Abstract
Aim:
In this study, antioxidant activities and antibacterial activities of acetone and chloroform extracts obtained from Rosa canina, Echinacea purpurea, Althaea officinalis and Glycyrrhiza glabra were explored.
Methods:
Disc diffusion method and minimum inhibition concentration (MIC) assays were used to reveal antibacterial activity of the extracts. Total phenolic content, total flavonoid content, total antioxidant capacity, DPPH and ABTS radical scavenging activity tests were performed to determine antioxidant activity of the extracts.
Results:
Acetone extracts of the studied plants showed higher activity than chloroform extracts. Both acetone and chloroform extracts of G. glabra produced higher inhibition zones compared to other plant extracts. The highest total phenol content was found in acetone extract of G. glabra while the lowest total phenol content was found in chloroform extract of R. canina. The highest and lowest total antioxidant capacity was determined as 247.28 ± 0.0557 µg ascorbic acid equivalent (AAE)/mL and 50.91 ± 0.0294 µg AAE/mL in chloroform extract of A. officinalis and acetone extract of A. officinalis, respectively.
Conclusions:
In the light of the obtained data, it was concluded that R. canina, E. purpurea, A. officinalis and G. glabra can be used as alternative natural antibacterial and antioxidant sources to synthetic antibacterial and antioxidant agents.
Keywords
Plant, antibiotic, reactive oxygen species, antioxidant, phenolic compoundIntroduction
More than 50% of the modern drugs contain natural sources like plants. Therefore, plants have a significant role in the pharmaceutical sector. Plants produce phytochemicals in response to environmental conditions or diseases. Phytochemicals are not important for plant metabolism but they have some therapeutic properties in humans [1].
Microbial resistance to antibiotic drugs is one of the biggest problems which threaten the health of societies. Many strategies have been proposed to cope with this problem. One of the strategies is to combine other molecules with the failing antibiotics. Because of having antibacterial properties plants could be used alone or in combination with antibiotics [2].
Antioxidants play a role in delaying or preventing oxidation of oxidizable substrates. Antioxidants can be synthesized in vivo or taken as dietary antioxidants. Plants are sources of dietary antioxidants. Ascorbic acid is the first discovered exogenous plant antioxidant. Since then, plants have gained fairly attention because of their antioxidant property [3].
Under stress conditions, plants produce reactive oxygen species (ROS) and these situations cause oxidative stress. In response to increased oxidative stress, plants produce or accumulate several low and high molecular weight antioxidants [3].
Rosa canina L. has biologically active compounds which have chemoprevention, antioxidant and anticarcinogenic activities. R. canina is generally utilized for treatment of colds used as tea [4], gastrointestinal disorders, infections, inflammatory diseases and chronic pains. Fruits are utilized in pharmaceutical, cosmetic and food industries [5].
Echinacea purpurea L. Moench has immunostimulatory and anti-inflammatory activity. It is also used to treat cold symptoms uses as tea, compress, tablet or capsule [6]. E. purpurea has also many beneficial effects such as antianxiety, antidepression, cytotoxicity and antimutagenicity [7].
Althaea officinalis L. has been utilized for the treatment of common colds, coughs, nasal congestion, and fever. Antimicrobial, anti-inflammatory, and other pharmacological effects of crude extract and/or purified compounds from flowers, roots and leaves from A. officinalis revealed by many researchers [8].
Glycyrrhiza glabra L. is beneficial for the treatment of sore throat and cough [9].
It aims to explore antioxidant activities and antibacterial activities of extracts of some medicinal plants such as R. canina, E. purpurea, A. officinalis and G. glabra used to treat colds.
Materials and methods
Plant samples
R. canina, E. purpurea, A. officinalis and G. glabra were brought from a herbal shop.
Microorganisms
It was used five reference microorganism of American type culture collection (ATCC). Listeria monocytogenes ATCC 7644, Salmonella enterica serovar typhimirium ATCC 14028, Staphylococcus aureus subsp. aureus ATCC 25923, Bacillus cereus 702 ROMA, Yersinia pseudotuberculosis ATCC 911, Enterococcus faecalis ATCC 29212, Bacillus subtilis IMG 22, Enterobacter aerogenes CCM, Gordonia rubripertincta (lab isolate), Klebsiella pneumoniae (lab isolate) and Proteus vulgaris (lab isolate) were used in the antibacterial activity experiments. Lab isolates were obtained from Yeditepe University. All bacterial strains were grown in Mhuller Hinton agar (MHA) and Mhuller Hinton broth (MHB) at 37°C overnight.
