Abstract
Glioblastoma (GBM) remains a formidable challenge in neuro-oncology due to its aggressive nature and propensity for therapeutic resistance. Anti-vascular endothelial growth factor (VEGF) therapies, although promising, often encounter resistance that limits their clinical efficacy. A multi-target and multi-drug approach has emerged as compelling strategies to address this resistance to enhance the treatment outcomes. This review examines the complex environment of anti-VEGF resistant GBM and analyses a multi-target therapeutic approach using natural products.
Keywords
Glioblastoma, anti-VEGF resistance, natural products, computational approachIntroduction
Cancer therapy consists of a combination of treatments which mainly include surgery with chemotherapy and radiation. In addition to these, immunotherapy, photodynamic therapy, and targeted therapy are also being practiced. In targeted therapy, the proteins that regulate the growth, division, and metastasis of cancer cells are targeted. The progression of tumor tissue growth is mainly due to the complex interaction among various signaling proteins and signaling pathways. Targeting these signaling proteins is a promising approach and involves the use of small molecule drugs and monoclonal antibodies which specifically target the proteins that drive cancer development.
Natural products are regarded as a rich reservoir of bioactive small molecules with medicinal potential due to the incredible chemical diversity in nature. Small molecules derived from plants have been reported to possess anti-cancer effects [1]. Several anti-cancer medications have been discovered and about 25% of all recently authorized anti-cancer medications are based on natural ingredients [2]. Plant secondary metabolites such as flavonoids, alkaloids, terpenoids, and tannins possess anti-cancer properties. Alkaloids such as vincristine and vinblastine were the first plant-based anti-cancer compounds [3]. There have been several natural substances implicated in the prevention of cancer, including curcumin, lycopene, taxol, camptothecin, resveratrol, berberine, baicalein, dioscin, wogonin, piperine, etc. [4]. However, there are some exceptions and it is possible to structurally modify natural compounds to reduce toxicity without affecting their biological activity.
Angiogenesis is a critical process in tumor growth. Targeting tumor angiogenesis has been adopted as a useful approach to arrest tumor growth. One of the therapeutic approaches is anti-vascular endothelial growth factor (VEGF) therapy, where small molecules or monoclonal antibodies are employed to block VEGF/VEGF receptor 2 (VEGFR2) signaling pathway, the key pro-angiogenic pathway. However, certain tumors such as glioblastoma (GBM) develop resistance against anti-VEGF therapy and capillaries continue to grow into the tumor site providing oxygen and nutrients for the tumor to grow. This anti-VEGF resistance is apparently due to alternate pathways and mechanisms that link with downstream components of VEGF/VEGFR2 signaling, triggering the expression of genes involved in key processes in endothelial cell (EC) activation relevant to angiogenesis. In our effort to get insights into this, we have carried out investigations to (a) develop a comprehensive signaling pathway map of VEGF/VEGFR2 signaling in EC; (b) examine the potential multiple molecules, particularly natural compounds that may block different arms of signaling pathway; (c) identify differentially expressed genes (DEGs) in the anti-VEGF resistant GBM tumors; and (d) examine the potential of multi-ligand multi-target approach based on traditional knowledge on the potential of phytocompounds from medicinal plants. In this review, we have tried to summarize the important findings of these studies and propose that a traditional knowledge-inspired natural compound-based, multi-ligand multi-target approach as an adjuvant therapy along with the anti-VEGF therapy.
Targeting angiogenesis in cancer therapy
Angiogenesis is a key process in the development of new blood vessels. The regulation of angiogenesis involves a delicate balance between pro-angiogenic and anti-angiogenic factors. Alterations in the balance of angiogenic factors can result in various pathological conditions. In adults, angiogenic processes are activated only under specific physiological conditions. However, excessive or insufficient development of blood vessels can lead to serious issues. Cancer cells trigger excessive angiogenesis as tumor tissue requires neovascularization to supply oxygen and nutrients for growth and metastasis. Targeting angiogenesis is a key therapeutic strategy in cancer therapy [5–9].
