Affiliation:
1School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 6111137, Sichuan, China
†These authors contributed equally to this work.
Affiliation:
2School of Chinese Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong 999077, China
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0002-3198-6066
Affiliation:
1School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 6111137, Sichuan, China
Email: feigao207@yeah.net
Affiliation:
3Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, Sichuan, China
Email: xcg718@aliyun.com
ORCID: https://orcid.org/0000-0001-9120-8605
Affiliation:
1School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 6111137, Sichuan, China
2School of Chinese Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong 999077, China
3Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, Sichuan, China
Email: abchen@hku.hk
ORCID: https://orcid.org/0000-0001-6494-5271
Explor Drug Sci. 2024;2:234–253 DOI: https://doi.org/10.37349/eds.2024.00044
Received: November 24, 2023 Accepted: February 12, 2024 Published: April 29, 2024
Academic Editor: Fernando Albericio, Universities of KwaZulu-Natal, South Africa; Universidad de Barcelona, Spain
Aim: Isoliquiritigenin (ISL) is a natural flavonoid found in many natural plants, which exhibits numerous pharmacological properties including anti-inflammatory, antioxidant, antitumor, and antiviral activities. However, the low bioavailability and stability of ISL limit its application in clinical practice. To overcome these limitations, ISL-zein phosphatidylcholine hybrid nanoparticles (ISL@ZLH NPs) have been developed to improve the bioavailability and stability of ISL. The present study aimed to evaluate the acute and subacute toxicity of ISL@ZLH NPs in Sprague-Dawley (SD) rats.
Methods: The ISL@ZLH NPs were prepared by the solvent evaporation method. The acute toxicity was evaluated by administering a single dose of 110 mg/kg and 160 mg/kg of ISL@ZLH NPs extracted in distilled water via oral gavage in rats and mice, respectively. The subacute toxicity was evaluated by administering doses of 27.5 mg/(kg∙day), 55 mg/(kg∙day), and 110 mg/(kg∙day) of ISL@ZLH NPs for 30 days and 90 days. The biochemical, hematological, and histopathological parameters were analyzed in both studies.
Results: In the acute toxicity study, no mortality or significant changes in the biochemical and hematological parameters were observed in both Kunming (KM) mice and SD rats. In the subacute toxicity study, no toxic reactions were observed in both species at all doses tested. Moreover, no significant changes in the biochemical, hematological and histopathological parameters were observed in both species.
Conclusions: The results of this study suggest that ISL@ZLH NPs are safe and non-toxic in both KM mice and SD rats. The nanoparticles (NPs) did not induce any adverse effects on the biochemical, hematological, and histopathological parameters in both acute and subacute toxicity studies. These results indicate that ISL@ZLH NPs are safe for prolonged consumption. Further studies are needed to evaluate the long-term toxicity and efficacy of these NPs in vivo.
Isoliquiritigenin (ISL) is a natural flavonoid found in many natural plants, like Spatholobus suberectus Dunn, licorice root, and so on, which has been shown to exhibit a range of pharmacological properties, including anti-inflammatory, antioxidant, antitumor, antiviral, anti-allergic, cardioprotective, hepatoprotective and neuroprotective properties [1, 2]. ISL has been shown to have a potential therapeutic effect in various diseases such as liver diseases, cardiovascular diseases, and neurological disorders [3–5]. It has been shown to inhibit cancer cell proliferation and induce apoptosis of various cancer cells, such as human Caucasian promyelocytic leukemia (HL, HL-60) cell line, colon cancer cells, gastric cancer cells, breast cancer cells, human prostate cancer, and human hepatoma cells [6–8]. Studies have principally focused on ISL’s ability to inhibit cell growth, with evidence showing its effectiveness against most of cancer cells through the induction of apoptosis and autophagy, as well as the regulation of diverse signaling pathways [9].
Additionally, ISL has been found to suppress metastasis of mouse renal, gastric and lung cell carcinoma [10, 11]. Despite its effectiveness in inducing cell apoptosis and autophagy, and regulating various signaling pathways against most cancer cells, the antitumor activity of ISL has been limited due to its low solubility [12]. Furthermore, the low bioavailability and stability of ISL limit its application in clinical practice [13]. To overcome these limitations, researchers have developed various drug delivery systems, including nanoparticles (NPs), to improve the bioavailability and stability of ISL [14].
NPs are particles with a size range of 1–1,000 nm, which have unique properties that make them ideal for drug delivery applications [15]. One such delivery system is the zein phosphatidylcholine hybrid (ZLH) NPs. Zein, a biodegradable and biocompatible protein derived from corn, has been widely used as a nanocarrier due to its ability to self-assemble into NPs under mild conditions [16]. Phosphatidylcholine is a common phospholipid found in cell membranes and has been shown to improve the stability and bioavailability of drugs, which facilitates drug delivery by enhancing transport across cell membranes and the intestinal epithelium [17]. Cholesterol acted as a membrane stabilizer, while lecithin ensured biocompatibility with oral NPs and bound poorly water-soluble drugs. Casein, with its amphiphilic nature, stabilized nanostructures during self-assembly and prevented hydrophobic aggregation in aqueous solutions in various drug delivery systems. As a result, ZLH NPs were developed as a drug delivery system in the laboratory for the purpose of encapsulating ISL, thereby enhancing its bioavailability and stability [12].
The use of NPs as drug delivery systems has gained significant attention in recent years, as they offer numerous advantages over conventional drug delivery systems [18–20]. For instance, NPs can improve the solubility and bioavailability of drugs, increase their half-life, and target specific tissues or cells [1, 13]. Furthermore, NPs can protect drugs from enzymatic degradation, reduce toxicity, and improve patient compliance by reducing the frequency of drug administration [21].
Several studies have investigated the toxicity of ZLH NPs in vitro and in vivo. Investigations have reported the safety and efficacy of ZLH NPs in delivering various drugs, including curcumin, resveratrol, and silymarin [22–25]. In vitro studies have shown that ZLH NPs are non-toxic to various cell lines, including liver cells, lung cells, and cancer cells [26, 27]. In vivo studies have also shown that ZLH NPs are safe and well-tolerated in animals [28, 29]. However, the toxicity of ISL-ZLH NPs (ISL@ZLH NPs) has not been extensively studied.
In addition to the advantages of NPs, there are also some potential risks associated with their use. NPs can accumulate in various organs, leading to toxicity and adverse effects [30, 31]. Therefore, it is essential to evaluate the toxicity and safety of NPs before their clinical application [32]. Toxicity studies are essential for the safety evaluation of any new drug or delivery system. Acute toxicity studies are designed to determine the potential adverse effects of a substance after a single exposure, while subacute toxicity studies evaluate the potential adverse effects of repeated exposure over a period of time [33–36]. Therefore, the present study aimed to evaluate the acute and subacute toxicity of ISL@ZLH NPs in Sprague-Dawley (SD) rats.
ISL was obtained from Damas-beta (Shenzhen, China). Casein was purchased from Adamas (Shanghai, China). Soybean phospholipid was acquired from Aiweituo Pharmaceutical Technology Co., LTD (Shanghai, China). Cholesterol was obtained from Aladdin Reagent Co., LTD (Shanghai, China). Zein was purchased from Tokyo Chemical Industry (Tokyo, Japan). Lecithin was obtained from Solarbio science and technology company (Beijing, China), hematoxylin-eosin staining solution (Sigma Aldrich, MO, USA), Triton x-100 (Sigma), paraformaldehyde (Sigma). The purity of the reagents above are all 98%. Solvents including dimethyl sulfoxide, ethanol, acetonitrile, and methanol (Sigma Aldrich, MO, USA), were of chromatographic grade. All other experiments were performed with Milli-Q deionized water (EMD Millipore, Billerica, MA, USA).
Both sexes of Kunming (KM) mice (15–20 g) and SD rats (105–120 g) were provided by SPF Biotechnology Co., Ltd, China. This study strictly adhered to the guidelines set forth in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. The research protocol received approval from the Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (approval number: 20220421). The room was maintained at a temperature of 25°C ± 1°C, with a relative humidity of 60%, and a 12-h daylight to darkness ratio. The animals had constant access to sufficient food and water. The animals were divided into six groups: Group 1 served as the control group, Group 2 received free ISL treatment, Group 3 was treated with the excipient (ZLH NPs), and Groups 4–6 were administered with respective ISL@ZLH NPs at doses of 27.5 mg/kg, 55.0 mg/kg, and 110.0 mg/kg body weight. All euthanasia procedures were carried out in accordance with the guidelines and regulations established by the institution’s animal care and use committee or equivalent regulatory body. For euthanasia, CO2 was used as the anesthetic for euthanasia purposes. The animal was placed into a euthanasia box and CO2 was infused at a rate of 30% of the box volume per minute until the animal stopped breathing and their pupils dilated. After turning off the CO2 supply, the animal was observed for an additional 2 min to confirm death. Then their organs (liver, spleen, lung, kidney, prostate, testes, urinary bladder, stomach, duodenum, jejunum, colon, thyroid, adrenals, pancreas, salivary gland, lymph nodes, and thymus) were dissected and measured for histopathological analysis.
