence [110, 111], probably due to emerging bacterial resistance to drugs, that impact prognosis [112].
Secondary prevention is recommended to help limiting the risk of a new ascites infection [113]. Primary prophylaxis is reserved for patients with Child Pugh C cirrhosis and a liver transplantation project [2].
This transudative pleural effusion occurs in 5% to 15% of patients with portal hypertension and is due to a unilateral transfer of ascites from peritoneum to pleural cavity through diaphragm breaches [114, 115]. A specific article concerns hepatic hydrothorax in this issue.
The diagnosis of hyponatremia in cirrhotic patients is generally considered under the 130 mmol/L threshold. Twenty-two percent of cirrhotic patients meet this definition, whereas 49% of patients have natremia under 135 mmol/L [15]. Mainly, hyponatremia is associated to hypervolemia and ascites, even if it can be present with hypovolemia (notably, when the diuretics treatment decreases of a too large volume.) or euvolemia. Hyponatremia presence is a risk factor for hepatic encephalopathy (OR: 2.4), spontaneous bacterial peritonitis (OR: 3.5) and HRS (OR: 3.5) and, overall, increases hospitalizations and mortality [15]. For these reasons, the MELD-Na score was built, notably for liver transplant allocations.
Management of hyponatremia differs according to clinicians [116]. Mostly, water restriction, and hypertonic saline or albumin perfusion are preferred and, more rarely, vaptans are used. Nevertheless, international recommendations propose water restriction only in case of mild hyponatremia (125–130 mmol/L). In case of hyponatremia under this threshold, water restriction to 1 litre/day is preferred: the risk of malnutrition and kidney impairment limits this restriction, but in certain cases, water restriction can be decreased to 500 mL/day [22]. Furthermore, diuretics must be stopped. Albumin intake is an option in this situation, particularly in case of hyponatremia under 120 mmol/L, contrary to hypertonic saline, which exposes to a hypervolemia increase [117]. The use of vaptans, vasopressin receptor antagonists, remains rare because of a low efficiency [118, 119]. Vaptans use can expose to dehydration also and, a potential overall hepatocellular risk was reported [120]. That is why its use is limited to 30 days [22].
Hyponatremia correction must not exceed 8 mEq/day of sodium, because of a potential risk of osmotic demyelination syndrome. A cautious follow-up is particularly necessary in liver transplantation, with large fluid movements, leading to a potential risk between 0.5% and 1.5% [121, 122].
Specific criteria of European Working Group on Sarcopenia in Older People assess sarcopenia, incorporating low handgrip strength (< 27 kg for men and < 16 kg for women) with low skeletal muscle index evaluated by CT (< 50 for men and < 39 for women) [123, 124]. Sarcopenia is frequent in cirrhosis, with a prevalence between 23% and 60% [125]. It is strongly associated with cirrhosis complications notably ascites [126], including refractory ascites and infectious complications [127]. Sarcopenia is also associated with a worse prognosis, notably for patients on a liver transplantation waiting list [128].
It is a major problem because of the impact on survival, before (mortality between 22% and 49% at 30 days) [129] or after liver transplantation [130, 131]. Its prevalence ranges between 27% and 53% in hospitalized patients with cirrhosis [132].
AKI is defined by an increase in serum creatinine > 0.3 mg/dL or ≥ 50% in 48 hours, in a 3-stage classification, dependent of creatinine concentrations [133]. Recently, definitions were adapted and HRS-AKI and non-HRS-AKI were distinguished: HRS-AKI corresponds to previous type I HRS, representing around 30% of AKI in cirrhotic patients [101]. Tables 3 and 4 summarize the definitions. Distinction between HRS-AKI and non-HRS-AKI can be difficult in clinical practice, HRS-AKI corresponding to a pre-renal AKI, with tests avoiding parenchymal causes. There is no specific serum or urine biomarker for HRS-AKI, despite investigations. Urinary neutrophil-gelatinase-associated lipocalin (NGAL) is a potential tool: with a cut-off of 220 µg/g, specificity is 85% and sensitivity is 88%, thanks to a dosage at AKI diagnosis and 3 days after [129].