ECs are principal cells involved in neovascularization in response to angiogenic stimuli; the activated ECs are more effective targets than tumor cells in treating cancer [10]. Neovascularization is activated by the paracrine loop between endothelial and non-endothelial cells through various growth factors. Information on angiogenic signaling pathways induced by these growth factors can be effectively used for anti-angiogenic therapies.
VEGF is an important angiogenic growth factor and it binds to VEGFRs on ECs to stimulate angiogenesis. There have been several strategies designed to target VEGF-mediated angiogenesis to date. Small molecule inhibitors of VEGFRs and anti-VEGF therapy are employed currently along with conventional therapeutics for treating cancer. The binding of monoclonal antibodies to VEGF prevents its binding to VEGFR, thereby inhibiting angiogenesis. Small molecule inhibitors of VEGF, VEGFRs, other angiogenic receptors, and specific angiogenic molecules block downstream signaling pathways relevant to angiogenesis. The most successful of these is bevacizumab/Avastin, a VEGF-neutralizing antibody approved by the Food and Drug Administration (FDA) in 2004 [11]. The second anti-angiogenic drug that has been approved by the FDA is the anti-VEGF aptamer pegaptanib/Macugen, which is used in the treatment of age-related macular degeneration [11, 12]. A small antibody fragment, ranibizumab/Lucentis, designed to bind to all VEGF isoforms, is in phase III clinical trials for the treatment of age-related macular degeneration [11–13].
In addition to these, several plant products have been reported to exhibit anti-angiogenic activity. Natural products such as polyphenols, alkaloids, terpenoids, and saponins have been reported to be effective in targeting tumor angiogenesis [14]. Some of the phytochemicals having anti-angiogenic properties include curcumin, resveratrol, quercetin, epigallocatechin-3-gallate, etc. [15]. Triphala churna (THL), an ayurvedic polyherbal formulation, and its active components, punicalagin and chebulagic acid inhibited VEGF induced angiogenesis [16]. Anti-angiogenic phytochemicals also possess the ability to act synergistically along with anti-cancer drugs [17].
Although anti-angiogenic therapy is a potential therapeutic approach against cancer, the development of resistance to anti-angiogenic therapy is a major drawback that affects the effectiveness of the treatment. One of the factors contributing to anti-angiogenic resistance is the activation of alternative angiogenic signaling pathways that maintain tumor vascularization and growth. Anti-angiogenic therapies targeting VEGF/VEGFR have proven to be effective, but resistance to anti-VEGF therapy has been reported [18, 19], indicating that modulation of angiogenesis occurs through VEGF-independent signaling pathways as well as the production of alternative pro-angiogenic factors. Reports showed that inhibition of one particular growth factor leads to the activation of other growth factors [20]. VEGF inhibition might lead to the activation of fibroblast growth factor (FGF), angiopoietin-1 (Ang-1), and epidermal growth factor (EGF) leading to stimulation of angiogenesis. It was reported that in mouse models of pancreatic tumors, inhibition of VEGF-VEGFR signaling using anti-VEGFR for a prolonged time leads to the activation of angiogenic factors like FGF and Ang-1 [21]. Targeting a single angiogenic factor may not be beneficial in anti-cancer therapy due to the development of resistance. Targeting multiple signaling pathways can be employed as a therapeutic strategy to overcome resistance to anti-angiogenic therapy, especially anti-VEGF therapy.
Glioma
Glioma is a general term used to describe primary brain tumors and is classified according to their expected cell of origin. These include oligodendrogliomas, ependymomas, astrocytic tumors (astrocytoma, anaplastic astrocytoma, and GBM), and mixed gliomas. They are the most commonly occurring tumors of the central nervous system (CNS), which account for almost 80% of all malignant primary tumors of the brain. GBM multiforme is the most malignant and frequently occurring type of primary astrocytomas. It accounts for more than 60% of all brain tumors in adults. Despite various modern therapies against GBM, it is still a deadly disease with an extremely poor prognosis. Patients usually have a median survival of approximately 14 to 15 months from the diagnosis [22].
