Affiliation:
1Group of Endocrinology, Diabetes and Nutrition, Institut de Recerca Sant Pau, 08041 Barcelona, Spain
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0001-6514-0731
Affiliation:
1Group of Endocrinology, Diabetes and Nutrition, Institut de Recerca Sant Pau, 08041 Barcelona, Spain
2CIBER of Diabetes and Associated Metabolic Diseases (CIBERDEM), Instituto de Salud Carlos III, 28029 Madrid, Spain
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-1500-6379
Affiliation:
1Group of Endocrinology, Diabetes and Nutrition, Institut de Recerca Sant Pau, 08041 Barcelona, Spain
ORCID: https://orcid.org/0000-0001-6748-3156
Affiliation:
1Group of Endocrinology, Diabetes and Nutrition, Institut de Recerca Sant Pau, 08041 Barcelona, Spain
Affiliation:
1Group of Endocrinology, Diabetes and Nutrition, Institut de Recerca Sant Pau, 08041 Barcelona, Spain
ORCID: https://orcid.org/0000-0002-5501-1052
Affiliation:
3Department of Biochemistry and Molecular Biology B and Immunology, Faculty of Medicine, University of Murcia, 30120 Murcia, Spain
ORCID: https://orcid.org/0000-0001-6913-0610
Affiliation:
4Group of Obesity, Diabetes and Metabolism, Instituto Murciano de Investigación Biosanitaria (IMIB), 30120 Murcia, Spain
ORCID: https://orcid.org/0000-0001-6804-5449
Affiliation:
5Genetic Research Unit, Faculty of Chemistry, Autonomous University of Querétaro, 76010 Querétaro, México
ORCID: https://orcid.org/0009-0005-2748-0192
Affiliation:
6LiMitAging, Institute of Metabolic and Cardiovascular Diseases, I2MC, Université de Toulouse, INSERM, Université Toulouse III - Paul Sabatier (UPS), UMR1297, 31432 Toulouse, France
7IHU HealthAge, 31000 Toulouse, France
ORCID: https://orcid.org/0000-0002-0985-3152
Affiliation:
8Faculty of Health Sciences, Universidad Rey Juan Carlos, 28922 Alcorcón, Spain
9CIBER of Cardiovascular Disease (CIBERCV), Instituto de Salud Carlos III, 28029 Madrid, Spain
Email: maria.galana@urjc.es
ORCID: https://orcid.org/0000-0002-4758-8388
Affiliation:
1Group of Endocrinology, Diabetes and Nutrition, Institut de Recerca Sant Pau, 08041 Barcelona, Spain
2CIBER of Diabetes and Associated Metabolic Diseases (CIBERDEM), Instituto de Salud Carlos III, 28029 Madrid, Spain
Email: jjulve@santpau.cat
ORCID: https://orcid.org/0000-0002-6531-2246
Explor Dig Dis. 2025;4:100566 DOI: https://doi.org/10.37349/edd.2025.100566
Received: October 28, 2024 Accepted: January 16, 2025 Published: February 26, 2025
Academic Editor: Luis E. Gomez-Quiroz, Universidad Autonoma Metropolitana, Mexico
The article belongs to the special issue Mitochondria and Lipid Signalling in Liver Diseases
The epidemic of metabolic dysfunction-associated steatotic liver disease (MASLD) is increasingly growing worldwide. Thus, there is an urgent need for novel, non-invasive, and reliable biomarkers to replace liver biopsy for the diagnosis and prognosis of MASLD. Circulating peripheral blood mononuclear cells (PBMCs) are highly responsive to various stimuli and physiological changes. Beyond their immunomodulatory role, PBMC may act as metabolic sensors in several cardiometabolic disorders, including MASLD, with their metabolic programs shifting accordingly. Recent evidence suggests a link between impaired mitochondrial bioenergetics in PBMC and MASLD. Additionally, impaired mitochondrial respiration is intricately linked to the intracellular depletion of the oxidized form of nicotinamide adenine dinucleotide (NAD+) in various cell types. Accumulating preclinical and clinical data show that NAD+-increasing strategies may protect against MASLD by restoring intracellular NAD+ pools and improving mitochondrial performance. This review will focus on [i] the relevance of mitochondrial dysfunction, including impaired bioenergetics, in PBMC as a marker for the diagnosis and prognosis of MASLD, and [ii] the potential benefits of NAD+ precursors in MAFLD and their relationship with improved mitochondrial respiration in blood immune cells.
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is a complex chronic metabolic dysfunction of the liver influenced by both genetic and non-genetic factors [1]. The recent renaming to MASLD expands the definition of NAFLD by requiring the presence of at least one of the components of metabolic syndrome, i.e., obesity, fasting hyperglycemia, elevated triglycerides, reduced HDL cholesterol, and hypertension, alongside hepatic steatosis [1].