ISL@ZLH NPs were prepared by the solvent evaporation method, which were formulated with distilled water to the required concentration for the test. Briefly, 30 mg of soybean phospholipid, 6 mg of cholesterol, 1 mg of zein, and 3 mg of ISL were dissolved in 2 mL of 90% ethanol (v/v). The resulting organic phase was slowly added to a 10 mL aqueous phase containing 1 mg of casein, while stirring gently at 500 r/min for 2 h under 40°C to evaporate the ethanol. After stirring, the ISL@ZLH NPs suspensions were centrifuged at 12,000 rpm for 10 min to remove non-encapsulated drugs, and then immediately filtered through a 0.45 μmol/L microporous membrane.
The study monitored the toxic effects of ISL@ZLH NPs in mice (15–20 g) and rats (105–120 g) with a sample size of 10 animals per group. The mice received ISL@ZLH NPs at a concentration of 160 mg/kg body weight via oral gavage, while the rats received 110 mg/kg body weight through the same route. Following administration, the animals were closely monitored for any signs of toxicity or adverse effects, including changes in behavior, appearance, and physiological parameters. Observations were made at regular intervals for a specified duration (e.g., from day 1 to day 7) to assess acute toxicity. The number of deaths and any morbidities were recorded, along with any abnormal behavior or clinical signs. The body weight of the animals was measured before the administration of ISL@ZLH NPs and at specified time points during the observation period. Food consumption was also monitored to assess any potential effects on appetite or feeding behavior. At the end of the observation period, surviving animals were euthanized, and a necropsy was performed. Organs such as the liver, kidney, heart, lungs, and spleen were collected and examined for macroscopic abnormalities. The median lethal dose (LD50) values were calculated using the Bliss method (1938) or any other specific method utilized for this calculation.
A total of 120 rats, aged 5–6 weeks, were divided into six groups of 20 animals each, with both sexes used in the study. Ten males and ten females were included in each group. Group 1 served as the control group, Group 2 received free ISL treatment [55 mg/(kg∙day)], Group 3 was treated with the excipient (ZLH NPs), and Groups 4–6 were administered with respective ISL@ZLH NPs at doses of 27.5 mg/kg, 55 mg/kg, and 110 mg/kg body weight. Subacute studies typically involve daily exposure of test subjects to a substance for a period of 30–90 days. These studies aim to identify the toxic effects of a substance that may occur within this relatively short exposure period. Changes in behavior, clinical signs, and body weight were monitored closely. Each rat was housed individually and provided with a rat diet. Before the endpoint of each period, the rats’ bladders were emptied by suprapubic manipulation immediately after saline or drug administration. Then, they were given 2 mL of water by gavage and placed in metabolism cages. After 5 h, urine was collected and a urine glutamic oxaloacetic transaminase (UGOT) assay was performed. At the end of each study, blood samples were taken from the orbital sinus for red blood cell (RBC) and white blood cell (WBC) counts, prothrombin time (PT), hemoglobin (Hb) and hematocrit (HCT) determinations, and blood glucose (GLU), blood urea nitrogen (BUN), and serum glutamic-pyruvic transaminase (SGPT) assays. At necropsy, the thyroid, adrenals, ovaries, uterus, prostate, testes, spleen, heart, kidneys, and liver were weighed, and the ratios of organ weight to body weight were calculated. The methodology involved evaluating the organ coefficients in ISL@ZLH NPs in comparison to free ISL and control-treated SD rats. The study measured the changes in the size and weight of organs in the rats to determine the effectiveness of the ISL@ZLH NPs. The formula used to calculate the organ coefficients was as follows: [organ weight (g) ÷ body weight] × 100%.
Representative tissues from organs including the liver, spleen, lung, kidney, prostate, testes, urinary bladder, stomach, duodenum, jejunum, colon, thyroid, adrenals, pancreas, salivary gland, lymph nodes, and thymus were fixed in formalin for histopathological examination.
The evaluation of the animals’ eyes, skin, fur, mucous membranes, respiratory system, circulatory system, autonomic nervous system, and central nervous system was performed according to the previous methods [37–40]. The animals’ eyes were visually inspected for any signs of redness, discharge, swelling, or other abnormalities. Additionally, the anterior and posterior segments of the eye, including the cornea, lens, and retina were also examined. The skin and fur were carefully examined for any signs of irritation, redness, swelling, lesions, or abnormal hair loss. The presence of any abnormalities, such as rashes or wounds, was noted. The mucous membranes, including those of the oral cavity and nasal passages, were observed for any signs of inflammation, ulcers, or discoloration. The animals’ respiratory system was assessed by monitoring their respiratory rate and observing any abnormal breathing patterns. The circulatory system was evaluated by monitoring the animals’ heart rate and pulse. Additionally, the color and temperature of the extremities were assessed. The autonomic nervous system was assessed by observing the animals’ response to various stimuli, such as light or sound. Changes in pupil size, salivation, lacrimation, urination, or defecation were noted. The central nervous system was evaluated through a comprehensive neurological examination, which included assessing the animals’ coordination, balance, reflexes, and overall behavior. Any signs of motor abnormalities, tremors, seizures, or changes in consciousness were recorded.
Statistical analysis was performed using GraphPad Prism 9.0 software. The data was presented as means ± standard deviations for 5 animals each. The Dunnett (1964) t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at P < 0.05.
Previously, ISL@ZLH NPs were prepared and characterized in the laboratory. These NPs had a size of approximately 132.06 nm, a polydispersity index of 11.2, and a zeta potential of −37.2 mV [12]. The stability of these nanomaterials was crucial for subsequent experiments. Therefore, ISL@ZLH NPs were stored at 4°C for a period of 14 days. The findings of the present study indicated that ISL@ZLH NPs exhibited consistent particle size, and there were no observable turbidity or impurity peaks. This suggests that the ISL@ZLH NPs were highly stable, even at 14 days of storage at 4°C.
In the acute toxicity study, a single oral dose of ISL@ZLH NPs at a high concentration (160 mg/kg for mice and 110 mg/kg for rats) did not result in mortality in both KM mice and SD rats. Normal patterns of behavior were observed in all groups (Table 1). There were no abnormalities in animals’ eyes, skin, fur, mucous membranes, respiratory system, circulatory system, autonomic nervous system, or central nervous system when treated with ISL@ZLH NPs. In addition, no significant difference was detected in body weights of these groups (Figures 1 and 2).
The body weights and clinical observations of treated KM mice and SD rats were recorded for a period of 7 days
| Groups | Body weight (g) | Death/treated individuals | Mortality latency | Clinical signs | |||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | ||||||
| KM mice | Control | Male | 16.20 ± 1.25 | 16.64 ± 1.13 | 17.14 ± 1.16 | 17.64 ± 1.06 | 0/5 | None | None |
| Female | 18.42 ± 1.75 | 18.90 ± 1.74 | 19.40 ± 1.64 | 19.92 ± 1.61 | 0/5 | None | None | ||
| 160 mg/kg | Male | 15.62 ± 1.15 | 16.16 ± 1.16 | 16.60 ± 1.11 | 17.24 ± 1.09 | 0/5 | None | None | |
| Female | 18.38 ± 0.84 | 18.08 ± 0.81 | 19.32 ± 0.87 | 19.86 ± 0.9 | 0/5 | None | None | ||
| SD rats | Control | Male | 107.06 ± 2.36 | 116.70 ± 2.02 | 126.14 ± 2.61 | 134.20 ± 2.53 | 0/5 | None | None |
| Female | 114.34 ± 2.08 | 123.68 ± 2.32 | 133.74 ± 2.08 | 141.84 ± 3.27 | 0/5 | None | None | ||
| 160 mg/kg | Male | 106.84 ± 0.91 | 116.44 ±1.66 | 127.18 ± 2.41 | 135.02 ± 1.05 | 0/5 | None | None | |
| Female | 116.20 ± 2.54 | 125.44 ± 3.24 | 134.80 ± 3.50 | 143.94 ± 2.30 | 0/5 | None | None | ||
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
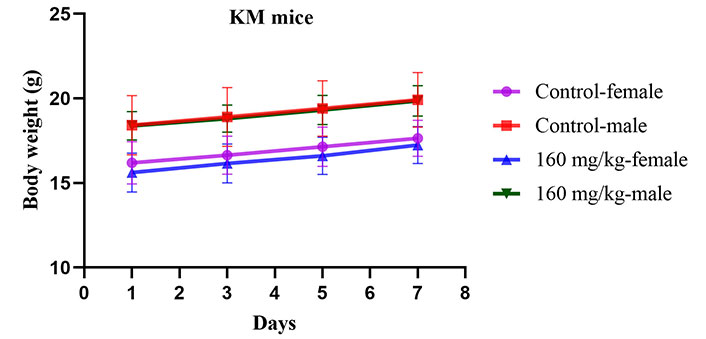
The changes in body weight (g) of KM mice during acute toxicity studies for 7 days. Presented as mean ± standard deviation, with a sample size of 5 mice per group
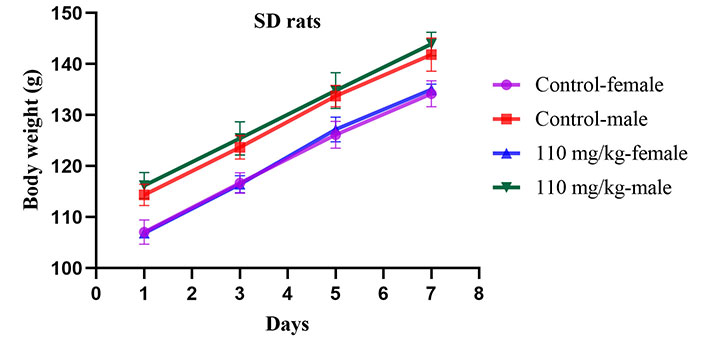
The changes in body weight (g) of SD rats during acute toxicity studies for 7 days. Presented as mean ± standard deviation, with a sample size of 5 rats per group
During the 30-day subacute toxicity studies (Table 2), the body weight of the SD rats remained consistent across all groups, including control, free ISL, excipient, and ISL@ZLH NPs (27.5 mg/kg, 55.0 mg/kg, 110.0 mg/kg body weight) groups, with no statistically significant differences observed among the groups. In male rats, measurements taken at the beginning of the study and at various intervals (1 day: 126.38 g ± 4.85 g, 10 days: 202.04 g ± 19.82 g, 20 days: 247.66 g ± 20.73 g, and 30 days: 276.52 g ± 17.16 g) showed no significant changes compared to the respective control group. Similarly, in female rats, there were no significant changes in body weight at various intervals (1 day: 113.88 g ± 3.91 g, 10 days: 147.42 g ± 8.51 g, 20 days: 170.98 g ± 13.04 g, and 30 days: 189.08 g ± 17.36 g) compared to the respective control group.