The current international standard for the nomenclature and diagnosis of gliomas is the World Health Organization (WHO) classification. Gliomas are classified into grades I to IV on the basis of the level of malignancy that is determined by the histopathological method. Grade I gliomas relate to lesions that have low proliferative potential and can be cured by surgical procedure, whereas, grade II to IV gliomas are highly malignant and invasive. GBM multiforme has been designated grade IV by WHO [23].
GBM is a solid tumor with structurally and functionally abnormal vasculature that is characterized by a dense network of vessels, with increased vessel diameter and abnormally thickened basement membranes. This tumor vasculature enhances tumor hypoxia and impairs the delivery of cytotoxic chemotherapy [24]. Radiation, chemotherapy, and surgery are the usual GBM treatment choices though prognosis even after these therapies remains grim. Immunosuppression and diverse variations in pathogenic characteristics are two important factors responsible for the failure of current therapies. Targeted treatments and immunotherapies have shown significant progress in the treatment of GBM during the past few years, with encouraging outcomes in clinical trials. Gene therapy, photodynamic techniques, nanotechnology, and local destruction of tumors by genetically altered bacteria or controlled hyperthermia are among further treatments under research [25].
GBM: susceptible to anti-VEGF resistance
The outcome for GBM patients remains lethal despite all the progress made in anti-GBM therapeutics. The overall survival of GBM patients ranges from 14.6 to 20.5 months [26]. GBM is a highly vascularized tumor and VEGF is the major angiogenic growth factor regulating its neovascularization. It is also highly resistant to anti-angiogenic therapies and several clinical studies reported that GBM patients are highly susceptible to anti-VEGF resistance [27]. Highly aggressive nature, heterogeneity, and genetic diversity contribute to the highly vascularized nature of GBM. GBM tumors are highly heterogeneous, consisting of various cell types with different genetic profiles and this heterogeneity allows some tumor cells to activate alternative angiogenic pathways, which can compensate for the blocked VEGF signaling, leading to anti-VEGF resistance [28, 29]. The hypoxic microenvironment in GBM may promote resistance mechanisms by activating alternative angiogenic pathways [30]. A therapeutic approach targeting multiple angiogenic pathways in tumor heterogeneity may be useful for improving treatment outcomes in GBM patients. Several phytochemicals have been reported to be useful in GBM therapeutics by targeting different biological processes such as cell cycle, proliferation, angiogenesis, and apoptosis (Figure 1) [31].
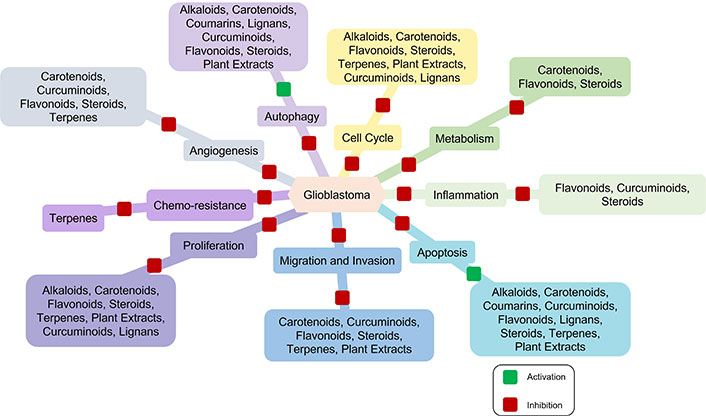
Natural products in glioblastoma (GBM) therapeutics. Multiple phytochemicals target multiple biological processes relevant to GBM therapeutics. These phytochemicals inhibit cell cycle, proliferation, metabolism, migration, and invasion, thereby reducing tumor tissue growth and metastasis. Some phytochemicals inhibit tumor tissue growth and development by blocking angiogenesis and inflammation. The induction of apoptosis and autophagy by phytochemicals ultimately leads to cell death. Furthermore, terpenes play a crucial role in overcoming chemo-resistance during therapies