The global prevalence of MASLD continues to rise steadily, paralleling the epidemics of obesity [2] and type 2 diabetes mellitus [3]. MASLD is primarily characterized by abnormal accumulation of triglycerides in the liver (hepatic steatosis). It can progress to metabolic dysfunction-associated steatohepatitis (MASH), which is mainly characterized by liver inflammation and injury, and in severe cases, hepatic fibrosis [4]. Both MASH and liver fibrosis are significant risk factors for the onset of cirrhosis and hepatocellular carcinoma, leading causes of liver transplantation [4, 5] and responsible for over 800,000 deaths worldwide [6]. Despite these serious implications, MASH is considered a silent disease, frequently diagnosed incidentally [7]. Therefore, early and accurate diagnosis of MASH along with the assessment of liver fibrosis, is crucial for effective disease management to prevent progression to more advanced stages of liver disease [8].
Mitochondrial performance may fail in target tissues under metabolically adverse conditions such as type 2 diabetes, obesity, dyslipidemia, and cardiovascular diseases [9]. Therefore, mitochondria have emerged as sentinels of cellular metabolic stress [10]. In this regard, mitochondrial dysfunction is increasingly recognized as a key feature of MASLD [11–17]. Accordingly, resmetirom, a recently FDA- and EMA-approved, oral, liver-directed, thyroid hormone receptor beta-selective agonist for treating biopsy-proven MASH with advanced fibrosis, has been shown to restore mitochondrial performance [18].
Accumulating data suggests that the cellular content of oxidized nicotinamide adenine dinucleotide (NAD+) content is frequently reduced in target tissues due to enhanced NAD+ consumption under metabolically adverse scenarios related to impaired insulin signaling conditions, i.e., diabetes, and obesity [19]. Because NAD+ reduction is frequently accompanied by impaired mitochondrial performance concomitant to tissue damage and dysfunction [20, 21], tissue NAD+ restoration has been proposed as a promising therapeutic target in mitochondria-driven strategies to treat metabolic diseases [19]. Supporting this, tissue NAD+ replenishment has been associated with improved mitochondrial function and protection against metabolic dysfunctions [19]. Particularly, hepatic NAD+ decline has been frequently related to MASLD [22], hepatic inflammation [23], and fibrosis [24–27] in experimental models, while its restoration is emerging as a therapeutic approach for MASLD treatment [23, 27–30].
Despite significant advances in MASLD diagnosis [31, 32], histological assessment through a liver biopsy remains the ‘gold standard’ for diagnosing, staging, and prognosing MASLD/MASH, as well as for accurately monitoring fibrosis progression [33]. However, its use is limited by factors such as invasiveness, rare but potentially fatal complications, sample variability, and high costs for the healthcare system [34]. Furthermore, due to the high prevalence of MASH in the general population, implementing a large-scale evaluation plan using liver biopsy is neither feasible nor advised in current clinical practice [35]. Therefore, it is crucial to identify new non-invasive and reliable biomarkers that can act as easily accessible diagnostic alternatives to liver biopsy for the regular monitoring of individuals at high risk of MASLD and its progression to MASH.
Notably, recent studies suggest that circulating peripheral blood mononuclear cells (PBMCs), obtained through minimally invasive approaches such as venipuncture, can serve as sensors of changes in the metabolic environment [36–42]. In the context of MASLD, PBMCs may reflect adaptive characteristics of internal target tissues, which are often challenging to obtain in response to disease states or therapeutic interventions [43, 44]. Supporting this notion, mitochondrial homeostasis is profoundly distorted in human PBMCs under various conditions frequently associated with chronic metabolic stress [36–38, 40], including MASLD [39, 41, 42]. Interestingly, mitochondrial dysfunction in PBMCs has also been recently related to another chronic manifestation of liver disease. Specifically, the degree of hepatic fibrosis has been linked to impaired mitochondrial performance and enhanced oxidative stress in PBMCs in postoperatively Fontan patients [45].
In this review, we will examine the potential of mitochondrial dysfunction in PBMCs as a potential diagnosis/prognosis marker for MASLD. Additionally, we will compile evidence regarding the effects of NAD+-boosting therapies—particularly those involving the administration of NAD+ precursors—on PBMC mitochondrial bioenergetics in the context of MASLD.
Mitochondria represent the main energy hub in hepatocytes, profoundly influencing oxidative metabolism and liver physiology [46, 47]. Under conditions of chronic metabolic stress, mitochondrial function becomes compromised in hepatocytes, including impaired mitochondrial fission and fusion dynamics [48–50]. This results in inefficient fatty acid oxidation and an inability to manage the excessive accumulation of reactive oxygen species (ROS) [46]. The generation and release of ROS, along with by-products derived from lipid peroxidation in the damaged hepatocytes, further triggers the release of inflammatory cytokines [46]. This cascade contributes to the inflammation, fibrosis, and necrosis associated with advanced stages of MASLD.