Body weight (g) changes in SD rats during subacute toxicity studies for 30 days
| Rats | Treatment (days) | Control | Free ISL | Excipient | ISL@ZLH NPs | ||
|---|---|---|---|---|---|---|---|
| 27.5 mg/kg | 55.0 mg/kg | 110.0 mg/kg | |||||
| Male | 1 | 129.56 ± 6.87 | 127.14 ± 4.28 | 133.24 ± 6.05 | 128.96 ± 1.69 | 129.50 ± 2.55 | 126.38 ± 4.85 |
| 10 | 194.04 ± 8.66 | 187.28 ± 9.75 | 187.46 ± 6.78 | 188.90 ± 4.29 | 192.40 ± 5.16 | 202.04 ± 19.82 | |
| 20 | 243.34 ± 12.88 | 236.66 ± 10.09 | 242.22 ± 7.90 | 242.36 ± 12.66 | 239.26 ± 16.43 | 247.66 ± 20.73 | |
| 30 | 277.56 ± 18.55 | 279.38 ± 13.68 | 278.24 ± 6.70 | 273.76 ± 8.35 | 268.58 ± 17.50 | 276.52 ± 17.16 | |
| Female | 1 | 117.84 ± 6.96 | 114.24 ± 4.73 | 118.72 ± 6.16 | 111.94 ± 9.25 | 118.02 ± 2.89 | 113.88 ± 3.91 |
| 10 | 149.30 ± 8.04 | 141.32 ± 5.16 | 146.08 ± 9.63 | 150.30 ± 12.13 | 146.76 ± 3.75 | 147.42 ± 8.51 | |
| 20 | 174.34 ± 9.84 | 166.76 ± 4.08 | 172.34 ± 16.06 | 174.16 ± 10.97 | 171.44 ± 6.35 | 170.98 ± 13.04 | |
| 30 | 191.26 ± 12.96 | 183.96 ± 6.25 | 191.06 ± 18.76 | 188.86 ± 10.29 | 191.10 ± 6.35 | 189.08 ± 17.36 | |
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
During the 90-day subacute toxicity studies (Table 3), the body weight of the SD rats remained consistent across all groups, including control, free ISL, excipient, and ISL@ZLH NPs groups, with no statistically significant differences observed among the groups. In both male and female rats, measurements taken at the beginning of the study and continued at 10-day intervals until 90 days showed no significant changes compared to the respective control group. The lack of change in body weight suggests that the administration of ISL@ZLH NPs and free ISL and excipient did not have any adverse effects on rats’ growth or metabolism. In the context of an excipient, it refers to the ZLH NPs administered group. This is a positive finding, as changes in body weight could indicate toxicity or other negative effects on rats’ overall health. The stable body weight also suggests that rats’ food intake and activity levels were not affected by the treatment. This supports the overall safety of ISL@ZLH as well as free ISL and excipients.
Body weight (g) changes in SD rats during subacute toxicity studies for 90 days
| Rats | Treatment (days) | Control | Free ISL | Excipient | ISL@ZLH NPs | ||
|---|---|---|---|---|---|---|---|
| 27.5 mg/kg | 55.0 mg/kg | 110.0 mg/kg | |||||
| Male | 1 | 127.92 ± 2.95 | 127.14 ± 3.53 | 127.16 ± 3.35 | 126.60 ± 5.27 | 127.98 ± 5.56 | 131.40 ± 4.49 |
| 10 | 182.10 ± 2.47 | 181.22 ± 3.38 | 180.88 ± 2.68 | 181.00 ± 5.27 | 182.38 ± 5.95 | 185.52 ± 4.74 | |
| 20 | 244.66 ± 1.59 | 243.24 ± 2.00 | 241.54 ± 5.19 | 237.28 ± 7.07 | 248.66 ± 14.30 | 249.48 ± 6.82 | |
| 30 | 294.76 ± 2.98 | 295.54 ± 5.20 | 292.16 ± 6.67 | 284.34 ± 9.65 | 300.64 ± 17.47 | 301.30 ± 6.36 | |
| 40 | 339.54 ± 2.22 | 341.56 ± 2.30 | 339.32 ± 5.39 | 336.54 ± 8.17 | 350.80 ± 13.68 | 347.24 ± 6.57 | |
| 50 | 370.60 ± 0.82 | 372.06 ± 3.48 | 369.32 ± 5.37 | 372.14 ± 5.96 | 381.90 ± 11.25 | 377.92 ± 7.43 | |
| 60 | 408.94 ± 2.05 | 410.00 ± 4.79 | 406.86 ± 4.70 | 411.66 ± 5.20 | 418.40 ± 9.28 | 414.32 ± 7.14 | |
| 70 | 459.06 ± 3.42 | 459.54 ± 5.72 | 457.66 ± 3.47 | 459.58 ± 4.01 | 467.04 ± 7.17 | 460.68 ± 6.43 | |
| 80 | 476.32 ± 3.22 | 476.98 ± 5.41 | 475.80 ± 2.66 | 477.92 ± 4.83 | 485.10 ± 6.03 | 479.76 ± 5.57 | |
| 90 | 499.08 ± 2.51 | 499.36 ± 6.13 | 497.78 ± 3.42 | 500.48 ± 5.31 | 506.58 ± 5.33 | 503.92 ± 5.03 | |
| Female | 1 | 117.26 ± 1.80 | 112.62 ± 7.08 | 113.90 ± 2.49 | 111.14 ± 2.54 | 116.56 ± 6.49 | 118.86 ± 5.67 |
| 10 | 144.16 ± 2.15 | 139.40 ± 6.64 | 139.34 ± 2.38 | 136.08 ± 2.24 | 141.66 ± 7.17 | 145.20 ± 6.41 | |
| 20 | 168.58 ± 4.87 | 161.16 ± 6.83 | 167.26 ± 4.37 | 161.90 ± 2.62 | 168.38 ± 10.08 | 167.44 ± 7.97 | |
| 30 | 203.18 ± 7.61 | 193.42 ± 6.73 | 203.62 ± 3.68 | 192.86 ± 3.24 | 201.62 ± 10.61 | 198.64 ± 9.06 | |
| 40 | 250.20 ± 8.02 | 240.74 ± 5.49 | 251.40 ± 7.72 | 240.04 ± 5.13 | 250.06 ± 10.16 | 248.84 ± 9.36 | |
| 50 | 268.64 ± 7.67 | 260.54 ± 5.27 | 269.48 ± 8.03 | 261.62 ± 5.04 | 268.96 ± 8.48 | 269.74 ± 8.40 | |
| 60 | 283.16 ± 6.86 | 276.06 ± 5.30 | 283.94 ± 6.31 | 277.70 ± 5.02 | 282.68 ± 7.08 | 284.78 ± 7.52 | |
| 70 | 300.38 ± 7.03 | 294.16 ± 5.63 | 302.30 ± 6.12 | 295.38 ± 4.92 | 299.52 ± 6.64 | 302.32 ± 8.11 | |
| 80 | 307.62 ± 6.09 | 303.02 ± 5.47 | 309.16 ± 5.34 | 303.30 ± 4.72 | 307.34 ± 6.43 | 309.88 ± 6.46 | |
| 90 | 320.30 ± 4.19 | 317.14 ± 4.91 | 321.78 ± 4.11 | 315.08 ± 4.63 | 320.20 ± 4.26 | 322.80 ± 6.39 | |
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
In the study, organ coefficients in SD rats were evaluated at two different time points: 30 days and 90 days. The results are presented in Tables 3 and 4. During the 30-day subacute toxicity studies (Table 4), the organ coefficients of SD rats remained consistent across all groups, including control, free ISL, excipient, and ISL@ZLH NPs (27.5 mg/kg, 55.0 mg/kg, 110.0 mg/kg body weight) groups, with no statistically significant differences observed. In male rats, organ coefficients were calculated on the 30th day and showed no significant changes compared to the respective control group for heart (0.371% ± 0.022%), thyroid (0.012% ± 0.001%), spleen (0.228% ± 0.032%), liver (2.497% ± 0.086%), kidneys (0.546% ± 0.012%), adrenals (0.019% ± 0.001%), prostate (0.124% ± 0.009%), and testes (0.660% ± 0.010%). Similarly, in female rats, there were no significant changes in organ coefficients on the 30th day for heart (0.372% ± 0.043%), thyroid (0.013% ± 0.001%), spleen (0.227% ± 0.027%), liver (2.563% ± 0.106%), kidneys (0.551% ± 0.005%), adrenals (0.019% ± 0.001%), ovaries (0.230% ± 0.016%), and uterus (0.055% ± 0.005%) compared to the respective control group. During the 90-day subacute toxicity studies (Table 5), the organ coefficients of SD rats remained consistent across all groups, indicating that the administration of different doses of ISL@ZLH NPs and free ISL-treated groups did not have any adverse effects on the organs compared to the control group.