Maceration of plants
25 g of R. canina, E. purpurea, A. officinalis and G. glabra were extracted in a shaker for 48 h utilizing 250 mL chloroform and acetone, separately [10].
Antibacterial activity
Disc diffusion method
Each plant extract was dissolved in dimethyl sulfoxide (DMSO) (plant extracts dissolved in 2.5% DMSO) at 30 mg/mL concentration. Chloroform and acetone were studied in different petri dishes. The sterile paper discs were put on inoculated petri dishes. Discs were loaded with 25 µL plant extract, separately. 25 μL DMSO was added to the disc for negative control. Gentamycine was used as the positive control. Diameter of zones was measured with a ruler [11, 12].
Determination of minimum inhibition concentration (MIC)
Chloroform and acetone extracts were prepared at 30 mg/mL concentration in DMSO. Minimum inhibition concentration (MIC) values of the extracts were determined with 96-well plates by the method of Yiğit et al. [13]. This 96-well plate was incubated at 37°C for bacteria overnight [13].
Antioxidant activity
The tests were performed in triplicate.
Total phenolic content
0.1 mL extract and 4.5 mL distilled water were mixed. Then, 0.1 mL Folin-Ciocalteu reagent (previously diluted 3-fold with distilled water) was put into the mixture. After 3 min, 0.3 mL Na2CO3 (2%) was added. The absorbance was measured at 760 nm after 90 min. Gallic acid (Sigma Aldrich, 842649) was used as the standard. Total phenolic content of the extracts was expressed as µg gallic acid equivalent (GAE)/mL by using the calibration curve [14].
Total flavonoid content
0.25 mL extract, 1.25 mL distilled water, and 75 μL NaNO2 (5%) were mixed. After 6 min, 150 μL of AlCl3·6H2O (10%) was added and the mixture was kept at room temperature for 5 min. Then, 0.5 mL NaOH (1 M) and 725 µL distilled water were added to the mixture. Absorbance was measured at 510 nm. Catechin (Sigma-Aldrich, C1788) was used as standard and the results were expressed as µg catechin equivalent (CE)/mL [15].
Total antioxidant capacity
0.3 mL extract and 3,000 µL reagent were incubated at 95°C for 90 min. Absorbance was read at 695 nm. Ascorbic acid (Sigma-Aldrich, 50-81-7) was used as the standard. The total antioxidant capacity was expressed as µg ascorbic acid equivalent (AAE)/mL [16].
DPPH radical scavenging activity
Extracts were added to 1.5 mL of a 6 × 10–5 M methanolic solution of DPPH (Sigma-Aldrich, D9132). BHT (Sigma-Aldrich, 1082708) and rutin (Sigma-Aldrich, 153-18-4) were used as positive controls [17]. The DPPH radical scavenging activity was calculated using the following equation:
DPPH radical scavenging activity (% inhibition) = [(A0 – A1) / A0] × 100
A0 = absorbance of control
A1 = absorbance of sample
ABTS radical scavenging activity
ABTS (Sigma-Aldrich, A1888) radical scavenging activity of extracts was determined according to the method of Arnao et al. [18] (2001). BHT and rutin were used as standards [18]. The ABTS radical scavenging activity was calculated using the following equation.
ABTS radical scavenging activity (% inhibition) = [(A0 – A1) / A0] × 100
A0 = absorbance of control
A1 = absorbance of sample
Results
Antibacterial activity
Inhibition zones created by the extracts are given in Table 1 and inhibition zones are presented in Figure 1. The highest antibacterial effect was found in acetone extract of G. glabra, while the lowest antibacterial effect was found in chloroform extract of R. canina. DMSO which was used as a negative control showed no antibacterial action. Acetone extract of G. glabra exhibited activities close to or higher than gentamycine against the tested bacteria. The antibacterial effect of the acetone extracts of plants was found higher than the chloroform extracts.