Numerous studies have proposed that PBMCs can act as sensors for tissue mitochondrial dysfunction in chronic diseases [51–54]. In most of these studies, the assessment of PBMCs as potential functional biomarkers of disease primarily relies on transcriptomic [43, 55–60] and metabolomic analyses [56, 61]. In the context of MASLD, assessing anaplerotic perturbations, inflammation, and oxidative stress in circulating PBMCs has been proposed as non-invasive biomarkers of liver fibrosis and MASH. Particularly, both transcriptomic and metabolomics approaches are widely used to decipher differentially expressed genes and patterns of metabolite intermediates in PBMCs, including those with a role in major bioenergetic pathways and mitochondrial homeostasis [59, 62].
Only a limited number of studies have explored the relationship between mitochondrial quantity and function in PBMCs and MASLD along with its related complications [12–14, 39, 41, 42, 59, 62] (Table 1). Various methodological approaches have been used to assess real-time mitochondrial bioenergetics in circulating PBMCs isolated from MASLD patients. These methods involve the use of mitochondrially-targeted compounds (e.g., oligomycin, FCCP) in conjunction with the measurement of oxygen consumption rate to assess mitochondrial respiration parameters (Seahorse XFp analyzer technology) [39, 62, 63], as well as high-resolution respirometry to evaluate mitochondrial oxidative phosphorylation (OXPHOS) capacity with complex I + II-linked substrates (Oroboros O2k-technology) [42]. Some of these studies have combined functional analyses of mitochondrial bioenergetics in PBMCs with either metabolomic studies to identify differentially-expressed mitochondrial-based molecular profiles [62] or genetic signatures [63] as markers for predicting disease progression in MASLD. In other studies, the assessment of mitochondrial dysfunction was limited to metabolomic patterns [56, 61].
Metrics of PBMCs mitochondrial function in MASLD
| MASLD outcome | Mitochondrial function metric | Key findings | Reference |
|---|---|---|---|
| MASLD (non-biopsed, diagnosed by ultrasound) | Live mitochondria-specific energy changes in PBMCs, determined by Seahorse XFp analyzer. | The real-time assessment showed reduced mitochondrial respiration capacity in PBMCs from MASLD patients. | Garrafa E, 2023 [39] |
| MASLD (simple steatosis, estimated by using the FLI score) | Mitochondrial OXPHOS capacity, determined by proteomics analysis. | Increased OXPHOS capacity with complex I + II-linked substrates in PBMCs from MASLD. | Shirakawa R, 2023 [42] |
| MASLD (diagnosed by biopsy) | Measurement of serum mitochondrial respirometry and hepatic bioenergetic profiles. | Co-presence of loss-of-function polymorphisms in PNPLA3, TM6SF2, and MBOAT7 significantly predisposes individuals to MASLD progression. | Paolini E, 2024 [63] |
| Maximal respiratory capacity. | |||
| MASLD [clinical diagnosis with at least 2 criteria (diabetes, biopsy, hypertriglyceridemia or under treatment, or CT or MRI imaging)] | Gene expression analysis related to inflammatory and metabolic pathways, determined by RNA sequencing. | The identified gene signatures showed enrichment in inflammation and metabolism pathways, suggesting their potential as diagnostic biomarkers for liver diseases. | Listopad S, 2022 [55] |
| MASLD/hepatic fibrosis (diagnosed by biopsy) | Live mitochondria-specific energy changes, determined by Seahorse XFp analyzer. | Significant reduction in mitochondrial energy consumption in PBMCs. | Ajaz S, 2021 [62] |
| MASH [diagnosed by biopsy (n = 43) or ultrasound] | mtDNA copy number as a proxy for mitochondrial mass, determined by transcriptomic analysis. | Reduced mtDNA copy number in PBMCs from MASLD patients compared to healthy subjects. | Lee AH, 2022 [41] |
| MASH (diagnosed by biopsy) | OPA1 (mitochondrial fusion marker), DRP1 (mitochondrial fission marker), and OPA1/DRP1 ratio (mitochondrial fusion/fission balance), determined by protein expression analysis by western blot. | Higher levels of OPA1 protein in MASH patients with significant fibrosis compared to those without fibrosis. Additionally, the OPA1/DRP1 ratio, indicating the balance between mitochondrial fusion and fission, was also higher in these patients. | Kunlayawutipong T, 2024 [59] |
DRP1: dynamin-related protein 1; FLI: fatty liver disease; MASH: metabolic dysfunction-associated steatohepatitis; MASLD: metabolic dysfunction-associated steatotic liver disease; MBOAT7: membrane bound o-acyltransferase domain-containing 7; mtDNA: mitochondrial DNA; OPA1: optic atrophy 1; OXPHOS: oxidative phosphorylation; PBMCs: peripheral blood mononuclear cells; PNPLA3: patatin-like phospholipase domain-containing 3; RNA: ribonucleic acid; TM6SF2: transmembrane 6 superfamily member 2
Mitochondria can adapt their number, mass, and activity through mitohormetic mechanisms in response to changes in energy demand and availability during the early stages of liver disease [10, 11, 64, 65]. However, at advanced stages of MASLD, this mitochondrial flexibility diminishes, leading to oxidative stress, inflammation, and release of mitochondrial damage-associated molecular patterns (mito-DAMPs), with mitochondrial DNA (mtDNA) fragments being one of their major components [66]. These factors collectively contribute to hepatocellular injury. Unfortunately, conventional methods for assessing mitochondrial function often necessitate a liver biopsy [67–69]. Interestingly, studies have reported a lower mtDNA copy number, which serves as a proxy for mitochondria quantity, in PBMCs isolated from patients with MASLD compared to healthy subjects [41]. While the number of mitochondria may not directly correlate with their quality, this finding highlights the potential of mtDNA as a mitochondrial-based metrics in this metabolic disorder.