In GBM, angiogenesis is associated with both hypoxia-dependent and hypoxia-independent mechanisms. The expression levels of the angiogenic growth factors impact tumor progression. These angiogenic factors are upregulated through various mechanisms such as oncogene activation, loss of tumor suppressor gene function, and/or hypoxic microenvironments [32]. Under hypoxia conditions, prolyl hydroxylases are inactivated resulting in the accumulation of hypoxia-inducible factor-1A (HIF-1A) which in turn causes up-regulation of VEGF [33]. VEGF is also upregulated by hypoxia-independent mechanisms as a result of dysregulated activation of mitogenic and survival pathways including Ras/mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K). Among the various angiogenic factors, VEGF is the most abundant and important mediator of angiogenesis in GBM. VEGF-A is upregulated in GBM cells and hence the expression of the receptors for VEGF-A (VEGFR1 and VEGFR2) is also upregulated on the ECs [33–35]. In addition to VEGF, GBM frequently expresses additional proangiogenic factors including FGF, platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), matrix metalloproteases (MMPs), integrins, hepatocyte growth factor/scatter factor, ephrins, Angs, and interleukin 8 (IL8). The prominent role played by VEGF in angiogenesis makes it an appealing target to be exploited in GBM therapeutics and a large number of clinical trials with anti-angiogenic agents are under various stages of study [36]. Anti-angiogenic treatment normalizes the vasculature, effectively decreasing vessel permeability, reducing tumor-induced edema, and eventually inhibiting the tumor cell proliferation in GBM. The prominent mechanisms in anti-angiogenesis involve VEGF pathway, VEGF-independent pathway, and inhibition of vascular EC migration.
Several anti-angiogenic agents are being evaluated in preclinical and clinical trial studies as an alternative or complementary approach to conventional cancer treatment. Most of the anti-angiogenic agents currently in phase I/II trials for primary and secondary brain tumors target the VEGF signaling pathway. Some of the prominent anti-angiogenic agents include monoclonal antibodies, bevacizumab, targeting VEGF-A, tyrosine kinase inhibitors (TKIs) such as cediranib, sunitinib, and vandetanib that inhibit VEGFR2, and soluble decoy receptors like aflibercept developed from VEGFR1 that selectively inhibits VEGF activity [37]. Bevacizumab or Avastin (Genentech, South San Francisco, CA), the first anti-angiogenesis agent approved for clinical use for brain cancer is a monoclonal antibody and it functions like the physiological antibodies that the human body naturally produces as part of the adaptive immune system [37]. Bevacizumab binds to VEGF and blocks its downstream signaling/suppresses new blood vessel growth. Several phase II clinical trials have examined the therapeutic efficacy of bevacizumab as a single agent or in combination with chemotherapy or radiation for recurrent GBM. Irinotecan, lomustine, and temozolomide (TMZ) are some of the combination drugs given along with bevacizumab against GBM.
Anti-VEGF treatment alters the abnormal characteristics of tumor vessels leading to devascularization that limits tumor growth. It is generally found to be well tolerated though a large fraction of patients develops toxicity and resistance to this treatment. Further, prolonged exposure to anti-angiogenic drugs blocks the blood supply to the tumors leading to a hypoxic environment, inducing chemo-resistance and tumor progression. The angiogenic inhibitors suppress the tumor growth, but do not necessarily kill all the cancer cells.
