Abstract
Coronavirus disease-2019 (COVID-19) has emerged as an aggressive viral infection in the last few years. Initially reported in the Wuhan area of the People’s Republic of China, it soon emerged across the globe. Researchers confront a worrying situation to rapidly develop effective strategies to combat this novel infection and its long-term aftereffects. To date, there have been myriad reports ranging from the repurposing of the classical antimalarial drug hydroxychloroquine to several other antiviral and anti-bacterial agents like remdesivir, favipiravir, and most recently azithromycin, which has entered clinical use in many countries for combating COVID-19 infections. Several studies have highlighted the nexus between COVID-19-associated morbidity and diabetes in a wide-ranging class of subjects ranging from pediatric cases to adults and patients with other co-morbidities. Metformin is a mainstay in the treatment of type 2 diabetes (T2D). It is safe, inexpensive, and effective and does more than merely control blood sugar levels. Important metabolites that encourage blood clotting and inflammation are also suppressed by metformin. Pro-inflammatory molecules are linked to obesity and T2D. Both are major risk factors for aggravated COVID-19. These characteristics gave rise to a hypothesis that metformin may find use as an efficacious treatment for COVID-19 especially if it decreases the inflammatory molecules that fuel the COVID-19 virus-induced effects. In this review, we attempt to elucidate the role of classical anti-diabetic medicine metformin in the treatment of COVID-19 infections by highlighting the pharmacological role of this drug during elevated glucose levels and insulin resistance. We examine how COVID-19 has correlations to diabetic physiology and thereby the possibility of repurposing metformin for COVID-19 treatment.
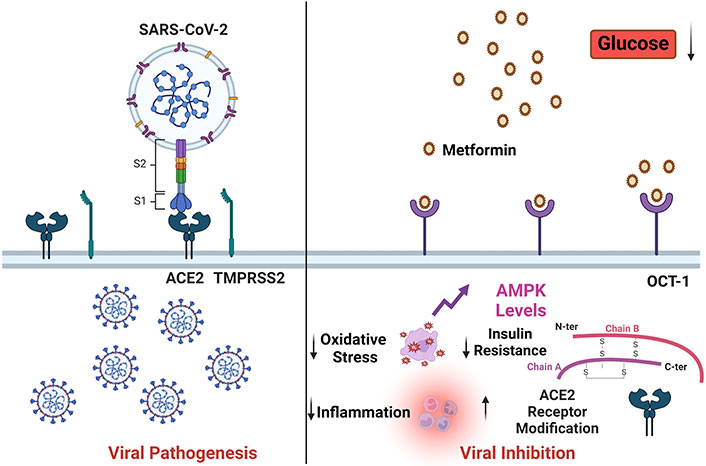
Metformin could affect the viral severity by modulating the ACE2 modification, inflammation, oxidative stress, and insulin resistance. SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane protease serine 2; AMPK: AMP-activated protein kinase; OCT-1: organic cation transporter 1. Created with BioRender.com
Keywords
COVID-19, SARS-CoV-2, metformin, blood glucose, insulin resistance, oxidative stress, gut microflora, hydroxychloroquineIntroduction
Coronavirus disease-2019 (COVID-19) is caused by the coronavirus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which spread rapidly, affecting over 85 million individuals across 220 countries [1]. The primary dispersal process of the virus is by the diffusion of respirational drops. The incubation period of SARS-CoV-2 is typically 2 to 14 days after exposure to the virus [2]. Larger respirational drops (> 5 μm) persist in the air for a relatively shorter time and travel shorter distances, generally up to 1 m while virus-burdened smaller (< 5 μm) aerosolized drops suspended in the air travel longer distances more than 1 m [3]. Symptoms of SARS-CoV-2 are milder than SARS and Middle East respiratory syndrome (MERS), but human-to-human transmission is much faster. SARS-CoV-2 dependent mortality rate is 3.4%. This is lower than the past outbreaks induced by SARS-CoV (9.6%) and MERS whose mortality was 35% [4]. Although the overall mortality rate is low, patients who have chronic diseases like diabetes mellitus (DM), hypertension, and asthma tend to suffer more severe disease outcomes [5, 6]. Diabetes is characterized by chronic high blood glucose ensuing from impairment in insulin sensitivity [7]. In 2014, it was estimated that globally 422 million individuals were suffering from diabetes and over the past decade, its prevalence was observed to be rising rapidly in low- and middle-income countries like India, Pakistan, Bhutan, etc. [8]. Approximately 90% to 95% of the diagnosed diabetic population comprises type 2 diabetes (T2D) as compared to type 1 diabetes [9]. Therefore, the risk of mortality due to COVID-19 is more prevalent in developing and poor countries. Apart from COVID-19, DM is linked with bad prognosis in other bacterial and viral infections, notably tuberculosis, typhoid fever, seasonal influenza, pandemic influenza A H1N1, SARS, and MERS [10]. Dysregulated immune responses to pathogens are indicated by T2D patients’ high vulnerability to infections. Weakened immune systems cause reductions in pathogen recognition, phagocytic activity impairments, and reduced immune cell production of chemokines and cytokines, which contribute to diabetic patients’ increased vulnerability to infection [11]. The priming of adaptive