NAD+ metabolism is complex and multicompartmental [70]. It involves the participation of intracellular and extracellular NAD+-synthesizing and -consuming enzymes, as well as specific cell transporters that facilitate the transport and uptake of nicotinamide-related metabolites [70]. Cellular NAD+ pools are constantly replaced using various precursors, including tryptophan, nicotinic acid (NA), or nicotinamide, through three distinct pathways (Figure 1): [i] the de novo pathway starting from tryptophan, [ii] the Preiss-Handler pathway from NA, and [iii] the salvage pathway, which uses nicotinamide to synthesize nicotinamide mononucleotide (NMN) in a reaction catalyzed by the action of nicotinamide phosphoribosyl transferase (NAMPT), followed by the conversion of NMN to NAD+ via NMN adenylyltransferases [71]. In turn, nicotinamide is produced by various NAD+-consuming enzymes, such as poly-adenosine diphosphate-ribose polymerase (PARPs) isoforms, which are involved in DNA repair [72], and sirtuins (SIRTs), also known as NAD+-dependent protein deacetylases, that are involved in numerous cellular processes, including inflammation and energy metabolism [73, 74]. As a result of NAD+ consumption, nicotinamide is regenerated and either recycled or converted into 1-methylnicotinamide (MeNAM). Additional precursors, such as nicotinamide riboside (NR), are directly converted to NMN, thereby contributing to the replenishment of NAD+. Collectively, these pathways support the dynamic regulation of cellular health [75, 76].
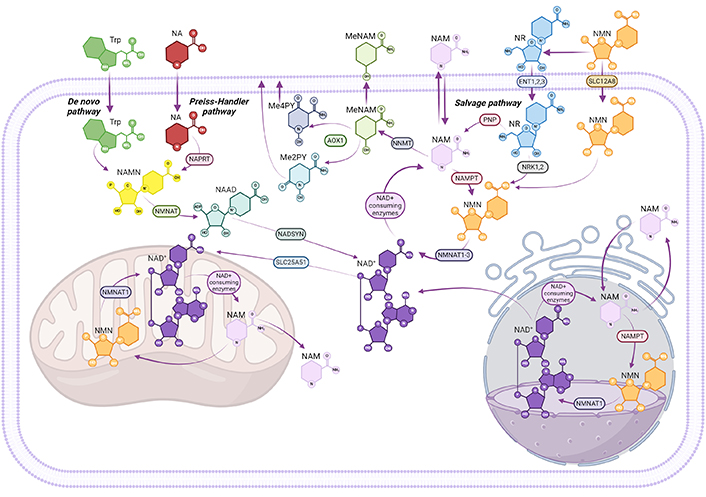
Overview of NAD+ biosynthesis and metabolism. Nicotinamide adenine dinucleotide (NAD+) could be synthesized through three main pathways: the de novo pathway from tryptophan (Trp), the Preiss-Handler pathway from nicotinic acid (NA), and the salvage pathway, from nicotinamide (NAM). In the de novo pathway, Trp is converted to NA mononucleotide (NAMN), and NAMN is further converted to NAD+ through NA adenine dinucleotide (NAAD) and NAD+ synthase (NADSYN). In the Preiss-Handler pathway, NA is converted to NAMN by NA phosphoribosyltransferase (NAPRT), followed by the same steps as the de novo pathway to form NAD+. In the salvage pathway, NAM converges into nicotinamide mononucleotide (NMN), via nicotinamide phosphoribosyltransferase (NAMPT). Nicotinamide riboside (NR) can also be transformed into NMN by nicotinamide riboside kinase 1,2 (NRK1,2). Besides, NMN can be transported into cells directly via the SLC12A8 transporter and converted into NAD+ by nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2). NAD+ is consumed by various enzymes, generating NAM as a byproduct, which can either be recycled or methylated to 1-methylnicotinamide (MeNAM), removing it from the NAD+ pool. NAM metabolites such as MeNAM and N-methyl-2-pyridone-5-carboxamide (Me2PY) are also produced, potentially contributing to distinct metabolic effects. Mitochondrial NAD+ pools are replenished through cytosolic NAD+ import, as mitochondria lack the NAMPT pathway. Created in BioRender. Niño, J. (2025) BioRender.com/y26g025
NAD+ has emerged as an essential molecule in cell physiology. This molecule is used as a key cofactor for hundreds of cellular redox reactions and serves as the mandatory substrate for NAD+-consuming enzymes that are critical players in the control of DNA repair (i.e., PARPs) or modulation of cellular energetic status, especially the activation of oxidative metabolism and stress resistance in mitochondria in various physiological or pathological conditions (i.e., SIRTs) [21, 71]. NAD+ content is negatively influenced by a wide range of metabolic complications and has recently emerged as a potential biomarker for metabolic diseases [19, 77]. Remarkably, NAD+ depletion has been identified as a characteristic feature of MASLD in experimental animals [78, 79], indicating that this molecule could serve as a potential therapeutic target for protecting against hepatic steatosis and further advanced stages such as MASH.