Anti-angiogenic therapy has been associated with prolonged progression-free and overall survival though most of the patients treated with these drugs eventually relapse. Additionally, there are also sets of patients who do not respond to bevacizumab. These patients may have a tumor that is not dependent on VEGF for growth and have an intrinsic resistance to anti-angiogenic therapy. Patients, who initially respond to treatment, may develop evasive/adaptive resistance by upregulation of alternate pro-angiogenic pathways, improved protection of tumor neovasculature by increased pericyte coverage, and increased invasiveness of tumor cells that co-opt native brain blood vessels [38]. Hypoxic tumors can recruit vascular progenitor cells and pro-angiogenic monocytes from the bone marrow that differentiate into endothelial and pericyte progenitors [39]. Evidence supporting these mechanisms come from pre-clinical models and efforts to identify tumor escape mechanisms in humans using blood or magnetic resonance imaging (MRI) biomarkers are emerging. Upregulation of stromal cell-derived factor-1α (SDF-1α), basic FGF (bFGF), PDGF, delta-like ligand 4 (DLL-4)/notch, and Tie-2 have been associated with the activation of alternate pro-angiogenic signaling pathways [40, 41]. Genetic alteration in the VEGF molecule itself or its receptor is also a commonly cited reason for acquired drug resistance in traditional chemotherapies, though the clear mechanism is yet to be understood [42]. Finding novel therapeutic approaches that target multiple signaling proteins and pathways simultaneously has gained attention to combat resistance and improve patient outcomes.
Targeting VEGF-VEGFR2 signaling pathway in ECs
The binding of VEGF to VEGFRs activates multiple downstream signaling pathways by regulating multiple signaling molecules leading to the activation of ECs, their proliferation, migration, and tube formation relevant to angiogenesis. We have developed a comprehensive VEGF/VEGFR signaling pathway map in ECs relevant to angiogenesis and its potential crosstalk with other signaling systems [43]. The pathway map documented 240 proteins and their diverse post translational modification (PTM)-dependent reactions involved in VEGF-A/VEGFR2 signal transduction. Seventy proteins involved in cell proliferation, 95 involved in cell survival, 103 in cell migration, and 31 proteins in receptor endocytosis were categorized. Also documented 14 receptors, 96 enzymes, and 28 transcription factors based on the function of proteins. This pathway also involved 95 protein-protein interactions (PPIs), 36 VEGF-induced direct phosphorylation, 5 dephosphorylation, 4 ubiquitination, 1 methylation, 1 glutathionylation, and 1 nitrosylation event. Later on, we improved this pathway map by incorporating an additional 198 proteins involved in angiogenesis, thereby reconstituting VEGF pathway map with 438 unique proteins [44]. VEGF-VEGFR signaling pathway map consists of 10 synchronously activated signaling modules that contain several signaling molecules. These signaling modules include Ras-related C3 botulinum toxin substrate (RAC), focal adhesion kinase (FAK), p38MAPK, Janus kinase (JAK), PI3K-protein kinase B-mammalian target of rapamycin (PI3K-Akt-mTOR), extracellular-signal-regulated kinases (ERK), phospholipase C-γ-protein kinase C (PLC-γ-PKC), Ras homolog family member A (RHOA), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and signal transducer and activator of transcription (STAT). Multiple signaling molecules in these modules crosstalk each other to regulate angiogenesis. Altogether, the VEGF-VEGFR signaling pathway map shows a series of connected and overlapping signaling events. It is also involved in critical junctions/branching points where various downstream signaling pathways converge/communicate with each other. Moreover, VEGF signaling pathways crosstalk with other extrinsic signaling pathways and function synergistically/antagonistically to modulate angiogenesis.
Due to the crosstalk and complementary effects of multiple signaling proteins among various signaling pathways related to angiogenesis, targeting multiple proteins in the pathway is crucial for regulating angiogenesis. This possibility was examined using plant products particularly medicinal plants which are used in traditional systems of medicine. THL is an ayurvedic herbal formulation used for the treatment of various diseases, including cancer. It is made up of dried fruits of three medicinal plants, Terminalia bellirica, Terminalia chebula, and Embelica officinalis, which contain a number of phytochemicals. THL and its active components, punicalagin and chebulagic acid, inhibit VEGF-induced angiogenesis [16]. These data suggest that inhibition of angiogenesis by this herbal extract involved the contribution of multiple compounds that act on different targets. This was shown by in silico molecular docking studies against different signaling molecules involved in VEGF-VEGFR2 signaling followed by experimental validation of the inhibition of the identified targets and inhibition of angiogenesis. The angiostatic effect of THL is mediated by the synergistic interaction of different THL phytochemicals with multiple signaling proteins in the VEGF/VEGFR2 signaling pathway. In addition to this, they also interact with other extrinsic angiogenic signaling pathways such as Wingless and Int-1 (Wnt)/β catenin signaling. Punicalagin modulates PI3K/Akt signaling and chebulagic acid modulates glycogen synthase kinase 3-beta (GSK3-beta)-β catenin signaling, two downstream signaling pathways relevant to angiogenesis (Figure 2) [45]. Another interesting fact is that these phytochemicals target Akt and β-catenin, which are key proteins at the converging points of multiple signaling pathways relevant to angiogenesis. Therefore, THL can be used as a potential herbal medicine modulating neovascularization as it targets multiple signaling proteins in angiogenesis.