immunity also gets delayed by impairment in the recruitment of antigen-presenting cells (APCs) and increased expression of programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) on lymphocytes of hyperglycemic individuals, resulting in reduced frequencies of Th1, Th2, and Th17 cells. Cytokines of T cells play a decisive role in the activation of macrophages and inflammatory response in bacterial as well as viral diseases [12]. Therefore, impaired immune response and defects in intracellular pathogen killing can potentially increase the pathogen load and lead to chronic inflammation and host cell death. The surface domain, S1, of the spike (S) protein must engage a cellular receptor for SARS-CoV-2 to enter host cells. This binding promotes viral attachment to target cell surfaces [13]. S protein priming by cellular proteases is another requirement for viral entry. This process involves cleaving S protein at the S1/S2 and S2’ sites and permits the union of viral and cellular membranes, which is catalyzed by the S2 subunit. SARS-S uses transmembrane protease serine 2 (TMPRSS2) for S protein priming and binds to angiotensin-converting enzyme 2 (ACE2) as the entrance receptor [14]. The S protein exhibits 76% amino acid similarity between SARS-S and SARS-2-S. SARS-CoV-2 exploits receptor ACE2 to enter the host cell and the S protein is primed by TMPRSS2 [15]. Coutard and co-workers [16] demonstrated that SARS-CoV-2 employs furin proteases for priming of S protein. Recently Cantuti-Castelvetri and co-workers [17] demonstrated that neuropilin-1 (NRP1) binds furin-cleaved substrates and enhances SARS-CoV-2 infectivity. Metformin, as a broadly used anti-hyperglycemic drug, is comparatively safer as compared to other anti-diabetic drugs, has controllable side effects, is relatively inexpensive, and causes a required amount of weight loss. The impact of metformin on different cellular pathways is of varied magnitude including enhancing the macromolecular catabolic processes including lipolysis, cellular apoptosis, pancreatic cell progenitors, and glycolysis a decreasing anabolic processes like lipogenesis and gluconeogenesis which is depicted in Figure 1. Numerous reports have depicted the potential of metformin as an anti-microbial. This has induced interest among the research community to explore its novel antimicrobial effects in treating MDR infections. These studies have shown the efficacy of metformin against Trichinella spiralis, Staphylococcus aureus, Pseudomonas aeruginosa, hepatitis B virus (HBV), HCV, and human immunodeficiency virus (HIV). In this review, we highlight the possible role of metformin in a wide range of infectious diseases and explore the possibility of repurposing the drug in the battle against COVID-19.
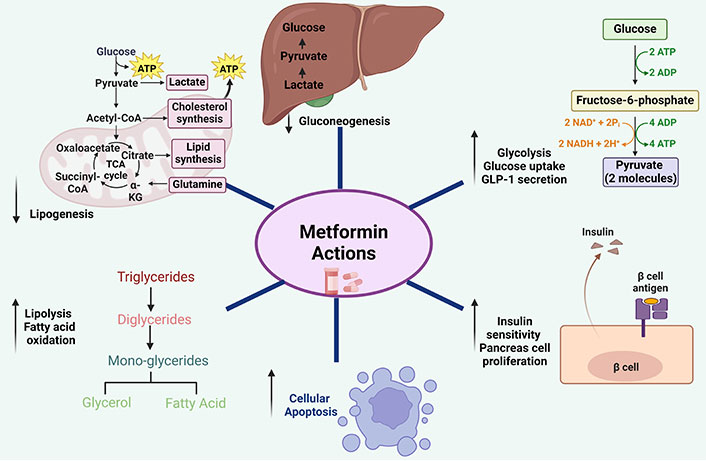
Action of metformin on different cellular pathways. Metformin enhances macromolecule’s catabolic cellular pathways while decreasing anabolic pathways. ATP: adenosine triphosphate; α-KG: α-ketoglutaric acid. Created with BioRender.com
Metformin: need for repurposing and pleiotropic action
Despite the availability of vaccines, the elimination of COVID-19 remains a challenge. The SARS-CoV-2 virus is a modified version of SARS-CoV and hijacks host cells via an unusual approach. With large parts of the world having to undergo lockdowns and health risks for millions, there is a need for an efficacious and effective drug till such time as a safe, affordable, easily deployable, and effective vaccine with no proven long-term adverse effects available. To address the current worldwide issue, it is not realistic to create new drugs from scratch, even though they might be beneficial in the long run. Drug repurposing is a new tactic in which pharmaceuticals that have been shown to be safe in human trials are repurposed to treat diseases that are difficult to cure. When combined with other therapeutic agents, these repurposed medications have the potential to yield considerable clinical advantages, even though they may not be beneficial when used alone. Metformin, an oral route administered anti-diabetic drug, belongs to the biguanide class. It exerts its effect by repressing glucose formation via the gluconeogenesis pathway and enhances peripheral glucose uptake [18]. Pleiotropic action of metformin includes the eradication of bacterial infection, viral infection, atherosclerosis, and vascular senescence [19]. In the current scenario, when there are no specific drugs available against COVID-19, re-purposing of drugs could be one of the primary options available to combat the SARS-CoV-2 virus infection.