Beyond its role in energy metabolism, NAD+ is also involved in regulating the immune response [77, 80, 81] and adaptation to systemic chronic inflammation and oxidative stress [77, 81–85]. In the context of MASH, aberrant immune activation is frequently observed in NAD+-deficient states [73]. For instance, the expression and activity of several NAD+-consuming enzymes (i.e., PARPs, CD38) in activated immune cells are frequently upregulated during inflammation [20, 21, 38, 82, 86, 87], thereby contributing to further NAD+ depletion. Concomitantly, several NAD+-dependent mechanisms, such as DNA repair and epigenetic reprogramming, are also upregulated in immune cells, further contributing to decreased cellular NAD+ pools [21, 82].
Intracellular NAD+ pools can be replenished through the administration of various nicotinamide-related metabolites, including NMN, NR, and nicotinamide, all of which serve as NAD+ precursors [71]. In the context of MASLD, the beneficial effects of NAD+-boosting strategies have been demonstrated in preclinical models of MASLD [23, 28, 29, 88–90] and MASH/hepatic fibrosis [27, 79] (Table 2).
Effects of NAD+ precursor-based clinical interventions on the liver and isolated PBMCs
| NAD+ precursor | Doses and study design | Liver effects | PBMC effects | Other effects | Clinical trial identifier | Reference |
|---|---|---|---|---|---|---|
| NR | Non-randomized, open-label pharmacokinetic study of 8 healthy volunteers; 250 mg NR were orally (capsules) administered on days 1 and 2, then up-titrated to peak dose of 1,000 mg twice daily on days 7 and 8. On the morning of day 9, subjects completed a 24-hour study after receiving 1,000 mg NR. | Not reported. | nd | No adverse side effects. | NCT02689882 (a) | Airhart SE, 2017 [91] |
| NR | One hundred and forty healthy male and female participants were enrolled in a randomized, double-blind, placebo-controlled parallel study; oral NR (100 mg/day, 300 mg/day, and 1,000 mg/day) were administered over 8 weeks. | Not reported. | nd | No adverse side effects; elevated values of NAD+ derived metabolites in blood and urine. | NCT0271593 (a) | Conze D, 2019 [92] |
| NR | A multicenter, three-arm, randomized, double-blinded, placebo-controlled study in a population of 120 healthy adults between the ages of 60 and 80 years; oral (capsules) combined NR (250 mg and 500 mg/day) and pterostilbene (50 and 100 mg/day) were administered (being NRPT 1× the recommended dose and NRPT 2×, the double of recommended dose, respectively); the intervention phase was 8 weeks with a 30-day follow-up period. | Not reported. | nd | No adverse side effects; elevated values of NAD+ in blood. | NCT02678611 (a) | Dellinger RW, 2017 [93] |
| NR | A randomized, placebo-controlled, double-blinded, and parallel-group designed clinical trial, forty healthy, sedentary men with a BMI > 30 kg/m2, age-range 40–70 years were randomly assigned to 12 weeks of NR (2,000 mg/day) or placebo. | Borderline decrease in hepatic triglycerides. | Not assessed. | No effects on glucose and insulin tolerance; increased urinary NR-derived metabolites. | NCT02303483 (a) | Dollerup OL, 2018 [94] |
| NR | A multicenter, randomized, double blind, placebo-controlled trial; the intervention phase was 26 weeks, with a 14-day follow-up period; participants were randomized into three arms (1:1:1): placebo, recommended dietary supplement dose (NRPT 1×), or double the recommended dose (NRPT 2×). | At the end of the study, no significant change was seen in the primary endpoint of hepatic fat fraction with respect to placebo; a time-dependent decrease in the circulating levels of the liver enzymes ALT and GGT was observed in the NRPT 1× group, and this decrease was significant with respect to placebo. | Not assessed. | A significant decrease in the circulating levels of the toxic lipid ceramide 14:0 was also observed in the NRPT 1× group versus placebo. | NCT03513523 (a) | Dellinger RW, 2023 [95] |
| NR | A randomized, double-blinded, placebo-controlled, crossover intervention study was conducted in 13 healthy overweight or obese men and women. Participants received NR for 6 weeks (1,000 mg/day) and placebo supplementation. | No effects on hepatic lipid accumulation. | nd | Increased skeletal muscle NAD+ metabolites, especially NAAD and MeNAM; supplementation with NR did not result in any change in mitochondrial respiration compared with the placebo state; affected skeletal muscle acetylcarnitine metabolism and induced minor changes in body composition and sleeping metabolic rate. | NCT02835664 (a) | Remie CME, 2020 [96] |