Inhibition zones which created by the extracts (mm)
| Bacteria | Acetone extract of E. purpurea | Acetone extract of A. officinalis | Acetone extract of R. canina | Acetone extract of G. glabra | Chloroform extract of E. purpurea | Chloroform extract of A. officinalis | Chloroform extract of R. canina | Chloroform extract of G. glabra | DMSO | Gentamycine |
|---|---|---|---|---|---|---|---|---|---|---|
| L. monocytogenes | 13 ± 1.41 | - | - | 14.5 ± 0.70 | - | - | - | - | - | 23 ± 0.00 |
| B. subtilis | 8 ± 0.00 | - | - | 20 ± 0.00 | 8.5 ± 0.70 | 9.5 ± 0.70 | 10 ± 0.00 | 16 ± 1.41 | - | 22.5 ± 0.70 |
| P. vulgaris | 7.5 ± 0.70 | 10 ± 0.00 | 10 ± 0.00 | 17.5 ± 0.70 | 13 ± 1.41 | 11 ± 0.00 | 11.5 ± 0.70 | 14 + 1.41 | - | 16.5 ± 0.70 |
| E. aerogenes | 8 ± 1.41 | - | - | 15 ± 1.41 | - | 9 ± 0.00 | - | 14.5 ± 0.70 | - | 22.5 ± 0.70 |
| B. cereus | 11 ± 1.41 | 11 ± 0.00 | - | 17.5 ± 0.70 | 6 ± 0.00 | 6 ± 0.00 | 9 ± 1.41 | 13.5 ± 0.70 | - | 21 ± 0.00 |
| K. pneumoniae | 9 ± 0.00 | 9 ± 0.00 | 10.5 ± 0.70 | 16 ± 1.41 | - | - | - | - | - | 17 ± 1.41 |
| G. rubripertincta | 13.5 ± 2.12 | 11 ± 1.41 | 14.5 ± 2.12 | 24.5 ± 0.70 | - | - | - | 9 ± 1.41 | - | 20 ± 1.41 |
| S. aureus subsp. aureus | 14 ± 0.00 | 12.5 ± 0.70 | 10 ± 0.00 | 17 ± 1.41 | 10 ± 0.00 | 8.5 ± 0.70 | 10 ± 1.41 | 14.5 ± 0.70 | - | 14 ± 1.41 |
| E. faecalis | 12.5 ± 0.70 | 14 ± 1.41 | 12 ± 1.41 | 23.5 ± 0.70 | - | - | - | 9.5 ± 0.70 | - | 21 ± 0.00 |
| S. enterica serovar typhimirium | 8 ± 0.00 | 12 ± 1.41 | 12.5 ± 0.70 | 17 ± 1.41 | 6.5 ± 0.70 | 6.5 ± 0.70 | - | 12 ± 1.41 | - | 22.5 ± 0.70 |
-: no activity. DMSO: dimethyl sulfoxide. All values are shown as mean ± standard deviation
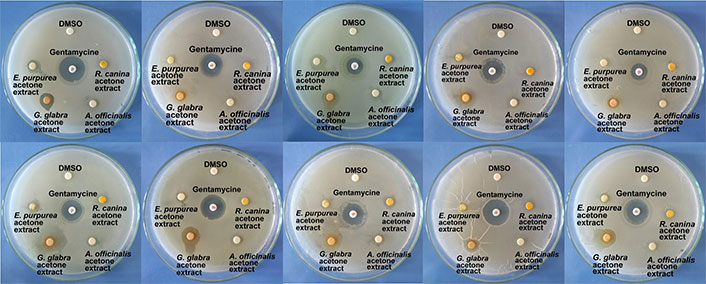
Inhibition zones created by acetone extracts of tested plants and gentamycine. DMSO: dimethyl sulfoxide
MIC values of the extracts are given in Table 2. MIC values of the extracts that formed inhibition zones of 10 mm and more than 10 mm were examined. MIC values were found to be lower in acetone extracts of plants compared to chloroform extracts. The lowest MIC value was determined as 0.00585 mg/mL against E. faecalis in the acetone extract of G. glabra. The highest MIC value was determined as 1.5 mg/mL against B. cereus in acetone extract of E. purpurea, as 1.5 mg/mL against Y. pseudotuberculosis in chloroform extract of G. glabra and chloroform extracts of A. officinalis.