Changes in organ coefficients (%) in SD rats during subacute toxicity studies for 30 days
| Rats | Organs | Control | Free ISL | Excipient | ISL@ZLH NPs | ||
|---|---|---|---|---|---|---|---|
| 27.5 mg/kg | 55.0 mg/kg | 110.0 mg/kg | |||||
| Male | Heart | 0.357 ± 0.016 | 0.371 ± 0.033 | 0.357 ± 0.008 | 0.359 ± 0.007 | 0.375 ± 0.020 | 0.371 ± 0.022 |
| Thyroid | 0.012 ± 0.001 | 0.011 ± 0.001 | 0.012 ± 0.001 | 0.013 ± 0.001 | 0.012 ± 0.001 | 0.012 ± 0.001 | |
| Spleen | 0.232 ± 0.020 | 0.222 ± 0.022 | 0.228 ± 0.008 | 0.241 ± 0.026 | 0.224 ± 0.027 | 0.228 ± 0.032 | |
| Liver | 2.631 ± 0.072 | 2.606 ± 0.161 | 2.527 ± 0.068 | 2.575 ± 0.116 | 2.564 ± 0.058 | 2.497 ± 0.086 | |
| Kidneys | 0.549 ± 0.011 | 0.550 ± 0.008 | 0.536 ± 0.017 | 0.547 ± 0.003 | 0.554 ± 0.017 | 0.546 ± 0.012 | |
| Adrenals | 0.019 ± 0.001 | 0.019 ± 0.001 | 0.018 ± 0.001 | 0.018 ± 0.001 | 0.019 ± 0.000 | 0.019 ± 0.000 | |
| Prostate | 0.122 ± 0.004 | 0.126 ± 0.005 | 0.126 ± 0.010 | 0.123 ± 0.002 | 0.119 ± 0.004 | 0.124 ± 0.009 | |
| Testes | 0.647 ± 0.012 | 0.657 ± 0.016 | 0.641 ± 0.027 | 0.658 ± 0.013 | 0.652 ± 0.015 | 0.660 ± 0.010 | |
| Female | Heart | 0.350 ± 0.013 | 0.375 ± 0.020 | 0.366 ± 0.015 | 0.374 ± 0.019 | 0.390 ± 0.036 | 0.372 ± 0.043 |
| Thyroid | 0.010 ± 0.005 | 0.012 ± 0.001 | 0.012 ± 0.001 | 0.013 ± 0.001 | 0.013 ± 0.001 | 0.013 ± 0.001 | |
| Spleen | 0.224 ± 0.027 | 0.232 ± 0.043 | 0.226 ± 0.024 | 0.204 ± 0.018 | 0.220 ± 0.033 | 0.227 ± 0.027 | |
| Liver | 2.518 ± 0.168 | 2.500 ± 0.114 | 2.603 ± 0.101 | 2.451 ± 0.109 | 2.609 ± 0.080 | 2.563 ± 0.106 | |
| Kidneys | 0.553 ± 0.013 | 0.549 ± 0.010 | 0.534 ± 0.045 | 0.572 ± 0.029 | 0.553 ± 0.012 | 0.551 ± 0.005 | |
| Adrenals | 0.018 ± 0.001 | 0.019 ± 0.001 | 0.020 ± 0.002 | 0.021 ± 0.003 | 0.018 ± 0.001 | 0.019 ± 0.001 | |
| Ovaries | 0.236 ± 0.010 | 0.248 ± 0.015 | 0.222 ± 0.013 | 0.224 ± 0.016 | 0.228 ± 0.011 | 0.230 ± 0.016 | |
| Uterus | 0.053 ± 0.001 | 0.055 ± 0.001 | 0.054 ± 0.004 | 0.053 ± 0.001 | 0.050 ± 0.002 | 0.055 ± 0.005 | |
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
Changes in organ coefficients (%) in SD rats during subacute toxicity studies for 90 days
| Rats | Organs | Control | Free ISL | Excipient | ISL@ZLH NPs | ||
|---|---|---|---|---|---|---|---|
| 27.5 mg/kg | 55.0 mg/kg | 110.0 mg/kg | |||||
| Male | Heart | 0.316 ± 0.004 | 0.319 ± 0.011 | 0.317 ± 0.008 | 0.323 ± 0.010 | 0.320 ± 0.007 | 0.331 ± 0.015 |
| Thyroid | 0.111 ± 0.001 | 0.107 ± 0.001 | 0.111 ± 0.004 | 0.109 ± 0.002 | 0.108 ± 0.002 | 0.111 ± 0.002 | |
| Spleen | 0.181 ± 0.008 | 0.179 ± 0.008 | 0.181 ± 0.012 | 0.182 ± 0.007 | 0.188 ± 0.002 | 0.188 ± 0.002 | |
| Liver | 2.358 ± 0.037 | 2.320 ± 0.076 | 2.347 ± 0.084 | 2.257 ± 0.083 | 2.360 ± 0.020 | 2.277 ± 0.092 | |
| Kidneys | 0.523 ± 0.013 | 0.513 ± 0.008 | 0.521 ± 0.005 | 0.517 ± 0.009 | 0.520 ± 0.011 | 0.512 ± 0.012 | |
| Adrenals | 0.021 ± 0.001 | 0.020 ± 0.001 | 0.021 ± 0.001 | 0.021 ± 0.001 | 0.020 ± 0.001 | 0.020 ± 0.001 | |
| Prostate | 0.101 ± 0.003 | 0.102 ± 0.006 | 0.101 ± 0.007 | 0.101 ± 0.002 | 0.099 ± 0.010 | 0.101 ± 0.005 | |
| Testes | 0.619 ± 0.008 | 0.635 ± 0.027 | 0.636 ± 0.026 | 0.638 ± 0.014 | 0.620 ± 0.000 | 0.642 ± 0.013 | |
| Female | Heart | 0.329 ± 0.012 | 0.332 ± 0.023 | 0.328 ± 0.005 | 0.319 ± 0.004 | 0.337 ± 0.008 | 0.339 ± 0.010 |
| Thyroid | 0.110 ± 0.004 | 0.110 ± 0.003 | 0.112 ± 0.005 | 0.110 ± 0.003 | 0.109 ± 0.004 | 0.109 ± 0.004 | |
| Spleen | 0.172 ± 0.002 | 0.176 ± 0.004 | 0.174 ± 0.005 | 0.177 ± 0.004 | 0.173 ± 0.003 | 0.178 ± 0.008 | |
| Liver | 2.343 ± 0.091 | 2.411 ± 0.083 | 2.312 ± 0.124 | 2.345 ± 0.063 | 2.355 ± 0.148 | 2.326 ± 0.150 | |
| Kidneys | 0.517 ± 0.016 | 0.525 ± 0.011 | 0.515 ± 0.006 | 0.509 ± 0.026 | 0.510 ± 0.017 | 0.515 ± 0.010 | |
| Adrenals | 0.020 ± 0.001 | 0.020 ± 0.001 | 0.021 ± 0.001 | 0.020 ± 0.000 | 0.020 ± 0.003 | 0.021 ± 0.002 | |
| Ovaries | 0.206 ± 0.004 | 0.192 ± 0.002 | 0.207 ± 0.007 | 0.207 ± 0.009 | 0.203 ± 0.010 | 0.208 ± 0.005 | |
| Uterus | 0.046 ± 0.001 | 0.044 ± 0.002 | 0.043 ± 0.001 | 0.044 ± 0.003 | 0.046 ± 0.002 | 0.044 ± 0.002 | |
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
Organ coefficients are a measure of the relative weight of an organ compared to the total body weight of an animal. It is an important parameter that is used to evaluate the toxicity of a substance. An increase in organ coefficients indicates that the organ is growing larger than expected, which could be a sign of toxicity. On the other hand, a decrease in organ coefficients could indicate that the organ is shrinking, which could also be a sign of toxicity. The fact that there were no significant differences in organ coefficients between the groups at both time points suggests that the substance did not cause any adverse effects on the organs of the rats. This is an important finding as it indicates that the substance is safe for use in animals.