| NR | A 2 × 6-week randomized, double-blind, placebo-controlled crossover clinical trial. Subjects (middle-aged and older men and postmenopausal women aged 55−79 years) ingested NR chloride (NIAGEN®; 500 mg, twice per day; ChromaDex, Inc.) and placebo capsules for 6 weeks each in a randomly determined order. | Not shown. | Elevated content of NAD+ in PBMCs by ~60% compared with placebo, especially NAAD and NAD+. | Increased blood cellular NAD+ concentrations. | NCT02921659 (a) | Martens CR, 2018 [97] |
| NR | Escalating doses of NR (250 mg twice a day for day 1, 500 mg twice a day for day 2, and 1,000 mg twice a day from day 3 on) for 5 to 9 days, 19 hospitalized patients with (stage D HFrEF) heart failure were compared with that of 19 healthy participants. | Not shown. | Improved mitochondrial respiration using standard Seahorse Mito Stress Test; attenuated proinflammatory activation. | nd | NCT03727646 (a) | Zhou B, 2020 [38] |
| NR | A 12-week, randomized, placebo-controlled trial, 30 participants with Stage C HFrEF were randomized to either NR or matching placebo (2:1 allocation ratio). | nd | Improved mitochondrial respiration using a Seahorse Extracellular Flux Analyzer; attenuated proinflammatory activation. | nd | NCT04528004 (a) | Wang DD, 2022 [98] |
| NR | A group of 8 healthy volunteers enrolled in a blood collection protocol to enable the collection of blood cells to test the effects of NR. This group consisted of 6 women and 2 men, with an age range of 23 to 48 years. These subjects had no history of acute or chronic disease. | nd | Increased maximal respiratory capacity assessed by using a Seahorse Extracellular Flux Analyzer; reduced inflammasome induction-mediated IL-1β secretion. | nd | NCT02122575 and NCT00442195 (a) | Traba J, 2015 [99] |
| NR | A 7-day NR supplementation on whole-body metabolism and exercise-induced mitochondrial biogenic signaling in skeletal muscle. Eight male participants received NR (1 g/day) or cellulose placebo supplementation for one week. | Not assessed or reported. | Not analyzed. | No effect of NR supplementation on skeletal muscle NAD+ concentration, but it did increase the concentration of deaminated NAD+ precursors NAR and NAMN and methylated nicotinamide break-down products (Me2PY and Me4PY); global acetylation, auto-PARylation of PARP1, acetylation of p53 Lys382, and MnSOD Lys122 were not affected. | Not reported, but the study was pre-approved by the National Health Service Research Ethics Committee Black Country, West Midlands, UK (17/WM/0321). | Stocks B, 2021 [100] |
| NMN | A 10-week, randomized, placebo-controlled, double-blind trial to evaluate the effect of NMN supplementation on metabolic function in postmenopausal women with prediabetes who were overweight or obese. | nd | Increased basal NAD+ content in PBMCs after treatment. | nd | NCT03151239 (a) | Yoshino M, 2021 [101] |
| NR | A randomized, double-blind, three-arm crossover pharmacokinetic study of oral NR chloride to 12 healthy, non-pregnant subjects (6 males and 6 females) (between the ages of 30 and 55 with body mass indices of 18.5–29.9 kg/m2) was performed at 100, 300 and 1,000 mg doses after overnight fasting were given on 3 test days separated by 7-day periods. | nd | Increased PBMC NAD+ metabolome, especially NAD+, Me2PY, and NAAD was elevated. | Increased NAD+ metabolome in human plasma and urine samples, especially MeNAM, Me2PY, and Me4PY. | NCT02191462 (a) | Trammell SAJ, 2016 [76] |
| NR | Oral administration in 12 aged men with 1 g NR per day for 21 days in a placebo-controlled, randomized, double-blind, crossover trial. | Not assessed; but hepatic ALT was not changed by NR administration. | Not assessed. | Elevations in NAD+ metabolites, and induction of transcriptomic and anti-inflammatory signatures in treated elder skeletal muscle biopsies. | NCT02950441 (a) | Elhassan, 2019 [102] |