MIC values of the extracts (mg/mL)
| Bacteria | Acetone extract of G. glabra | Acetone extract of A. officinalis | Acetone extract of E. purpurea | Acetone extract of R. canina | Chloroform extract of G. glabra | Chloroform extract of A. officinalis | Chloroform extract of E. purpurea | Chloroform extract of R. canina |
|---|---|---|---|---|---|---|---|---|
| E. faecalis | 0.00585 | 0.1875 | 0.75 | 0.375 | 0.375 | 0.375 | - | - |
| S. enterica serovar typhimirium | 0.04687 | 0.1875 | - | - | 0.1875 | - | - | - |
| L. monocytogenes | 0.09375 | - | 0.1875 | - | - | - | - | - |
| B. subtilis | 0.02343 | - | - | - | 0.09375 | 0.375 | - | 0.375 |
| P. vulgaris | 0.375 | 0.75 | - | 0.375 | 0.75 | 0.75 | 0.75 | 0.75 |
| E. aerogenes | 0.04687 | - | - | - | 0.09375 | - | - | - |
| B. cereus | 0.02343 | 0.375 | 1.5 | - | 0.375 | - | - | - |
| K. pneumoniae | 0.02343 | - | - | 0.75 | - | - | - | - |
| G. rubripertincta | 0.02343 | 0.375 | 0.75 | 0.375 | - | - | - | - |
| S. aureus | 0.1875 | 0.1875 | 0.75 | 0.75 | 0.75 | - | 0.75 | - |
| Y. pseudotuberculosis | - | - | - | - | 1.5 | 1.5 | 0.75 | 0.75 |
-: no activity. MIC: minimum inhibition concentration
Antioxidant activity
Plant phenolics show antioxidant properties due to their redox properties. They act as reducing agents, hydrogen donors, singlet oxygen inhibitors and metal chelators [19].
Total phenol contents of the studied plant extracts are given in Table 3. The highest total phenol content was found in acetone extract of G. glabra (273.45 ± 0.0932 µg GAE/mL), while the lowest total phenol content was found in chloroform extract R. canina (17.03 ± 0.0129 µg GAE/mL). In addition, it was determined that the acetone extracts of the studied plants had higher total phenolic content compared to the chloroform extracts.
Total phenolic content (µg GAE/mL), total flavonoid content (µg CE/mL) and total antioxidant capacity of extracts (µg AAE/mL)
| Extract | Total phenolic content (µg GAE/mL) | Total flavonoid content (µg CE/mL) | Total antioxidant capacity (µg AAE/mL) |
|---|---|---|---|
| Acetone extract of E. purpurea | 54.97 ± 0.0419 | 88.95 ± 0.0710 | 126.38 ± 0.0780 |
| Acetone extract of A. officinalis | 96.45 ± 0.0321 | 54.28 ± 0.0141 | 50.91 ± 0.0294 |
| Acetone extract of R. canina | 181.33 ± 0.0946 | 48.63 ± 0.0596 | 81.58 ± 0.0618 |
| Acetone extract of G. glabra | 273.45 ± 0.0932 | 119.55 ± 0.0766 | 87.88 ± 0.0434 |
| Chloroform extract of E. purpurea | 20.09 ± 0.0103 | 16.71 ± 0.0424 | 101.96 ± 0.0372 |
| Chloroform extract of A. officinalis | 87.72 ± 0.0541 | 58.7 ± 0.0124 | 247.28 ± 0.0557 |
| Chloroform extract of R. canina | 17.03 ± 0.0129 | 30.81 ± 0.0129 | 208.26 ± 0.0200 |
| Chloroform extract of G. glabra | 58.51 ± 0.0396 | 65.05 ± 0.080 | 178.88 ± 0.0327 |
AAE: ascorbic acid equivalent; CE: catechin equivalent; GAE: gallic acid equivalent. All values are shown as mean ± standard deviation
Flavonoids play an important role as antioxidants. Most flavonoids inhibit the formation of lipid peroxidation-causing radicals and lipid peroxy radicals [20]. Total flavonoid contents of the studied plant extracts are given in Table 3. The highest total flavonoid content was found in acetone extract of G. glabra (119.55 ± 0.0766 µg CE/mL), while the lowest total phenol content was found in chloroform extract of E. purpurea (16.71 ± 0.0424 µg CE/mL). In addition, it was determined that acetone extracts of the studied plants (except A. officinalis acetone extract) had higher total flavonoid content than chloroform extracts.