Metformin: a wonder drug with multiple therapeutic actions
The oral anti-diabetic medication, metformin, can help with concomitant diabetes problems. Metformin has been shown in clinical trials and observational studies to significantly prevent or treat several conditions, including inflammation, inflammatory bowel disease (IBD), tuberculosis, cancer, neurodegenerative diseases, obesity, diabetes, and polycystic ovarian syndrome (PCOS) [20]. Nuclear factor-kappa B (NF-κB) is inhibited by metformin both independently and in association with AMP-activated protein kinase (AMPK) signaling. This modulates inflammation. Numerous inflammatory mediators are expressed because of NF-κB nuclear translocation and P65 phosphorylation. In individuals with diabetes, metformin has been linked to a lower incidence of heart failure, diminished mortality, and a lower rate of readmission due to chronic kidney disease (CKD) and congestive heart failure (CHF) [21]. Interestingly, metformin stimulates autophagy and the AMPK signaling pathway, which can significantly alleviate neurological conditions and speed up neuron regeneration [22]. Diabetes raises the chance of developing multiple malignancies, including hepatocellular carcinoma, prostate cancer, endometrial cancer, lung cancer, and colorectal cancer. Metformin may prevent cancer in a dose-dependent manner. Comparing metformin use to other antidiabetic drugs and insulin, a 31% reduction in total cancer risk has been found [23]. Among diabetes patients, the mean survival in the case of hepatocellular carcinoma and pancreatic cancer co-morbidities has been reported [24, 25]. Teenage PCOS is linked to an increased risk of metabolic syndrome, especially in patients who are overweight or obese. Teenage girls with PCOS experienced better menstrual cycles and ovulation after taking metformin (1,700 mg/day) for six months [26, 27].
Metformin as an adjunct anti-COVID-19 agent
Due to the paucity of safe and effective COVID-19 medicines, there is a pressing need for treatment options given the current pandemic. For clinical testing, established medications should be chosen over new ones because of their low cost, clinical management, and safety profiling. Although the three most popular vaccines, Modera, Pfizer NSE, and Oxford-AstraZeneca are currently more effective against SARS-CoV-2 infection, their acquisition and storage are quite expensive for developing and impoverished nations that desperately need them. However, inexpensive vaccination options are not very effective. In this scenario, there is a need for affordable medication candidates that are already available and can be added to less expensive vaccinations to boost their effectiveness. Metformin, a medication used to treat diabetes, may improve the effectiveness of vaccinations. New research has shown that memory T cells adapt their energy source to withstand viral or other infections. Rather than burning glucose as do other cells, they begin to burn fat. Certain engineered mice strains were unable to produce memory T cells because they lacked the capacity to alter their fuel sources. However, when metformin was administered to these specially bred mice, the medicine helped the animals produce memory T cells [28]. Therefore, this commonly used medication may improve immunization campaigns. Metformin is being viewed as an adjuvant therapy to anti-tuberculosis medication since it decreases the growth of Mycobacterium tuberculosis intracellularly [29]. It is possible that it could also be potentially effective for anti-COVID-19 therapy.
Metformin and high blood glucose crosstalk
T2D is characterized by a loss of glycemic control often due to insulin resistance (IR) in metabolic tissues such as adipose and muscle [30]. In a recent study, Ye et al. [31] claimed that elevated circulatory glucose elevates Kaposi sarcoma herpes virus (KSHV) lytic gene expression mediated by hydrogen peroxide and viral replication in various cell types and that induction of the KSHV lytic gene expression by high glucose is mediated by H2O2. In 2020, Cuong and Thoa [32] demonstrated that high glucose-fed adult zebrafish increased their mRNA levels for inflammatory cytokines and demonstrated elevated endoplasmic reticulum (ER) stress after being challenged with KSHV. High glucose also induces replication of the HCV in the host cells [33]. Hyperglycemia-induced enzymatic glycosylation can affect the action of numerous proteins [34]. Glycosylation of ACE2 promotes virus linkage to its cellular receptor and affects the severity of the disease [35]. ACE2 activity in the lung did not increase in the non-obese diabetic (NOD) mice model, although ACE2 protein levels rose. This is in accordance with an increase in ACE2’s glycosylated form [36]. Increase of SARS-CoV-2 viral binding sites may also be caused by potentially elevated and aberrant ACE2 glycosylation in respiratory system tissues including lungs, nasal airways, nasopharynx, and tongue due to uncontrolled hyperglycemia thereby enhancing the risk of COVID-19 infection and severity of the disease [37]. Metformin, a conventional drug, responds against increased glucose and helps restore the glucose equilibrium like most other anti-diabetic drugs to maintain a glycemic state. All the above reports indicate a possible role for metformin against COVID-19. A recent study highlights the role of COVID-19 infections in promoting type 1 diabetes among children [38–40].
Metformin and IR crosstalk
IR is a multi-component dependent physiological state where increasing quantities of insulin are required for maintaining glycemic homeostasis and sufficient glucose consumption in insulin target tissues. IR occurs when cells of muscle, fat, and liver tissue do not respond adequately to insulin and cannot utilize blood glucose for energy metabolism. To make up for the deficit, the pancreas produces more insulin. Over time, the patient’s blood sugar levels rise [41]. Pro-inflammatory cytokines from T helper-1 cells are known to promote IR in muscle and adipose tissue [42]. It has long been recognized that infection, thermal injury, and a variety of traumatic conditions markedly increase IR [43]. HCV infection poses the danger of developing IR. HCV-induced IR is due to the core protein of HCV that induces proteasomal degradation of insulin receptor substrates 1 and 2 (IRS-1 and IRS-2) and blocks intracellular insulin signaling. It is commonly known that people with diabetes have a higher risk of influenza-associated morbidity and mortality [44]. This risk is linked to the onset of ketoacidosis, a metabolic condition, and a higher risk of secondary bacterial pneumonia compared to non-diabetics. HIV-positive individuals frequently experience IR, especially those receiving protease inhibitor therapy. When using anti-retroviral (ARV) therapy for HIV infection, the prevalence of hyperglycemia and DM is much greater than in the general population [45]. Working with a mouse model of diet-induced obesity, Hadjadj and co-workers [46] demonstrated that IR causes a substantial ACE2 over-expression in lungs. This negatively correlates with the expression of sterol response element-binding proteins 1 and 2 (SREBP1 and 2). The anti-hyperglycemic effect of metformin is a result of the drug’s action on the reduction of IR in liver, muscle, and adipose tissue. It is likely that the interaction among the effects across three different tissues brings about the overall beneficial effect of metformin [47] and suggests a potential role in the battle against COVID-19.