| NMN | An open-label, single-arm exploratory study on 10 healthy individuals, including five males and five females (age, 20–70 years), recruited from the Tokyo Tsukishima Clinic; after a 12-hour fast NMN was intravenously administered until the end of the clinical trial; intravenous drip infusion was performed at 5 mL/min by dissolving 300 mg of NMN in 100 mL of saline and inserting an extension tube through a vein in the middle of the arm. | Clinical variables of liver function were within the normal range. | nd | Increased blood NAD+; reduced blood triglycerides. | Not reported but the study was approved by the Japanese Organization for Safety Assessment of Clinical Research (#20210623-02; 23/06/2021) and registered with the University Hospital Medical Information Network (UMIN; Japan) (UMIN-ID: UMIN000047134; 09/03/2022) | Kimura S, 2022 [103] |
| NMN | A single-center, single-arm, open-label trial; twenty-eight healthy adult Japanese (40–60 years) male volunteers; a dose of 250 mg/day was administered for 8 weeks. | Not observed; no changes in hepatic transaminases were seen. | NAD+ levels in PBMCs increased over the course of NMN administration. | No adverse effects were observed. | Japanese 031180242 (b) | Yamaguchi S, 2024 [104] |
| NAM | Seventy diabetic MASLD patients were randomly assigned either to the nicotinamide group (n = 35) who received nicotinamide 1,000 mg once daily for 12 weeks in addition to their antidiabetic therapy or the control group (n = 35) who received their antidiabetic therapy only. | Decreased serum ALT, but no effect on liver fibrosis or steatosis. | nd | nd | NCT03850886 (a) | El-Kady RR, 2022 [105] |
ALT: alanine aminotransferase; BMI: body mass index; GGT: gamma-glutamyl transferase; HFrEF: heart failure with reduced ejection fraction; MeNAM: 1-methylnicotinamide; Me2PY: N-methyl-2-pyridone-5-carboxamide; MnSOD: manganese superoxide dismutase; NAAD: nicotinic acid adenine dinucleotide; NAD+: nicotinamide adenine dinucleotide; NAMN: nicotinic acid mononucleotide; NAR: nicotinic acid riboside; nd: not described; NMN: nicotinamide mononucleotide; NR: nicotinamide riboside; NRPT: nicotinamide riboside and pterostilbene; PARP1: poly-adenosine diphosphate-ribose polymerase 1; PBMCs: peripheral blood mononuclear cells; p53: tumor protein 53. (a) registered at ClinicalTrials.gov (https://clinicaltrials.gov/). (b) registered at jRCT
Pioneering studies primarily conducted with healthy participants over short-term periods were designed to assess the dose tolerance and safety of NAD+ precursors, particularly NR [76] (Table 2). The effect of various NAD+ precursors has been associated with signs of improved liver function in human volunteers [94, 95, 105]. For instance, in one of these studies, while no effects of nicotinamide on MASLD and liver fibrosis were observed, a significant decrease in circulating alanine transaminase levels was reported [105]. In contrast, two other studies reported a significant reduction in hepatic fat content among participants treated with NR compared to those receiving a placebo [94, 95].
Although oral administration of NR did not have a direct impact on liver status, as revealed by the circulating levels of liver enzymes [97], levels of relevant NR-derived metabolites, especially NAD+ and nicotinic acid adenine dinucleotide (NAAD), were significantly elevated in circulating PBMCs of treated human participants [76, 97]. Interestingly, concomitant increases in metabolites related to methylated and oxidized waste products of nicotinamide were also observed in PBMCs, especially MeNAM, N-methyl-2-pyridone-5-carboxamide (Me2PY), and N-methyl-4-pyridone-5-carboxamide (Me4PY) [76] (Table 2). Consistent findings were reported in an independent study showing significant elevations of cellular NAD+ in isolated PBMCs from subjects treated with NMN [104].
Only a few intervention studies have reported that NAD+ precursors, particularly NR, enhance mitochondrial respiration in isolated PBMCs using Seahorse technology [38, 98, 99] (Table 2). This effect was also associated with reduced mitochondrial ROS (mtROS) production and inhibition of the NLRP3 inflammasome, as revealed by decreased IL-1β production from isolated leukocytes obtained from NR-treated human healthy volunteers [99]. However, the impact of this improved bioenergetic and anti-inflammatory profile in PBMC, driven by NR, on MASLD was not explored, as these studies were not focused on MASLD, and liver function was assessed only through routine laboratory tests as part of the safety protocols, with no significant changes reported [99, 104].