The results of the volume of urine and UGOT did not show any differences over the 30-day and 90-day periods, as presented in Table 6. During the 30-day subacute toxicity studies (Table 6), the urine volume and UGOT of SD rats remained consistent across all groups, including control, free ISL, excipient, and ISL@ZLH NPs (27.5 mg/kg, 55.0 mg/kg, 110.0 mg/kg body weight) groups, with no statistically significant differences observed. In male rats, urine volume was 1.37 mL ± 0.03 mL and UGOT levels were 3.13 mL ± 0.05 mL on the 30th day, and 3.37 mL ± 0.08 mL and 3.21 mL ± 0.06 mL, respectively, on the 90th day. The data indicated that there were no significant changes in the volume of urine or UGOT compared to the respective control group in male rats. In female rats, urine volume was 1.25 mL ± 0.05 mL and UGOT levels were 2.73 mL ± 0.07 mL on the 30th day, and 2.12 mL ± 0.04 mL and 2.90 mL ± 0.06 mL, respectively, on the 90th day (Table 5). The data revealed no significant alterations in the volume of urine or UGOT compared to the corresponding control group in female rats. These findings imply that the experimental treatment did not exert significant effects on these measures in male or female rats, thus indicating no significant detrimental impacts on the rats’ health throughout the study.
Evaluation of the changes in urine volume (mL) and UGOT (U/L) of SD rats after 30 days and 90 days of subacute toxicity studies
| SD rats | Period of treatments | Tests | Control | Free ISL | Excipient | ISL@ZLH NPs | ||
|---|---|---|---|---|---|---|---|---|
| 27.5 mg/kg | 55.0 mg/kg | 110.0 mg/kg | ||||||
| Male | 30 days | Urine volume (mL) | 1.38 ± 0.03 | 1.37 ± 0.02 | 1.39 ± 0.03 | 1.39 ± 0.04 | 1.36 ± 0.03 | 1.37 ± 0.03 |
| UGOT (U/L) | 3.15 ± 0.07 | 3.10 ± 0.03 | 3.14 ± 0.06 | 3.13 ± 0.07 | 3.15 ± 0.06 | 3.13 ± 0.05 | ||
| Female | 30 days | Urine volume (mL) | 1.26 ± 0.04 | 1.25 ± 0.03 | 1.24 ± 0.04 | 1.24 ± 0.04 | 1.23 ± 0.04 | 1.25 ± 0.05 |
| UGOT (U/L) | 2.78 ± 0.06 | 2.74 ± 0.04 | 2.75 ± 0.08 | 2.73 ± 0.09 | 2.75 ± 0.10 | 2.73 ± 0.07 | ||
| Male | 90 days | Urine volume (mL) | 3.35 ± 0.04 | 3.34 ± 0.07 | 3.32 ± 0.06 | 3.34 ± 0.09 | 3.36 ± 0.04 | 3.37 ± 0.08 |
| UGOT (U/L) | 3.26 ± 0.04 | 3.23 ± 0.07 | 3.25 ± 0.07 | 3.22 ± 0.05 | 3.24 ± 0.07 | 3.21 ± 0.06 | ||
| Female | 90 days | Urine volume (mL) | 2.12 ± 0.03 | 2.11 ± 0.04 | 2.13 ± 0.05 | 2.12 ± 0.03 | 2.12 ± 0.05 | 2.12 ± 0.04 |
| UGOT (U/L) | 2.87 ± 0.07 | 2.89 ± 0.08 | 2.88 ± 0.08 | 2.91 ± 0.05 | 2.86 ± 0.09 | 2.90 ± 0.06 | ||
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
The results of the hematological parameters did not show any differences over the 30-day and 90-day periods, as presented in Tables 7 and 8. During the 30-day subacute toxicity studies (Table 7), the hematological parameters of SD rats remained consistent across all groups, including control, free ISL, excipient, and ISL@ZLH NPs (27.5 mg/kg, 55.0 mg/kg, 110.0 mg/kg body weight) groups, with no statistically significant differences observed. In male rats, hematological parameters were determined on the 30th day and showed no significant changes in RBC [(7.06 ± 0.07) × 1012/L], WBC [(12.29 ± 1.10) ×109/L] Hb (218.49 g/L ± 3.94 g/L), HCT (45.24% ± 0.43%), GLU (9.62 mmol/L ± 0.32 mmol/L), BUN (5.62 mmol/L ± 0.23 mmol/L), SGPT (51.40 U/L ± 2.36 U/L), and PT (12.90 s ± 0.43 s) compared to the respective control group. Similarly, in female rats, there were no significant changes in hematological parameters on the 30th day for RBC [(7.06 ± 0.07) × 1012/L], WBC [(12.29 ± 1.10) × 109/L], Hb (218.49 g/L ± 3.94 g/L), HCT (45.24% ± 0.43%), GLU (9.62 mmol/L ± 0.32 mmol/L), BUN (5.62 mmol/L ± 0.23 mmol/L), SGPT (51.40 U/L ± 2.36 U/L), and PT (12.90 s ± 0.43 s) compared to the respective control group.
Changes in the hematological parameters of SD rats in subacute toxicity studies for 30 days
| Rats | Organs | Control | Free ISL | Excipient | ISL@ZLH NPs | ||
|---|---|---|---|---|---|---|---|
| 27.5 mg/kg | 55.0 mg/kg | 110.0 mg/kg | |||||
| Male | RBC (× 1012/L) | 7.13 ± 0.06 | 7.09 ± 0.04 | 7.10 ± 0.04 | 7.12 ± 0.04 | 7.12 ± 0.04 | 7.06 ± 0.07 |
| WBC (× 109/L) | 11.56 ± 0.22 | 12.34 ± 1.04 | 11.69 ± 0.12 | 11.53 ± 0.63 | 12.32 ± 0.94 | 12.29 ± 1.10 | |
| Hb (g/L) | 216.03 ± 1.30 | 214.61 ± 3.99 | 214.40 ± 2.21 | 214.73 ± 3.69 | 217.63 ± 4.15 | 218.49 ± 3.94 | |
| HCT (%) | 45.55 ± 0.36 | 45.71 ± 0.25 | 45.29 ± 0.78 | 45.20 ± 0.60 | 45.81 ± 0.26 | 45.24 ± 0.43 | |
| GLU (mmol/L) | 9.57 ± 0.21 | 9.68 ± 0.38 | 9.84 ± 0.29 | 9.70 ± 0.36 | 9.70 ± 0.29 | 9.62 ± 0.32 | |
| BUN (mmol/L) | 5.71 ± 0.21 | 5.60 ± 0.18 | 5.77 ± 0.09 | 5.63 ± 0.25 | 5.58 ± 0.18 | 5.62 ± 0.23 | |
| SGPT (U/L) | 52.78 ± 2.06 | 52.68 ± 1.80 | 52.32 ± 1.91 | 52.46 ± 2.05 | 51.82 ± 2.40 | 51.40 ± 2.36 | |
| PT (s) | 12.66 ± 0.22 | 12.70 ± 0.21 | 12.72 ± 0.13 | 12.30 ± 0.42 | 12.42 ± 0.31 | 12.90 ± 0.43 | |
| Female | RBC (× 1012/L) | 7.08 ± 0.06 | 7.10 ± 0.04 | 7.05 ± 0.04 | 7.16 ± 0.05 | 7.08 ± 0.04 | 7.06 ± 0.07 |
| WBC (× 109/L) | 11.22 ± 0.43 | 11.70 ± 1.06 | 11.68 ± 0.90 | 11.47 ± 0.64 | 11.77 ± 1.05 | 12.29 ± 1.10 | |