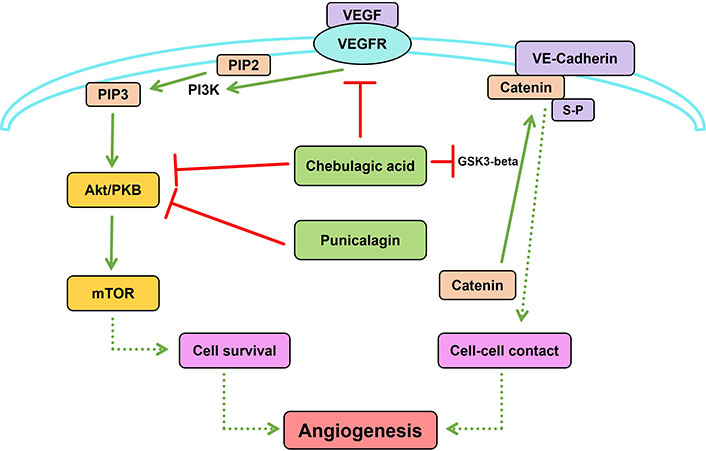
Synergistic interaction of Triphala churna (THL) phytochemicals with multiple signaling proteins in angiogenesis. Punicalagin inhibits phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling and chebulagic acid inhibits both Akt and glycogen synthase kinase 3-beta (GSK3-beta)-β catenin signaling, two downstream signaling pathways in angiogenesis. GSK3-beta induced phosphorylation of β-catenin at serine residue is essential for its binding with VE-cadherin, promoting cell-cell contact relevant to angiogenesis. Solid lines indicate direct modulation of one molecule by another. Dashed lines indicate that multiple signaling molecules are involved in the modulation of the target process/molecule. Created using PathVisio [46]. VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor; PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; mTOR: mammalian target of rapamycin; S-P: serine phosphorylation
Plant products as a novel therapeutic strategy in anti-VEGF resistant GBM
GBM therapeutics improvement is necessary due to the unfavorable outcomes of current approaches. Studies on plant-based products are an interesting field of investigation because of the presence of multiple bioactive compounds in plants. As an alternative approach, phytochemicals can be employed as a potentially less harmful and effective therapeutic strategy, particularly as adjuvant therapy. Plant products include polyherbal formulations that contain multiple small phytochemicals. They act together synergistically against multiple target proteins and thus correspond to a “multi-ligand multi-target” treatment strategy which would be beneficial for overcoming resistance to a particular single drug. Polyherbal formulations have been reported to be effective in cancer treatment [47]. Natural small molecules such as apigenin, quercetin, curcumin, etc. have been targeting NF-kB signaling in GBM [48]. Natural compounds such as alkaloids, carotenoids, flavonoids, coumarins, terpenes, lignans, natural steroids, and tannins possess modulatory effects on GBM [49, 50]. These reports suggest the promising role of a combination of phytochemicals in the treatment of anti-VEGF resistant GBM.
Several natural products have shown anti-cancer activity in GBM pre-clinical models, such as withaferin A, curcumin, berberine, saponin, ginsenoside, and iridin. In vitro and in vivo studies demonstrated that rutin and procyanidins inhibit GBM cell proliferation and angiogenesis by downregulating VEGF. Ginsenoside, together with TMZ, decreased angiogenesis by inhibiting VEGF in GBM mouse xenograft models [51].