Metformin-autophagy-mTOR complex-1 crosstalk
When intracellular components like protein aggregates, damaged organelles, and pathogens are engulfed into double-membrane structures called autophagosomes, they are degraded by an evolutionarily conserved process known as macroautophagy or autophagy. These autophagosomes then fuse with lysosomes to form autolysosomes [48]. A significant amount of interest has been paid in the last fifteen years to the role autophagy plays in coronavirus infection, largely because of the 2002–2003 SARS outbreak. It has been shown that either autophagy related gene 5 (ATG5) or ATG7, two essential autophagy proteins involved in the regulation of autophagosome synthesis, are needed to prevent viral replication in cells infected with mouse hepatitis virus (MHV) or SARS-CoVs [49]. The viral replication rate was not inhibited by cells that had either the ATG5 or ATG7 deletion. Recently in 2021, Gassen et al. [50] reported that SARS-CoV-2 infection limits autophagy by interfering with multiple metabolic pathways. In addition, compound-driven intervention aimed at autophagy induction to reduce SARS-CoV-2 propagation in vitro is via activation of the mammalian target of rapamycin (mTOR) pathway. Rapamycin is the most potent inducer of autophagy. It operates via down-regulation of the mTOR complex-1 and researchers propose low-dose rapamycin is required for protecting the elderly from COVID-19 [51]. In addition to regulating the antibody response for cross-protective immunity against infectious influenza viruses, mTOR signaling is a key player in the pathophysiology of influenza. Metformin can also indirectly influence the mTOR pathway in addition to rapamycin [52]. Gordon and co-workers [53] predicted that metformin could be used as an anti-COVID drug. Metformin inhibits the mTOR pathway by activating AMPK through liver kinase B1 (LKB1). Additionally, it phosphorylates IRS-1, which inhibits the mTOR signaling cascade and indirectly attenuates AKT activation [54]. Singhal et al. [29] reported that metformin inhibits the intracellular growth of M. tb by inducing autophagy selectively via enhanced mitochondrial reactive oxygen species (ROS) production. Metformin, therefore, could be a game-changer in the management of COVID-19 infection.
Metformin modulates the severity of virus infections
HIV attacks CD4+ immune cells [55]. These lymphocytes course through the body, detecting any infections as well as anomalies in other cells. When HIV targets and infects these cells, it compromises the body’s ability to combat other diseases. AIDS is the most advanced stage of HIV infection. HIV patients have an increased risk of diabetes, cardiovascular disease, cancer, and tuberculosis [56]. Metformin treatment was found to ease arterial blockage and slow the advancement of coronary artery calcification in HIV-positive patients, without affecting CD4 counts or viral loads [57]. It is still uncertain what mechanism underlies this phenomenon. Liver failure, malignancy, and scarring are all possible outcomes of hepatitis B infection. If treatment is not given, it is lethal [58]. Hepatitis B surface antigen (HBsAg) levels in culture supernatants and HepG2 cell lysates decreased following metformin treatment, according to one study [59]. Hepatitis B e antigen (HBeAg) expression, HBV replication, and HBsAg production were all dramatically reduced by metformin. These findings imply that metformin may lessen the long-term consequences of HBV, even though the exact mechanism needs to be explored further. Hepatitis C is another viral infection that causes liver inflammation, sometimes leading to serious liver damage [60]. The HCV spreads through contaminated blood. Many HCV-positive individuals were unable to take the weekly injections and oral drugs required for hepatitis C treatment due to other medical conditions or intolerable side effects [61]. Research on the impact of metformin on patients with chronic hepatitis C has yielded inconsistent findings. In a study involving the treatment of chronic hepatitis C, Sharifi et al. [61] demonstrated improvement in sustained viral response (SVR) when metformin was added to the antiviral therapy [pegylated interferon (IFN) and ribavirin]. It remains to be seen if metformin would inhibit the SARS-CoV-2 virus by stimulating these pathways.
Metformin and ACE2 receptor crosstalk
The mechanism by which SARS-CoV-2 enters host cells through ACE2 receptors is now well established [62]. A catalytic enzyme called ACE2 is expressed on the plasma membrane of cells found in the kidney, intestines, heart, lungs, and arteries [63]. By catalyzing the conversion of angiotensin (Ang) II, a vasoconstrictor peptide, into Ang 1–7, a vasodilator, ACE2 reduces blood pressure [64]. Due to the higher expression of the ACE2 receptor gene, Asian men have been shown to be more susceptible to SARS-CoV-2 infection than women and people of other races [65]. But compared to adults, children are less vulnerable to COVID-19. Lower ACE2 expression is the cause of this [66, 67]. According to Wrapp and co-workers [68], the S protein from SARS-CoV-2 has a 10–20-fold higher affinity for ACE2 than the S protein from SARS-CoV.