| Hb (g/L) | 216.85 ± 2.09 | 212.51 ± 2.62 | 215.21 ± 3.66 | 216.39 ± 3.69 | 215.83 ± 5.01 | 218.49 ± 3.94 | |
| HCT (%) | 44.95 ± 0.47 | 45.34 ± 0.52 | 45.85 ± 0.73 | 45.43 ± 0.29 | 45.23 ± 0.38 | 45.24 ± 0.43 | |
| GLU (mmol/L) | 9.68 ± 0.29 | 9.87 ± 0.18 | 9.71 ± 0.16 | 9.67 ± 0.28 | 9.52 ± 0.22 | 9.62 ± 0.32 | |
| BUN (mmol/L) | 5.68 ± 0.25 | 5.62 ± 0.20 | 5.76 ± 0.15 | 5.64 ± 0.23 | 5.75 ± 0.21 | 5.62 ± 0.23 | |
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
Changes in the hematological parameters of SD rats in subacute toxicity studies for 90 days
| Rats | Organs | Control | Free ISL | Excipient | ISL@ZLH NPs | ||
|---|---|---|---|---|---|---|---|
| 27.5 mg/kg | 55.0 mg/kg | 110.0 mg/kg | |||||
| Male | RBC (× 1012/L) | 7.39 ± 0.13 | 7.29 ± 0.12 | 7.34 ± 0.14 | 7.30 ± 0.18 | 7.24 ± 0.15 | 7.31 ± 0.10 |
| WBC (× 109/L) | 11.94 ± 0.38 | 11.81 ± 0.42 | 11.91 ± 0.66 | 11.81 ± 0.48 | 11.49 ± 0.62 | 12.03 ± 0.68 | |
| Hb (g/L) | 214.45 ± 4.93 | 213.00 ± 4.81 | 219.44 ± 2.95 | 218.78 ± 3.60 | 218.95 ± 6.26 | 218.39 ± 6.58 | |
| HCT (%) | 45.99 ± 1.07 | 45.59 ± 0.81 | 45.57 ± 1.06 | 45.35 ± 0.40 | 45.92 ± 1.34 | 45.70 ± 1.02 | |
| GLU (mmol/L) | 9.76 ± 0.29 | 9.80 ± 0.25 | 9.53 ± 0.20 | 9.53 ± 0.37 | 9.85 ± 0.08 | 9.86 ± 0.23 | |
| BUN (mmol/L) | 5.82 ± 0.31 | 5.78 ± 0.36 | 5.82 ± 0.25 | 5.86 ± 0.48 | 5.70 ± 0.14 | 5.77 ± 0.41 | |
| SGPT (U/L) | 51.76 ± 5.27 | 53.72 ± 2.28 | 54.08 ± 3.81 | 51.98 ± 4.69 | 53.40 ± 4.78 | 52.28 ± 3.51 | |
| PT (s) | 12.52 ± 0.33 | 12.46 ± 0.34 | 12.32 ± 0.32 | 12.50 ± 0.35 | 12.38 ± 0.40 | 12.28 ± 0.37 | |
| Female | RBC (× 1012/L) | 7.19 ± 0.16 | 7.30 ± 0.24 | 7.35 ± 0.14 | 7.27 ± 0.16 | 7.23 ± 0.23 | 7.19 ± 0.19 |
| WBC (× 109/L) | 11.58 ± 0.49 | 11.78 ± 0.92 | 12.34 ± 0.34 | 11.60 ± 0.45 | 12.14 ± 0.43 | 11.64 ± 0.26 | |
| Hb (g/L) | 213.53 ± 4.30 | 215.77 ± 4.55 | 212.99 ± 3.78 | 217.18 ± 5.77 | 211.75 ± 7.59 | 215.57 ± 3.75 | |
| HCT (%) | 46.59 ± 0.53 | 46.09 ± 0.99 | 45.84 ± 0.83 | 46.16 ± 0.80 | 46.56 ± 0.65 | 46.11 ± 1.36 | |
| GLU (mmol/L) | 9.68 ± 0.17 | 9.61 ± 0.27 | 9.82 ± 0.25 | 9.77 ± 0.33 | 9.62 ± 0.20 | 9.52 ± 0.19 | |
| BUN (mmol/L) | 5.80 ± 0.30 | 5.96 ± 0.39 | 5.82 ± 0.33 | 5.92 ± 0.25 | 5.54 ± 0.25 | 5.86 ± 0.38 | |
| SGPT (U/L) | 52.78 ± 3.25 | 52.48 ± 3.51 | 52.00 ± 3.95 | 52.24 ± 2.54 | 54.92 ± 4.77 | 56.06 ± 2.87 | |
| PT (s) | 12.58 ± 0.40 | 12.30 ± 0.35 | 12.62 ± 0.26 | 12.92 ± 0.69 | 12.86 ± 0.21 | 12.44 ± 0.40 | |
Values are expressed as mean ± standard deviation, n = 5. The Dunnett t-test was used to determine the significance of data variability between the treated and control groups. Statistical significance was considered at *P < 0.05
During the 90-day subacute toxicity studies (Table 8), the hematological parameters of SD rats remained consistent across all groups, indicating that the administration of different doses of ISL@ZLH NPs and free ISL-treated groups did not have any adverse effects on the blood parameters compared to the control group. These findings suggest that the subacute toxicity test over both 30 days and 90 days did not have any significant effects on the hematological parameters in rats. It is important to note that hematological parameters are crucial indicators of the overall health of the animals, and the lack of any significant changes in these parameters indicates that the subacute toxicity test did not have any adverse effects on the health of the rats.
Upon microscopic examination of the lung tissue, it was observed that the cellular structures of the bronchiole, alveoli, alveolar duct, and blood vessels were normal in rats administered ISL@ZLH NPs and ISL with excipient, as compared to the control group. Similarly, the architecture of cardiac muscle cells and connective tissue was found to be normal in the heart tissue of treated rats. The liver tissue of treated rats showed no abnormalities in the cellular structures of hepatocytes, sinusoids, and the central vein, which were comparable to those of the control group. In the kidney, the epithelial lining of glomerular tufts and renal tubules were observed with no abnormalities. Furthermore, there were no abnormalities observed in the acinar and islet cells of the pancreas of rats treated with ISL@ZLH NPs, as compared to rats in the control group. These findings suggest that the administration of ISL@ZLH NPs and ISL with excipient did not result in any significant structural changes in the cellular tissue of the lung, heart, liver, kidney, and pancreas of rats. Furthermore, there were no abnormalities or any significant structural changes in the cellular tissue of the salivary glands, stomach, duodenum, jejunum, colon, urinary bladder, lymph nodes, thymus, spleen, mammary gland, ovaries, uterus, prostate, testicles, thyroid and adrenal glands. Based on the findings, it can be concluded that the treatment of ISL@ZLH NPs did not lead to any significant adverse effects on the morphology of these tissues. This information is supported by the data presented in Figures 3, 4, 5, and 6.