MASLD and its related complications can be defined as a complex condition involving both genetic and non-genetic factors, which influence its progression through the MASLD spectrum [106]. Emerging evidence suggests that circulating PBMCs act as sensors of metabolic and immunologic changes associated with the onset of MASLD/MASH [36, 42]. It has been proposed that PBMCs are sensitive to metabolic alterations in internal tissues, including the liver, and can have the capability to modulate their immunomodulatory properties in response to MASLD/MASH progression [43, 44, 107]. Since PBMCs can be obtained through minimally invasive methods, this characteristic has garnered clinical interest in investigating their potential to differentiate between stages of MASLD. However, the contribution of PBMCs to MASLD/MASH diagnosis has been assessed in only a limited number of studies [56, 108, 109].
Mitochondrial performance is tightly linked to the immune response of PBMCs. For instance, recent evidence shows that transcriptomic and metabolomic markers related to mitochondrial dynamics and cell death are differentially expressed in PBMCs from subjects with MASLD/MASH, as demonstrated through transcriptomic [43, 55–59] and metabolomic analyses [61]. Supporting this concept, mitochondrial dysfunction in circulating PBMCs features enhanced immunomodulation in patients with advanced acute-chronic liver diseases [110, 111]. Recent investigations suggest mitochondrial dysfunction in PBMCs could serve as a diagnostic and functional biomarker of MASLD [12–14, 39, 41, 42, 59, 62]. Several studies have used the Seahorse technology to assess mitochondrial function [38, 39, 61, 98, 99], introducing a novel automated methodology to precisely sense ex vivo metabolic alterations associated with MASLD.
The use of NAD+ precursors offers potential new avenues for therapeutic intervention approaches targeting activated immune cells; however, the mechanisms by which NAD+ and its precursors regulate immune cell function are poorly understood, and the contribution of NAD+ metabolizing enzymes to this process remains unclear. Currently, very little is known about the impact of NAD+-based interventions on the metabolic reprogramming and improved mitochondrial respiration of PBMCs in MASLD. Emerging data suggests that NAD+-increasing therapeutic interventions targeting mitochondrial performance in PBMC may show promise in monitoring MASLD [38]. Further research in this area is therefore warranted.
MASH: metabolic dysfunction-associated steatohepatitis
MASLD: metabolic dysfunction-associated steatotic liver disease
Me2PY: N-methyl-2-pyridone-5-carboxamide
MeNAM: 1-methylnicotinamide
mtDNA: mitochondrial DNA
NA: nicotinic acid
NAD+: nicotinamide adenine dinucleotide
NAFLD: non-alcoholic fatty liver disease
NMN: nicotinamide mononucleotide
NR: nicotinamide riboside
PARPs: poly-adenosine diphosphate-ribose polymerase
PBMC: peripheral blood mononuclear cells
SIRTs: sirtuins
JNN, JR, MIRL, E Moreira, E Mateus, and EMR: Writing—original draft, Writing—review & editing. AJRA, BRM, and LOM: Writing—original draft, Writing—review & editing, Funding acquisition. MG: Conceptualization, Writing—original draft, Writing—review & editing. JJ: Conceptualization, Writing—original draft, Writing—review & editing, Funding acquisition. All authors read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This research was supported by Consorcio Centro de Investigación Biomédica en Red – CIBER- de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) (group [CB15/00071], leading group) and CIBER de Enfermedades Cardiovasculares (CIVERCV) [CB16/11/00257], Ministerio de Ciencia e Innovación Instituto de Salud Carlos III (ISCIII), Spain. MIRL holds a predoctoral grant from Pla Estratègic de Recerca i Innovació en Salut (PERIS) 2021-2024 of Generalitat de Catalunya [SLT017/20/000107]. JJ has received financial support from Agencia Estatal de Investigación (MCIN/AEI/10.13039/501100011033 and European Union “NextGeneration EU”/PRTR) within the action “Consolidación Investigadora 2022” [CNS2022-135559], and from Ministerio de Sanidad y Consumo, ISCIII [PI21/00770, PI24/00156], and Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa”. BRM is supported by the “Miguel Servet” program, ISCIII, Spain; co-funded by the FEDER [CP19/00098]. AJRA was funded by Biomedical Research Institute of Murcia [IMIB/CI/09]. LOM has received funding from the IHU HealthAge [ANR-23-IAHU-0011]. Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau (Institut de Recerca SANT PAU) is accredited by the Generalitat de Catalunya as Centre de Recerca de Catalunya (CERCA). JJ also belongs to the XARTEC Salut network, and is part of the coordinated consolidated group AGAUR [2021 SGR 00857, and 2021 SGR 01211]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3054
Download: 46
Times Cited: 0
Sanda Win ... Filbert Win Min Aung
Daniel L. Pouliquen
Vicent Ribas
Laura Fàbrega ... Carmen Garcia-Ruiz