The higher infectivity that results from this, according to the authors’ theory, may help to explain why the two epidemics’ evolutionary paths diverge. In addition to COVID-19, ACE2 guards against sepsis, acid aspiration, SARS, and fatal avian influenza A H5N1 virus infection, which can all cause severe acute lung injury [69]. DM, cancer, and cardiovascular disorders are among the conditions where the energy sensor AMPK exhibits abnormal expression. AMPK signaling induction increases glucose homeostasis in diabetes, which is crucial for reducing hyperglycemia. AMPK can help with liver disorders, nephropathy, neuropathy, and reproductive changes brought on by DM. To achieve these protective effects, AMPK signaling engages in the PI3K/Akt pathway and has interactions with transcription factors, including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and NF-κB [70]. Based on the literature, it is speculated that AMPK could regulate the expression of ACE2. But this effect may be tissue- or cell-type-dependent, and it can vary between tissues. Utilizing cell culture models, Liu et al. [71] showed that AMPK increased the expression of ACE2 by hampering its ubiquitination and proteasomal degradation. In addition, they also observed increased receptor stability due to phosphorylation at Ser680 [71]. It is conceivable, that this addition of a phosphate may cause conformational and functional changes in the ACE2 receptor that can inhibit the binding of S protein to the ACE2 receptor.
In a separate investigation, oral Ang (1–7) and resveratrol feeding to mice on a high-fat diet (HFD) resulted in enhanced AMPK activity and decreased expression of ACE in white adipose tissue [72]. AMPK may control the expression of ACE and elevating AMPK phosphorylation can inhibit the expression of ACE2. It is known that metformin causes cells’ AMPK to become active [73]. The investigation of Gilbert et al. [74] revealed a substantial correlation between urine ACE2 and metabolites such as elevated triglycerides, cholesterol, and blood sugar. Enzyme activity and protein level expression of urinary ACE2 were found to be attenuated by the administration of insulin, rosiglitazone, and metformin, agents known to control hyperglycemia [74]. Therefore, metformin by inducing post-translational modifications in the ACE2 receptor via activation of AMPK-signaling may prevent the cellular entry of SARS-CoV-2 and may prevent the development of cardinal signs of COVID-19, which is exemplified in Figure 2.
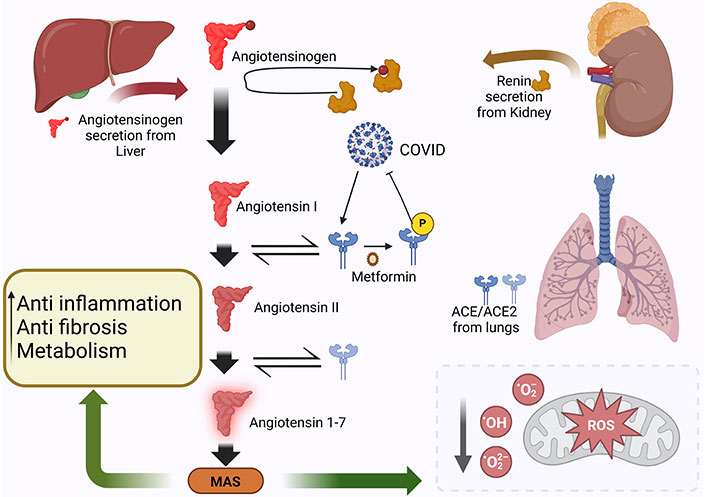
Metformin could affect the COVID-19 severity via post-translational modification of the ACE2 receptor via activation of AMPK-signaling pathway. COVID-19: coronavirus disease-2019; ROS: reactive oxygen species; MAS: Mas receptor; ACE2: angiotensin-converting enzyme 2. Created with BioRender.com
Metformin and anti-inflammatory crosstalk
SARS-CoV-2 also inflicts damage to organs like heart, liver, kidneys, as well as to the immune system along with pneumonia-like symptoms. Severely afflicted patients eventually die due to multiple organ failure, shock, acute respiratory distress syndrome (ARDS), heart failure, arrhythmias, and renal failure [75]. Multi-organ failures are usually brought upon by “cytokine storm”, overwhelming the host immune response to SARS-CoV-2 infection [76, 77]. Recently, Theobald et al. [78] showed that SARS-CoV-2 S protein drives inflammasome activation and interleukin-1 beta (IL-1β) secretion. Neutrophil extracellular traps (NETs) are networks of extracellular fibers, consisting of DNA from neutrophils, which bind pathogens [79]. According to recent research, serum from COVID-19 patients has greater levels of NETs, including citrullinated histone H3 (Cit-H3), myeloperoxidase (MPO), and cell-free DNA. These have been linked to inflammatory markers [80]. In patients with diabetes, metformin has been linked to a reduction in the neutrophil-lymphocyte ratio and a decrease in the neutrophil count in PCOS [81]. Metformin also ameliorated NETosis in patients with diabetes [82]. Several preclinical and clinical studies suggested that metformin improves chronic inflammation by improving metabolic parameters such as hyperglycemia, IR, atherogenic dyslipidemia along with direct anti-inflammatory action [83]. Anti-inflammatory effect of metformin is induced by the inhibition of NF-κB via AMPK-dependent and independent pathways [84]. In another study, metformin, and the inhibitor of kappa B kinase beta (IKKβ) inhibitor BI605906 both inhibited TNF-α dependent IκB degradation and expression of pro-inflammatory mediators’ IL-6, IL-1β, and CXCL1/2 in primary hepatocytes [81]. Metformin reduces IFNγ levels and increases forkhead box P3 (FOXP3) mRNA expression in mice models of systemic lupus erythematosus (SLE) [85]. Jing et al. [86] showed metformin altered macrophage polarization by reducing TNF-α expression from macrophages. Another study by Duan et al. [87] demonstrated that metformin suppresses T cell proliferation and inhibits the differentiation of Th1 and Th17 cells while promoting the development of Tregs in vitro in a dose-dependent manner. Recently, Bharath and co-workers [88] reported that metformin ameliorated Th17-based inflammation by increasing autophagy and improving mitochondrial bioenergetics. Metformin not only reduces inflammatory response but also improves the phagocytosis by immune cells [89] which is a component of the innate immune system. Bramante et al. [90] showed that metformin may lower the risk of COVID-19 fatality in women. Metformin reduced the inflammation marker C-reactive protein (CRP) twice as much in women than in men and significantly decreased levels of TNF-α, an inflammatory cytokine that appears to make the effects of COVID-19 more severe. Since metformin inhibits pro-inflammatory cytokines production, it could be a direct modulator of the innate as well as adaptive immune response, thus preventing viral replication and pathogenesis.