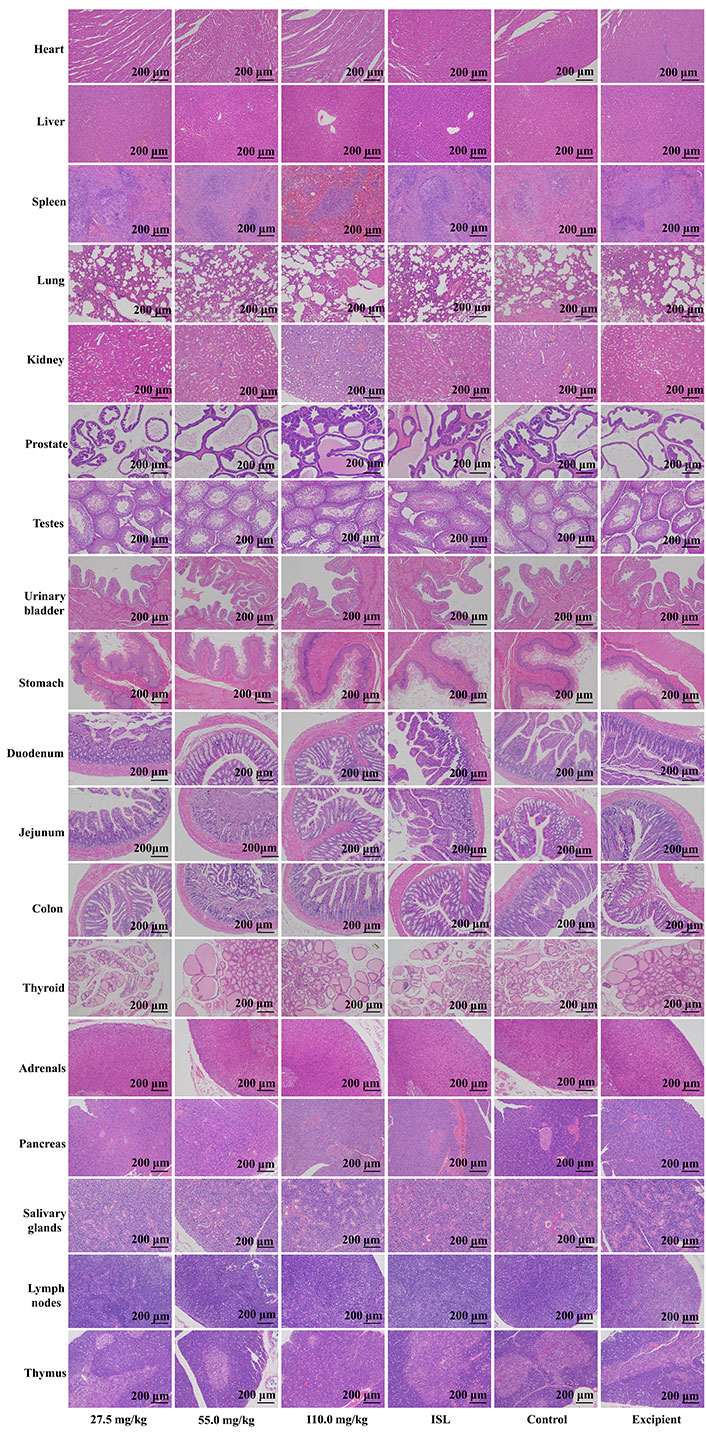
Histology of tissue from the subacute toxicity testing of ISL@ZLH NPs in male rats of 30 days. All histological sections of the heart, liver, spleen, lung, kidney, prostate, testes, urinary bladder, stomach, duodenum, jejunum, colon, thyroid, adrenals, pancreas, salivary glands, lymph nodes, and thymus (hematoxylin and eosin staining, ×10) of rats
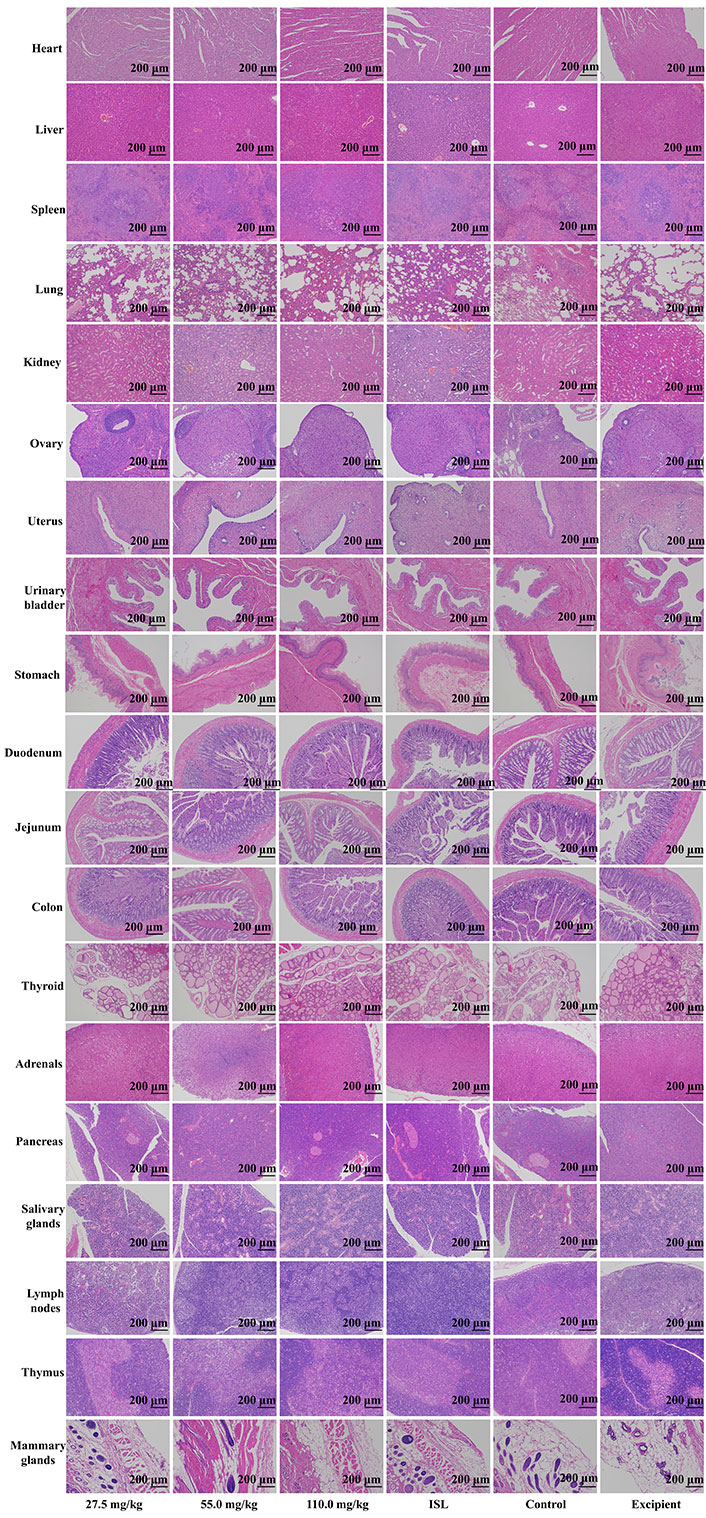
Histology of tissue from the subacute toxicity testing of ISL@ZLH NPs in female rats of 30 days. All histological sections of the heart. livers, spleen, lungs, kidneys, ovaries, uterus, urinary bladder, stomach, duodenum, jejunum, colon, thyroid, adrenals, pancreas, salivary glands, lymph nodes, and thymus (hematoxylin and eosin staining, ×10) of rats
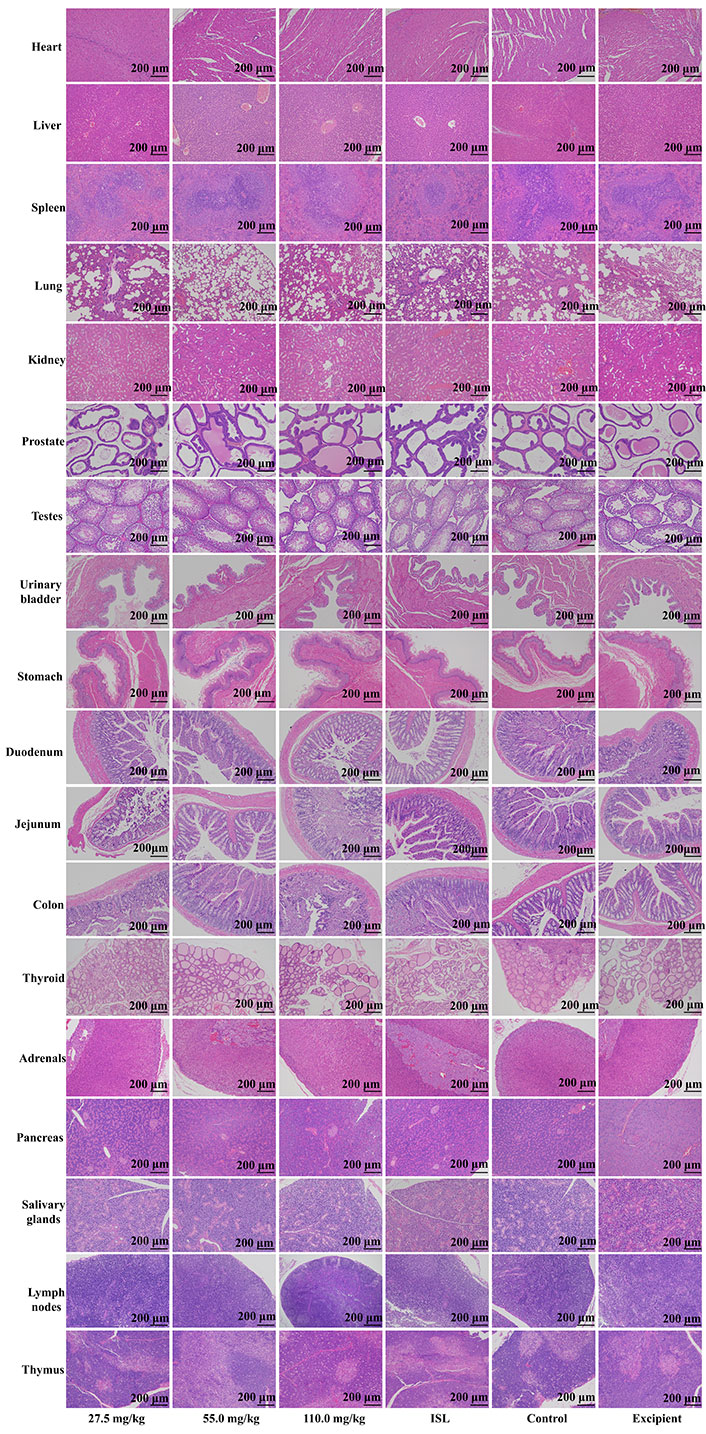
Histology of tissue from the subacute toxicity testing of ISL@ZLH NPs in male rats of 90 days. All histological sections of the heart. livers, spleen, lungs, kidneys, prostate, testes, urinary bladder, stomach, duodenum, jejunum, colon, thyroid, adrenals, pancreas, salivary glands, lymph nodes, thymus (hematoxylin and eosin staining, ×10) of rats
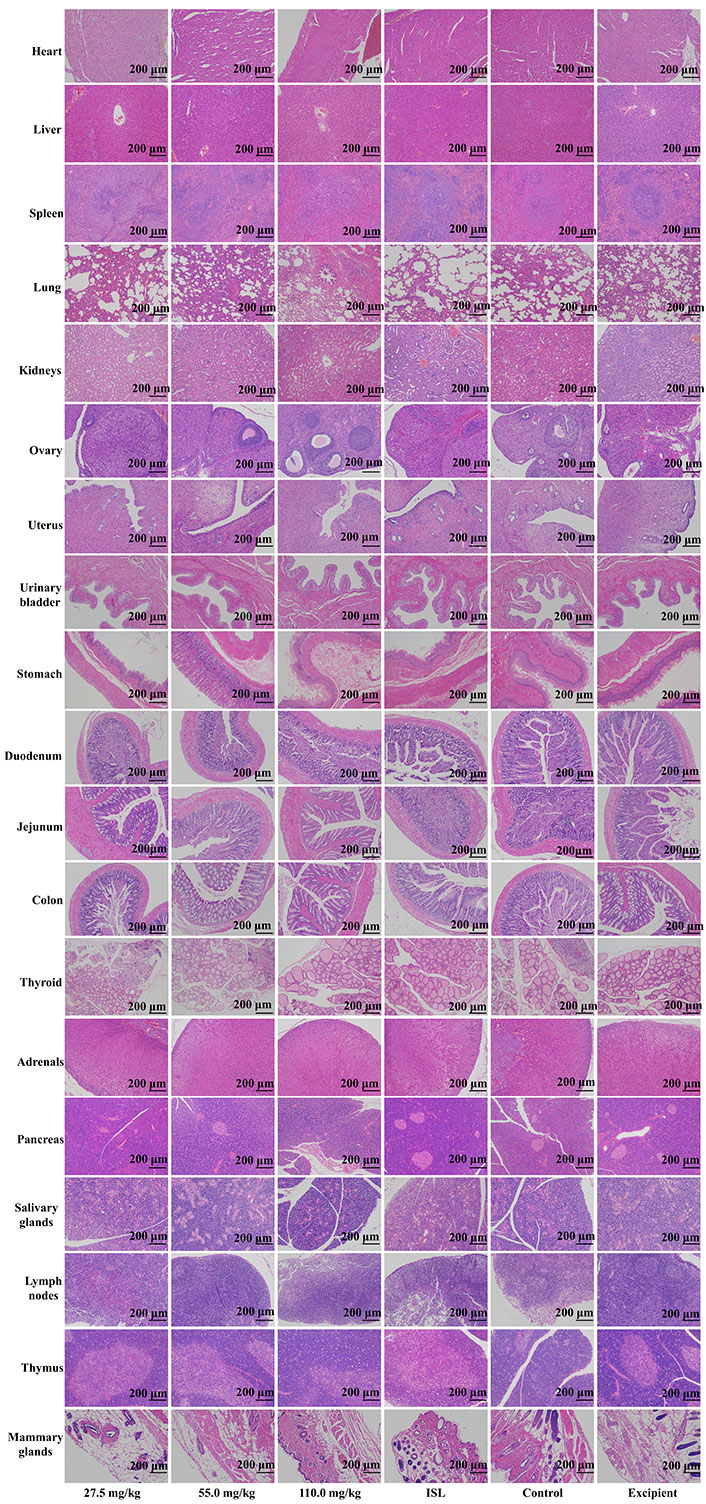
Histology of tissue from the subacute toxicity testing of ISL@ZLH NPs in female rats of 90 days. All histological sections of the heart. livers, spleen, lungs, kidneys, ovaries, uterus, urinary bladder, stomach, duodenum, jejunum, colon, thyroid, adrenals, pancreas, salivary glands, lymph nodes, thymus, mammary glands (hematoxylin and eosin staining, ×10) of rats
The use of traditional medicine as a primary pharmacotherapy for Chinese people is often met with skepticism by Western medical professionals. Concerns have been raised regarding the potential toxicity of herbal remedies, as there have been reports of allergic reactions, liver and kidney damage, and heart problems associated with their use in recent years [34, 35, 41]. Polyherbal drugs prescribed by traditional practitioners offer potential solutions to managing various diseases. However, it is crucial to conduct scientifically rigorous in vivo studies to investigate the safety profile of these commercially available herbal drugs. If these remedies can prove their efficacy and absence of toxicity, it would provide confidence in recommending polyherbal remedies for clinical practice [30, 42, 43].
The integration of herbal medicine and low toxicity in nanomedicine has gained significant attention in recent years. This is due to the potential benefits it offers in terms of efficacy, safety, and sustainability [30]. NPs can encapsulate herbal active compounds, protecting them from degradation and improving their bioavailability. NPs have been traditionally employed as drug carriers that enable speedy drug absorption. It can accurately transport various small molecules such as DNA, mRNA, drugs, and peptides to the target sites. This allows for targeted delivery to specific cells or tissues, increasing therapeutic efficacy while minimizing side effects [12].
The normal changes in body weight of the experimental rats indicated a healthy nutritional status. Body weight is a significant anthropometric parameter for evaluating overall nutrition [44–46]. Studies conducted earlier have shown that free ISL present in a mixture of plants can induce weight loss through various mechanisms [44]. Earlier, the studies showed that different doses of ISL to the rats and observed them for signs of toxicity over a period of 14 days. The study found that free ISL did not cause any significant toxic effects even at high doses, suggesting a low acute toxicity profile [44]. Similarly, the rats were orally administered with free ISL for 90 days, and various parameters such as body weight, organ weight, hematological and biochemical parameters were assessed [45]. The study concluded that free ISL did not cause any significant toxic effects on the tested parameters, indicating a favorable subchronic toxicity profile [46]. The present findings are also consistent with earlier studies that ISL@ZLH NPs or free ISL did not cause any noteworthy toxic effects on the tested parameters. The assessment of hematological parameters is utilized to ascertain the impact of the tested extract on the blood functions of healthy rats [47]. The findings of the current investigation indicate that the ISL@ZLH NPs did not have any significant effects on the erythropoiesis, morphology, or osmotic fragility of erythrocytes. The ISL@ZLH NPs-treated rats showed no significant changes in white cell counts compared to healthy control rats, indicating an intact immune system and absence of tissue damage. Additionally, insignificant alterations in platelet count in rats treated with the ISL@ZLH NPs or free ISL suggested no substantial effects on fibrin fibers or damage to blood vessels. Evaluating biochemical parameters is important in detecting potential toxic effects of herbal drugs on the liver, kidney, and other organ functions [48].