In vitro GBM based cell culture studies show the effect of certain individual phytocompound in combating drug resistance in GBM conditions. Inhibition of VEGF and TGF-β by rutin, a flavonoid compound, in GBM cells may help overcome chemoradiotherapy resistance. Curcumin combats drug resistance in GBM by regulating angiogenesis and apoptosis through the modulation of the PI3K-Akt signaling pathway. Quercetin increased the sensitivity of GBM cell lines to TMZ by suppressing the heat shock protein that causes drug resistance. Resveratrol decreased TMZ resistance by modulating NF-kB signaling in GBM cells. Tagitinin C, from Tithonia diversifolia, inhibited the growth of GBM cells by downregulating the anti-apoptotic protein survivin, which is associated with drug resistance [51].
Recent trends in plant-based drug discovery: computational approaches
Numerous substances of plant origin with anti-cancer properties or distinctive structural benefits in investigating druggable targets have been reported. Despite these successes, it has remained difficult to convert bioactive natural materials into medications, in part due to the challenges associated with large-scale isolation, mechanistic understanding, and pharmaceutical development. As a result, major pharmaceutical corporations all over the world have scaled back or altogether stopped investing in natural product medication development in favor of enormous libraries of chemically created molecules or biologics. Recently, with the rapid expansion of our understanding of cancer therapy and the development of cutting-edge technologies, it has become possible to overcome the challenges in improving the effectiveness of drug discovery, identifying the precise targets of natural products, and resolving the complexity of the multifaceted pharmacological effects [52], particularly using computational approaches.
We employed a high throughput approach to identify alternate pathways of angiogenesis that contribute to anti-VEGF resistance in GBM conditions by identifying the DEGs in resistant GBM. After identifying the DEGs relevant to anti-VEGF resistant GBM, a traditional knowledge-driven study approach is performed to identify phytocompounds targeting the proteins coded by the genes expressed during anti-VEGF resistant GBM conditions.
Hub genes and key pathways associated with anti-VEGF resistant GBM
Transcriptome analyses are valuable tools to analyze genome-wide cellular alterations. Microarray and RNA-sequencing (RNA-Seq) analyses are two of the main methods to study transcriptomics [53]. In recent years, high throughput approaches have been developed to capture DEGs in various conditions including drug resistance. Microarray-based gene expression profiling and sequence-based techniques like RNA-Seq analysis provide useful information about the DEGs, key pathways, and the signature genes with respect to different conditions. Most of these data sets are now publicly available. Recently published studies employed gene expression data based computational approaches to characterize the genetic alterations at genome level, which helps identify DEGs and their possible physiological or pathological relevance.
As indicated in the earlier section, the anti-VEGF resistance is apparently due to alternative mechanisms leading to VEGFR2 activation and signaling in a VEGF independent manner [54]. It can also be due to the activation of other signaling pathways which link with the downstream components of the VEGF/VEGFR2 signaling pathway. The key strategy in such a situation would be to target key components of such pathways complementing anti-VEGF therapy. We have employed a novel approach of gene expression data analysis for identifying the potential genes associated with the resistance to anti-VEGF therapy in GBM [55]. This study analyses the gene expression data in xenografts from anti-VEGF-resistant GBM using bioinformatics tools, to understand the molecular basis of resistance to anti-VEGF therapy. The results suggest that the cells adapt to such conditions by changing gene expression and restoring angiogenesis. The analysis of the microarray data from fourth generation xenografts of anti-VEGF resistant GBM patients showed upregulation of 359 genes and downregulation of 514 genes, indicating differences in gene expression during the development of anti-VEGF resistance. The Gene Ontology (GO) function and pathway enrichment analysis of DEG showed significant enrichment in the biological processes such as cell proliferation, cell migration, and angiogenesis, indicating the ability of the tumor to have acquired angiogenic phenotypes. A further analysis of the DEGs showed enrichment in the molecular functions such as receptor-binding and growth factor activity; the signaling pathways such as TNF signaling pathway, PI3K-Akt pathway, and cytokine receptor pathway, were enriched particularly in up-regulated DEGs. The PPIs on a genome-wide scale aids in understanding the physiological mechanisms by which proteins are controlled with their function. The PPI network analysis showed enrichment in the key angiogenic pathways, such as the HIF-1 pathway, PI3K-Akt pathway, and cell cycle pathway, critical in angiogenesis and cancer development. The centrality measures help in identifying the genes that are crucial in the network and function as hub genes. Network centrality analysis identified 21 hub genes, including IL6, VEGF-A, and SRC. The survival analysis showed that the high expression of four hub genes IL6, VEGF-A, isocitrate dehydrogenase 1 (IDH1), and C-X-C motif chemokine ligand 8 (CXCL8) were associated with a shorter overall survival time of GBM patients.