Metformin-aging-COVID-19 crosstalk
Human aging can alter the body in a variety of ways, which can subsequently impact the immune system. For example, aging can cause the body to react more violently to antigens that have a reduced ability to thwart infections. Individuals over 60 years of age have a 4.5% mortality rate, while individuals under 60 have a 1.4% mortality rate. As a result, therapeutic approaches may be less effective in older patients. To lower the death rate, managing older COVID-19-infected individuals has thus become a significant concern [91]. A typical medication used to treat T2D in people is metformin. Moreover, metformin contains anti-inflammatory properties, which may help explain why it can delay the aging process. Reducing inflammation can enhance health and prolong life because persistent inflammation is a sign of aging and age-related disorders. Martin-Montalvo and co-workers [92] have reported that metformin (0.1% w/w in food) given to male mice over an extended period beginning in middle age increases their lifespan and quality of life, while a higher dose (1% w/w) was harmful. Without lowering caloric consumption, metformin treatment mimics some of the advantages of calorie restriction, including enhanced insulin sensitivity, decreased levels of low-density lipoprotein and cholesterol, and improved physical performance [92]. There has been speculation that the low number of deaths in India can be attributed to two factors: the virus’s less virulent strain and the country’s comparatively youthful population when compared to western societies which have a higher percentage of elderly individuals. The vulnerability of Indians to COVID-19 may be reduced by several factors pertaining to the type of pathogen, host, and environment conditions. These include genetic polymorphisms of ACE2 receptors, host factors such as innate immunity, genetic diversity in immune responses, epigenetic factors, and the universal BCG vaccination. Environmental factors such as high temperature and humidity can also affect the viability and transmissibility of the strain. Finally, some ongoing mutations that alter the virulence of the circulating SARS-CoV-2 strains are also included [93].
Metformin and type 1 IFN crosstalk
The well-researched cytokines type 1 IFNs have antiviral and immune-modulating properties [94]. A secreted substance known as “interferon” was discovered by Isaacs et al. [95] in 1957, and it was shown to be able to make influenza virus-infected chick cells resistant to infection. There are several IFNα, IFNβ, ε, κ, and ω subtypes in the type 1 IFN family, in addition to the δ and τ subtypes that are specific to sheep and pigs, respectively. By attaching to the type 1 IFNα/β receptor (IFNAR), which is found in a range of cell types, these cytokines trigger antiviral responses [96]. Despite variations in their pathophysiology, proteome, and epidemiology, MERS-CoV, SARS-CoV, and SARS-CoV-2 share similar features [96]. Intriguingly SARS-CoV-2 is relatively more sensitive to type 1 IFN than SARS-CoV suggesting the modulation of type 1 IFN to reduce SARS-CoV-2 infection [97]. The combination of type 1 IFN agonists with lopinavir/ritonavir, ribavirin, or remdesivir could improve its efficacy [98]. Unfortunately, SARS-CoV-2 infection causes impairment in type 1 IFN activity in patients [46]. Recently, Blanco-Melo et al. [99] showed that SARS-CoV-2 infection induces low IFN-I and IFN-III levels with a moderate IFN-stimulated gene response. In 2010, Tsai and Chung [100] showed metformin activates type 1 IFN signaling and inhibits the replication of herpes virus via activation of AMPK. It may be possible to induce the production of type 1 IFN and metformin could be a candidate small molecule for COVID-19 therapy.
Metformin-mitochondria-cell death crosstalk
Mitochondria are membrane-bound cell organelles that provide most of the chemical energy required for the cell’s metabolic activities. Adenosine triphosphate (ATP) stores the chemical energy generated by oxidative phosphorylation that takes place in mitochondria. In addition to generating most of the energy required for cellular processes, mitochondria also play a key role in the control of cellular homeostasis, cell metabolism, innate immunity, cell death, and epigenetics [101]. Approximately one thousand distinct proteins, each with a unique function, are found within mitochondria. These proteins rely on the exchange of ions and metabolites between the mitochondria and the cytoplasm. Consequently, it is necessary for metabolites to pass through both the inner and outer mitochondrial membrane (IMM and OMM). Metabolites can be transferred across the OMM by the voltage-dependent anion channel 1 (VDAC1). The IMM is furnished with numerous transporters or carrier proteins, each of which oversees moving a particular metabolite across the IMM [102, 103]. Thompson and co-workers [104] demonstrated a specific T cell population seen in COVID-19 patients that is marked by strong VDAC1 and H3K27me3 epigenetic marker overexpression. According to Loubiere et al. [105], metformin has also been shown to enhance VDAC1 expression levels in LNCaP cells. It has also been shown to do the same in rats treated with metformin that resemble those with PCOS [105]. Zhang et al. [106] showed that in PCOS-like rats treated with metformin, there was an increase in VDAC expression and a decrease in superoxide dismutase 1 (SOD1) in comparison to control rats. Furthermore, it was observed that metformin modulates pathological conditions in which VDAC1 is overexpressed. VDAC1 overexpression induces mitochondrial dysfunction, which leads to cell death [107]. Therefore, metformin could play a decisive role in COVID-19 via modulating the VDAC1 expression and cell death of SARS-CoV-2-infected cells.