The liver and kidney have complex mechanisms to eliminate toxic substances from the body, making their assessment critical. Biochemical parameters are crucial in evaluating hepatic and renal functions to determine possible toxic effects of herbal drugs. Despite their remarkable detoxification abilities, the functions of these organs can be compromised during the process of eliminating toxins [49, 50]. Routine liver enzymes can be used to screen for alterations in liver functions, while quantitative estimation of kidney function parameters in experimental rats can affect drug dosing and enable clinical trials for novel therapeutic agents [50]. Urea and creatinine are first-line screening tests for renal functions, and serum total protein evaluation can estimate nutritional status and diagnose alterations in kidney functions [33]. Kidney dysfunction can result in inefficient excretion of urea and creatinine, causing their accumulation in the blood. The present findings reveal that the ISL@ZLH NPs did not cause liver, renal, and other organ toxicity in healthy rats.
Histological analysis of the liver, kidney, and other tissues is an important tool for detecting toxic damage caused by substances. Parameters such as interstitial edema, epithelial changes, tubular degeneration, capillary congestion, and leukocyte infiltration are used to assess toxicity [51, 52]. In addition to these specific studies, it is important to consider the general toxicity profile of flavonoids, as ISL belongs to this class of compounds. Flavonoids are widely distributed in nature and have been extensively studied for their safety [53, 54]. They are generally considered safe for consumption and have even been associated with various health benefits.
Based on the present toxicity studies, ISL@ZLH NPs appear to have a low or minimal acute and subacute toxicity profile in rats. The subacute toxicity evaluation revealed that the ISL@ZLH NPs did not cause any adverse or toxic effects, as confirmed by biochemical, hematological, and histological assessments. Therefore, the drug appears to be a promising candidate for clinical use.
The findings of this study should be interpreted with caution due to the use of animal models, which may not fully capture the complexity and variability of human biology and disease conditions. Extrapolation of these results to human populations should be done cautiously. Additionally, longer-term studies are necessary to gain a better understanding of the sustained effects and potential long-term safety of ISL@ZLH NPs. Conducting long-term follow-up studies would be valuable to assess any potential delayed or cumulative effects. Furthermore, future studies should explore the optimal dosage range and potential dose-dependent effects of ISL@ZLH NPs. Although this study evaluated the anti-inflammatory effects of ISL@ZLH NPs, it did not measure specific inflammatory markers such as C-reactive protein or interleukins [ILs (IL-1, IL-6)]. Incorporating these measurements in future studies would provide a more comprehensive assessment of the anti-inflammatory activity of ISL@ZLH NPs.
Animal models, including KM mice and SD rats, were utilized to assess the acute and subacute toxicity of ISL@ZLH NPs. The findings demonstrated that ISL@ZLH NPs exhibit safety and non-toxicity in these animal models, thereby indicating their potential for clinical application. The study observed no noteworthy alterations in biochemical, hematological, and histopathological parameters in both species across all tested doses, highlighting the promising potential of ISL@ZLH NPs as a drug delivery system for ISL. This innovative approach could enhance therapeutic efficacy and bioavailability while upholding safety considerations. However, further extensive investigations are warranted to validate these findings and establish the full extent of their clinical utility.
BUN: blood urea nitrogen
GLU: blood glucose
Hb: hemoglobin
HCT: hematocrit
ILs: interleukins
ISL: isoliquiritigenin
ISL@ZLH NPs: isoliquiritigenin-zein phosphatidylcholine hybrid nanoparticles
KM: Kunming
NPs: nanoparticles
PT: prothrombin time
RBC: red blood cell
SD: Sprague-Dawley
SGPT: serum glutamic-pyruvic transaminase
UGOT: urine glutamic oxaloacetic transaminase
WBC: white blood cells
ZLH: zein phosphatidylcholine hybrid
KY and KG equally contributed to: Conceptualization, Methodology, Validation, Investigation, Writing—original draft, Writing—review & editing. FG and CX: Methodology, Software, Validation, Formal analysis, Data curation. JC: Supervision, Project administration, Funding acquisition. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The research protocol received approval from the Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (20220421).
Not applicable.
Not applicable.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This work was supported by the Midstream Research Programme for Universities from the Innovation and Technology Commission of Hong Kong [MRP/027/18X]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