The total antioxidant capacities of the studied plant extracts are given in Table 3. The highest and lowest total antioxidant capacity was determined as 247.28 ± 0.0557 µg AAE/mL and 50.91 ± 0.0294 µg AAE/mL in chloroform extract of A. officinalis and acetone extract of A. officinalis, respectively. In addition, it was determined that chloroform extracts of the studied plants had higher total antioxidant capacity than acetone extracts (except E. purpurea acetone extract).
When DPPH solution is mixed with a substance that can give hydrogen atoms, the reduced form of the radical is released and as a result DPPH. A lightening is seen in the dark purple color of the radical [21].
DPPH radical scavenging activity of the studied plant extracts is given in Table 4. Acetone extracts showed higher activity compared to chloroform extracts. Acetone extract of R. canina showed higher activity than rutin which is used as standard antioxidant substance. No activity was observed at 250 µg/mL concentrations of acetone extract of G. glabra, chloroform extract of A. officinalis and 250, 500 and 750 µg/mL concentrations of chloroform extract of E. purpurea. DPPH radical scavenging activities of plant extracts, BHT and rutin at 1,000 µg/mL concentration increase as following order: chloroform extract of E. purpurea < chloroform extract of A. officinalis < chloroform extract of G. glabra < acetone extract of E. purpurea < acetone extract of A. officinalis < chloroform extract of R. canina < acetone extract of G. glabra < BHT < rutin < acetone extract of R. canina.
DPPH and ABTS radical scavenging activity of the extracts and standards
| Plant Extract | Concentration (µg/mL) | DPPH radical scavenging activity (% inhibition) | ABTS radical scavenging activity (% inhibition) |
|---|---|---|---|
| Acetone extract of A. officinalis | 250 | 11.37 ± 0.024 | 54.5 ± 0.019 |
| 500 | 27.24 ± 0.022 | 72.92 ± 0.015 | |
| 750 | 35.54 ± 0.034 | 84.41 ± 0.008 | |
| 1,000 | 37.93 ± 0.005 | 93.03 ± 0.039 | |
| Acetone extract of R. canina | 250 | 88.53 ± 0.007 | 94.16 ± 0.015 |
| 500 | 91.77 ± 0.012 | 94.25 ± 0.005 | |
| 750 | 92.49 ± 0.006 | 94.96 ± 0.016 | |
| 1,000 | 94.48 ± 0.003 | 95.7 ± 0.005 | |
| Acetone extract of G. glabra | 250 | NA | 91.11 ± 0.014 |
| 500 | 13.78 ± 0.015 | 94.86 ± 0.013 | |
| 750 | 34.74 ± 0.013 | 95.77 ± 0.020 | |
| 1,000 | 58.18 ± 0.022 | 97.18 ± 0.022 | |
| Acetone extract of E. purpurea | 250 | 12.76 ± 0.025 | 22.69 ± 0.038 |
| 500 | 23.38 ± 0.025 | 24.89 ± 0.056 | |
| 750 | 26.45 ± 0.022 | 41.3 ± 0.064 | |
| 1,000 | 30.93 ± 0.008 | 50.76 ± 0.011 | |
| Chloroform extract of A. officinalis | 250 | NA | NA |
| 500 | 4.95 ± 0.013 | 16.13 ± 0.018 | |
| 750 | 5.04 ± 0.013 | 32.55 ± 0.034 | |
| 1,000 | 5.71 ± 0.021 | 33.03 ± 0.002 | |
| Chloroform extract of R. canina | 250 | 13.92 ± 0.018 | 17.14 ± 0.012 |
| 500 | 22.38 ± 0.009 | 33.24 ± 0.015 | |
| 750 | 29.33 ± 0.010 | 49.96 ± 0.041 | |
| 1,000 | 38.21 ± 0.022 | 58.27 ± 0.007 | |
| Chloroform extract of G. glabra | 250 | 6.73 ± 0.021 | 30.08 ± 0.037 |