Metformin partially inhibits complex 1 of the electron transport chain in the mitochondria [108]. This results in an irregular electron-to-oxygen flow and the accumulation of ROS in the mitochondrial matrix. ROS are thought to play a role in the etiology of several illnesses, such as inflammation and cancer. Additionally, ROS are required for the differentiation, secretion, growth, and killing of tumor cells. Nonetheless, apoptosis and senescence of tumor cells might be brought on by elevated ROS levels. Additionally, ROS cause the membrane potential (∆ψm) to collapse, which sets off a sequence of events related to mitochondria, such as apoptosis and necroptosis [109–111]. Metformin also induces the killing of cancer cells via targeting the extracellular signal-regulated kinases (ERK) pathway [112]. This suggests that metformin could potentiate the killing of SARS-CoV-2 infected cells and help in the clearance of the virus.
Metformin and oxidative stress axis
A redox imbalance of oxidative stress (OS) may be connected to cytokine generation, inflammation, cell death, and other pathophysiological processes associated with respiratory virus infections. It is commonly known that excessive formation of ROS and suppression of antioxidant mechanisms are essential for the proliferation of viruses [113]. Increased ROS and Ca++ disruptions brought on by unfolded protein response (UPR), which is mediated by ER stress, are linked to viral infections, including SARS-CoV [52]. Viral infections, bacterial toxins, and air pollutants can cause high quantities of ROS to be generated in the lung airways. At the site of viral infection, excessive ROS production causes the recruitment of inflammatory cells [114]. Patients with COVID-19 infection have been shown to have significantly elevated blood levels of cytokines (cytokine storm) and chemokines due to increased immune cell recruitment [31]. The cytokine storm contributes to the catastrophic outcomes of COVID-19 patients by causing a pro-inflammatory milieu that is highly correlated with severe tissue damage [115]. The non-structured protein 3CLpro, a viral protease, of SARS-CoV was shown by Lin and colleagues [116] to be involved in cell death and to significantly boost ROS generation in HL-CZ cells. Chen et al. [117] showed that K+ influx and ROS overproduction are adequate triggers for the activation of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP-3) inflammasome caused by SARS-CoV’s protein 3a. In cerebral ischemia, metformin pretreatment stimulates Nrf2 antioxidant pathways and suppresses inflammatory responses by inducing the AMPK pathway [118]. A study by Prasad and co-workers [119] revealed that metformin activates the Nrf2 pathway, which significantly diminishes the toxicity of smoking cigarettes at the cerebrovascular level. Nrf2 is a basic leucine zipper (bZIP) protein that controls antioxidant protein production to prevent oxidative damage brought on by inflammation and injury [120]. McCord et al. [121] showed that Nrf2 activator PB125 down-regulates ACE2 and TMPRSS2 mRNA expression in human liver-derived HepG2 cells and controls the OS response. All these reports indicate that metformin could be a potential drug candidate for COVID-19 treatment.
Metformin and gut microflora crosstalk
The human gut microbiota harbors ~1014 microorganisms (bacteria, archaea, viruses, and fungi) [122]. Most bacteria in gut belong to: Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes phyla [123]. The major families are: Bacteroidaceae, Prevotellaceae, Rikenellaceae, Lachnospiraceae, and Ruminococcaceae [124]. Gut microbiota influence and regulate energy metabolism and inflammation. Fluctuations in microbiota cause obesity, escorted by low-grade inflammation, and pose the risk of gut-associated and other diseases like type 2 DM [125]. Recently Zuo et al. [126] reported that microbiota changes during the time of hospitalization, for Coprobacillus, Clostridium ramosum, and C. hathewayi (pro-inflammatory bacteria) correlated with COVID-19 severity. However, an inverse correlation was found among the abundance of Bifidobacterium and Faecalibacterium prausnitzii (anti-inflammatory bacteria) and disease severity. Imaoka et al. [127] showed that probiotic Bifidobacterium strains in fermented milk enhance IL-10 production in peripheral blood mononuclear cell (PBMC) and inhibit IL-8 secretion in intestinal epithelial cells, suggesting that Bifidobacterium strains have anti-inflammatory effects against ulcerative colitis. Its metabolic effects are also believed to be caused by altering the gut flora in response to metformin. Most investigations have focused on, how metformin boosts the number of microbiota members, particularly Escherichia coli, B. bifidum, Butyrivibrio, Megasphaera, subspecies of Prevotella, Lactobacillus, and Akkermansia muciniphila [128, 129]. Utilizing an in vitro culture model, it has been observed that metformin also encourages the growth of A. muciniphila and B. adolescentis [130]. Thus, by reducing the pro-inflammatory condition-provoking bacteria in the gut, metformin could be a potential weapon in the battle against COVID-19 (Figure 3).