Acute kidney injury (AKI) stage according to International Ascites Club [133]
| AKI stage | Definition |
|---|---|
| 1 | Increase of serum creatinine ≥ 0.3 mg/dL or up to 2-fold from baseline |
| 2 | Increase in serum creatinine between 2-fold and 3-fold from baseline |
| 3 | Increase in serum creatinine > 4 mg/dL (353.6 µmol/L) with an acute increase ≥ 0.3 mg/dL (26.5 µmol/L) or > 3-fold from baseline or serum creatinine or initiation of renal replacement therapy |
Diagnostic criteria of hepatorenal syndrome-acute kidney injury (AKI) according to International Ascites Club [133]
| Criteria of hepatorenal syndrome-AKI |
|---|
| Cirrhosis with ascites |
| Diagnosis of AKI according to International Ascites Club-Acute Kidney Injury criteria |
| No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin infusion (1 g/kg body weight per day) |
| Absence of shock |
| No current or recent use of nephrotoxic drugs (aminoglycosides, NSAIDs, or iodinated contrast media) |
| No signs of structural kidney injury, as indicated by proteinuria (> 500 mg per day), microhematuria (> 50 red blood cells per high-power field), and/or abnormal renal ultrasonography |
HRS management cornerstone is prevention, particularly in case of spontaneous bacterial infection and large volume paracenteses. Also, potential nephrotoxic medications, such as NSAID, must be avoided [134]. In case of HRS-AKI, diuretics, such as beta-blockers, should be stopped according to Baveno consensus, as previously written here [45]. The specific treatment combines terlipressin, if possible, at 3 mg/day as continuous infusion, and albumin [135–138]. Terlipressin must be used cautiously, because of potential side effects: vasoconstriction, notably in peripheral or coronary arteries, hyponatremia, heart arrhythmia, respiratory failure. For these reasons, terlipressin is generally avoided in patients with unstable coronary or ischemic diseases [139]. Norepinephrine is an alternative, but with lower efficiency [140]. Despite this treatment, mortality remains high, reaching nearly 50% [132]. Also, alternatives remain limited as renal replacement therapy is often associated with a high mortality [141]. It is generally reserved to patients with a liver transplantation project or a simultaneous liver-kidney transplantation. This option remains rare but possible, with specific criteria [142, 143]. TIPS insertion is not recommended in this situation, because of a lack of significant results [144].
Ascites is a prognostic stage in cirrhosis natural history and is often associated to potentially lethal complications. Management is based on ascites control, thanks to sodium intake reduction and diuretics, and prevention of complications. Clinicians have also the choice of radical invasive treatments, such as TIPS, liver transplantation and more rarely, low flow pump, but side effects are not negligible in cirrhotic patients. Ongoing and future works will target this early phase, with ascites or just portal significant hypertension, to limit the progression of the illness, with beta-blockers, SGLT2 inhibitors, statins, and eventually TIPS insertion.
AKI: acute kidney injury
BNP: brain natriuretic peptide
HRS: hepatorenal syndrome
MELD: model for end-stage liver disease
PPCD: post paracentesis circulatory dysfunction
SGLT2: sodium glucose co-transporter 2
SOC: standard of care
TIPS: transjugular intrahepatic portosystemic shunt
We thank Sarah Legrand-Demai for her English language review and Céline Rigaud for her precious help.
PC: Conceptualization, Writing—original draft, Writing—review & editing. VLR and MDG: Validation, Writing—review & editing. LE: Validation, Writing—review & editing, Supervision.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Guillermo Alejandro Costaguta, Fernando Álvarez
Andrew Johnson, Shahid Habib
Thierry Thevenot ... Hilary M. DuBrock
Balasubramaniyan Vairappan ... Mukta Wyawahare