| 500 | 7.58 ± 0.013 | 47.90 ± 0.041 | |
| 750 | 7.69 ± 0.025 | 68.30 ± 0.009 | |
| 1,000 | 8.11 ± 0.031 | 85.61 ± 0.011 | |
| Chloroform extract of E. purpurea | 250 | NA | 7.67 ± 0.018 |
| 500 | NA | 21.37 ± 0.011 | |
| 750 | NA | 36.08 ± 0.011 | |
| 1,000 | 5.09 ± 0.020 | 53.85 ± 0.043 | |
| Rutin | 250 | 86.80 ± 0.008 | 78.54 ± 0.048 |
| 500 | 87.91 ± 0.003 | 81.94 ± 0.019 | |
| 750 | 90.60 ± 0.004 | 85.26 ± 0.010 | |
| 1,000 | 91.89 ± 0.011 | 87.63 ± 0.006 | |
| BHT | 250 | 88.85 ± 0.012 | 93.48 ± 0.011 |
| 500 | 89.55 ± 0.005 | 93.92 ± 0.006 | |
| 750 | 90.27 ± 0.011 | 94.43 ± 0.004 | |
| 1,000 | 91.55 ± 0.008 | 96.65 ± 0.008 |
NA: no activity. All values are shown as mean ± standard deviation
ABTS radical scavenging activity test is performed by spectrophotometrically measuring the color loss of ABTS+ colored chromophore, which is formed as a result of oxidation of ABTS with potassium persulfate [21]. ABTS radical scavenging activity of the studied plant extracts is given in Table 4.
Acetone extracts showed higher ABTS radical scavenging activity than chloroform extracts. Acetone extracts of G. glabra exhibited higher activity than BHT and rutin used as standard antioxidant agents at 1,000 ug/mL concentration. Except for the 250 µg/mL concentration of A. officinalis chloroform extract, all other extracts and concentrations showed ABTS radical scavenging activity. ABTS radical scavenging activities of plant extracts, BHT and rutin at 1,000 µg/mL concentration increase as following order: chloroform extract of A. officinalis < acetone extract of E. purpurea < chloroform extract of E. purpurea < chloroform extract of R. canina < chloroform extract of G. glabra < rutin < acetone extract of A. officinalis < acetone extract of R. canina < BHT < acetone extract of G. glabra.
Discussion
There are many studies about antibacterial efficiencies of E. purpurea, A. officinalis, R. canina and G. glabra in literatures. For example: Taştekin [22] (2017) found that fruit of R. canina which grow in Samsun exhibited antibacterial activity at varying degrees against E. faecalis, Escherichia coli, S. aureus, Enterococcus faecium, Staphylococcus epidermidis and Pseudomonas aeruginosa. In our study, acetone extract of fruit of R. canina showed activity against E. faecalis and S. aureus but chloroform extract of fruit of R. canina showed no activity. Berber et al. [23] (2013) revealed methanol extract of R. canina exhibited activity against E. faecalis but no activity against Bacillus cereus, E. coli, S. aureus and Micrococcus luteus. In a study conducted by Hassan et al. [24] (2020), it was determined methanol extract of E. purpurea inhibited E. coli and Streptococcus faecalis.
Stanisavljevic et al. [25] (2009) found classic and ultrasound extracts of E. purpurea were active against E. coli, P. aeruginosa, B. subtilis and S. aureus. In our current study, we found acetone and chloroform extracts of E. purpurea weren’t effective against B. subtilis but effective against S. aureus [25].