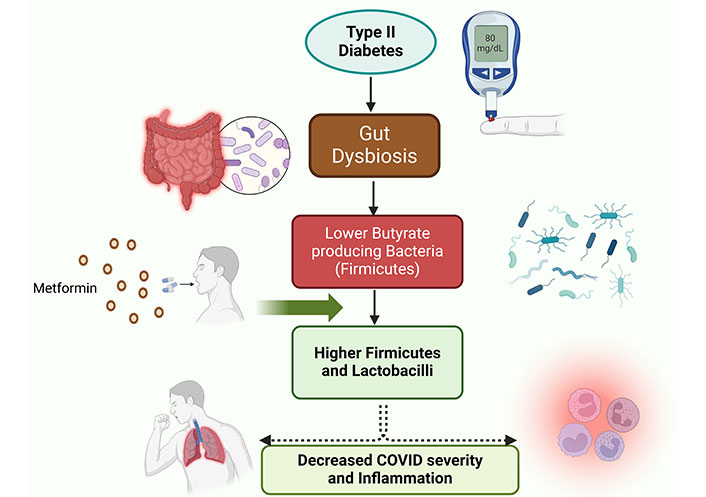
Metformin could rewire the dysbiosis of the gut microflora affected by type 2 diabetic condition. This rewiring i.e., increases in Firmicutes and lactobacilli populations in gut microflora decreases coronavirus disease (COVID) severity and cellular inflammation and decreases COVID-19 severity. Created with BioRender.com
Side effects and drug-to-drug interactions of metformin
No drug is perfect and the same is the case with metformin. It may have some adverse effects. The side effects of metformin that occur most frequently are headache, nausea, vomiting, and diarrhea. It can also occasionally induce lactic acidosis in patients. If metformin is taken for an extended period, vitamin B12 insufficiency may result. This may cause patients to feel extremely exhausted, short of breath, and dizzy. Patients with a glomerular filtration rate of less than 30 mL/min/1.73 m2 are not advised to take metformin [131]. Substrates for the human organic cation transporters 1 and 2 (OCT-1 and OCT-2) include metformin [132]. Medication that blocks these transporters may raise the risk of metformin-related side effects and increase the persistence of the drug in the body.
Conclusions
COVID-19 is a disruptive pandemic that still lacks a comprehensive treatment regime. Though mRNA and protein subunit-based vaccines have been used for the past 2–3 years, 100% efficacy is still not achieved. FDA-approved drugs used for the treatment of other diseases are being repurposed in trials against COVID-19. These drugs include antivirals, antimalarial, ACE inhibitors (ACEIs), Ang II receptor blockers (ARBs), dexamethasone, statins, and monoclonal antibodies [133–137]. Unfortunately, these drugs have shown extensive side effects in various clinical studies. Metformin is a classical anti-diabetic drug. It has emerged as a key candidate drug in overcoming other abrupt physiological changes in post-infection host subjects. To add to the drug’s appeal to prevent severe COVID-19 is its wide availability, low cost, minimal need for follow-up, and extensive safety data even among pregnant subjects. It accomplishes its beneficial effects by stimulating a host of cellular responses such as: promoting autophagy, controlling ACE2 expression levels, OS management, anti-inflammatory effects in response to infection-induced cytokine storm, etc. Various studies have demonstrated that metformin plays a role in the reduction of pro-inflammatory factors by modulating the gut microbiota. COVID-19 infection also promotes pro-inflammatory effects which can be potentially reduced by metformin. Indeed, metformin could act as a potential drug candidate in the treatment of COVID-19 infection and associated morbidity. Such a repurposing of metformin to be used in combinatorial therapy may provide an additional tool in the current arsenal available against this virus leading to improvement in current treatment strategies for treatment of the viral pandemic.
Abbreviations
| ACE2: | angiotensin-converting enzyme 2 |
| AMPK: | AMP-activated protein kinase |
| Ang: | angiotensin |
| ATG-5: | autophagy related gene 5 |
| COVID-19: | coronavirus disease-2019 |
| DM: | diabetes mellitus |
| HBV: | hepatitis B virus |
| HIV: | human immunodeficiency virus |
| IFN: | interferon |
| IL-1β: | interleukin-1 beta |
| IMM: | inner mitochondrial membrane |
| IR: | insulin resistance |
| IRS-1: | insulin receptor substrate 1 |
| KSHV: | Kaposi sarcoma herpes virus |
| MERS: | Middle East respiratory syndrome |
| mTOR: | mammalian target of rapamycin |
| NETs: | neutrophil extracellular traps |
| NF-κB: | nuclear factor-kappa B |
| OS: | oxidative stress |
| PCOS: | polycystic ovarian syndrome |
| ROS: | reactive oxygen species |
| S: | spike |
| SARS-CoV-2: | severe acute respiratory syndrome coronavirus-2 |
| T2D: | type 2 diabetes |
| TMPRSS2: | transmembrane protease serine 2 |
| VDAC1: | voltage-dependent anion channel 1 |
Declarations
Acknowledgments
Critical comments provided by colleagues are well appreciated to this work. Support via fellowship by Indian Council of Medical Research (ICMR) to Manoj Raje is acknowledged. Additionally, we extend our gratitude to the CSIR-IMTECH for its institutional support. We genuinely thank both institutions for their facilities and resources, which helped us do this project successfully. This is IMTECH communication No. 07/2021.
Author contributions
MR, GKC, and RD: Conceptualization, Writing—original draft, Writing—review & editing. AD, ST, and RM: Visualization, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
The study is supported via fellowship by Indian Council of Medical Research, Government of India [No. HRD/Head/IES/2023]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2024.