Lateef Al-Awsi et al. [26] (2021) stated methanol extract of A. officinalis which was prepared at 200 mg/mL concentration inhibited S. aureus, P. aeruginosa, Klebsiella pnemoniae and Streptococcus pnemoniae. In our research, acetone and chloroform extracts of A. officinalis which were prepared at 30 mg/mL concentration showed antibacterial action against S. aureus (both acetone and chloroform extract) and K. pneumoniae (only acetone extract).
Durmaz et al. [27] (2018) reported water and ethanol extracts of G. glabra inhibited B. subtilis, S. typhimurium, Proteus mirabilis, Salmonella enteritidis, K. pneumoniae, S. aureus and E. coli but not inhibited B. cereus. In our study, acetone and chloroform extracts of G. glabra inhibited B. subtilis, S. typhimirium, K. pneumoniae and S. aureus ve B. cereus.
In a study carried out by Mohammed et al. [28] (2021), it was found that ethanol extract of G. glabra inhibited S. aureus, metisilline resistant S. aureus, E. faecalis, E. coli and P. aeruginosa.
The emergence of different results in the literature and our research might be due to the use of different solvents during extraction, the collection of the same plant species from different geographies and the difference in the amount of extract applied to microorganisms.
There are many studies in the literature on the antioxidant activities of E. purpurea, A. officinalis, R. canina and G. glabra.
Pehlivan et al. [29] (2018) determined that R. canina samples collected from Erzincan exhibited high antioxidant activity. In our study, acetone and chloroform extracts of R. canina were found to have antioxidant activity.
Çömlekcioğlu et al. [30] (2022) examined the antioxidant activities of fresh and dry R. canina extracts and determined that dry R. canina extracts had higher antioxidant capacity.
Erenler et al. [31] (2015) determined that the water extract of E. purpurea exhibited strong DPPH radical scavenging activity, ABTS radical scavenging activity and reducing potency activity. In our research, it was found that acetone extract of E. purpurea has both DPPH and ABTS radical scavenging activity, while chloroform extract exhibits ABTS radical scavenging activity, but not DPPH radical scavenging activity.
Stanisavljevic et al. [25] (2009) examined E. purpurea extracts obtained by classical and ultrasound extraction methods and determined that the extracts obtained by classical extraction had higher total antioxidant capacity and higher total phenol and flavonoid content.
In a study conducted by Elmastas et al. [32] (2004), it was noted that the ethanol extract of A. officinalis showed strong total antioxidant capacity, reducing power activity, superoxide radical anion scavenging activity, DPPH radical scavenging activity and metal chelating activity. In our study, it was determined that acetone and chloroform extracts of A. officinalis also exhibited DPPH radical scavenging activity. Xue et al. [33] (2022) determined that the methanol extract of A. officinalis showed high antioxidant activity.
Babich et al. [34] (2022) found ABTS and DPPH radical scavenging activities of methanol extract of G. glabra obtained by Soxhlet extraction as 117.62 ± 7.91 µmol Trolox equivalent/g and 58.16 ± 3.90 µmol Trolox equivalent/g, respectively. Chopra et al. [35] (2013) determined the IC50 value of the DPPH radical scavenging activity of the methanol extract of G. glabra collected from India as 359.45 µL/mL.
In conclusion, chloroform and acetone extracts of R. canina, E. purpurea, A. officinalis, and G. glabra showed antibacterial and strong antioxidant properties. Hence, these plants could be an alternative to synthetic antioxidants and antibacterial agents. Additional studies are required to isolate and determine active compounds and understand the action mechanism of pharmaceutical properties.
Abbreviations
| AAE: | ascorbic acid equivalent |
| ATCC: | American type culture collection |
| CE: | catechin equivalent |
| DMSO: | dimethyl sulfoxide |
| GAE: | gallic acid equivalent |
| MIC: | minimum inhibition concentration |
Declarations
Author contributions
SA: Data curation, Writing—original draft, Resources, Investigation, Writing—review & editing.
Conflicts of interest
The author declares that there is no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Requests for accessing the datasets should be directed to Sinem Aydin, sinem.aydin@giresun.edu.tr.
Funding
This study is financially supported by Giresun University Scientific Project [FEN-BAP-A-250221-51]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2024.