Traditional knowledge-driven approach for drug discovery in anti-VEGF resistant GBM
Traditional medicine is gaining increasing interest across the globe, including in India, Japan, Korea, China, and Thailand. In India, the traditional system of ayurvedic treatment, which places an emphasis on “balance” in daily lives, has been in practice for more than 5,000 years [56]. Particularly, herbal therapy based on natural products is currently anticipated to be a novel treatment for a number of diseases, including cancer, due to its effectiveness and lack of major side effects [57–61].
Most of the existing drugs in the market are either directly or indirectly obtained from the medicinal plants used in traditional practice [62, 63]. Rasayana therapy given for general physiological and psychological well-being, aims to promote the absorption and circulation of essential nutrients required for nourishment to cells and tissues in the body. Immunity, longevity, cognitivity, intellect, and rejuvenation are some of the secondary attributes offered by Rasayana therapy [64]. Naimittika Rasayana, Ajasrika Rasayana, and Kamya Rasayana are three major classes of which Medhya Rasayana, is a sub-class of medicinal plants used for managing neurological disorders and for enhancing memory and intellect. The Sanskrit word “Medhya”, means “intellect”, and “Rasayana”, means “rejuvenation”. This Rasayana therapy consists of 13 medicinal plants, Centella asiatica Linn, Glycyrrhiza glabra Linn, Tinospora cordifolia, Bacopa monniera, Acorus calamus, Celastrus paniculatus, Benincasa hispida, Nardostachys jatamansi, Plumbago zeylanica, Convolvulus pluricaulis Choisy, Clitoria ternatea, Evolvulus alsinoides, and Canscora decussate, used in single or in combination to manage cognitive disorders. It is evident from many scientific investigations that all these plants individually exhibit neuroprotective effects against cognitive impairment [50, 65–69].
The ayurvedic approach to disease management has recently received renewed attention with the aid of molecular biology and the application of computational tools and techniques paving the way to the new field of Ayurinformatics. The concept of computer-aided drug discovery (CADD)-based drug-target interaction has been widened in Ayurinformatics to encompass a group of phytochemicals against one or more targets (multi-ligand-multi-target interaction) involved in the disease mechanism. We have demonstrated the therapeutic potential of neuroprotective ayurvedic formulation, Medhya Rasayana, in anti-VEGF resistant GBM therapeutics (unpublished data). By using computational approaches, we identified the key targets of anti-VEGF resistant GBM and also key plants and phytochemicals in Medhya Rasayana that interact with these targets. In Ayurveda, treatment is based on the individual’s prakriti type, where different plant combinations are used in the formulation. The network pharmacological approach was used to identify, which plant/phytochemical is highly effective for a possible adjuvant therapy. To understand the contribution of Medhya Rasayana plants and their phytochemical in reducing the resistance, a plant-phytochemical-target interaction network was generated (Figure 3). Multiple phytocompounds from different Medhya Rasayana plants interact with multiple targets involved in resistance to anti-VEGF therapy in GBM, which is particularly relevant in the context of ayurveda concept of therapy. Formulations of multiple medicinal plants containing multiple phytocompounds are prescribed in a personalized manner depending on the “biological constitution” (prakruthi) of each individual, rather than monotherapy, facilitating multi-target therapy.
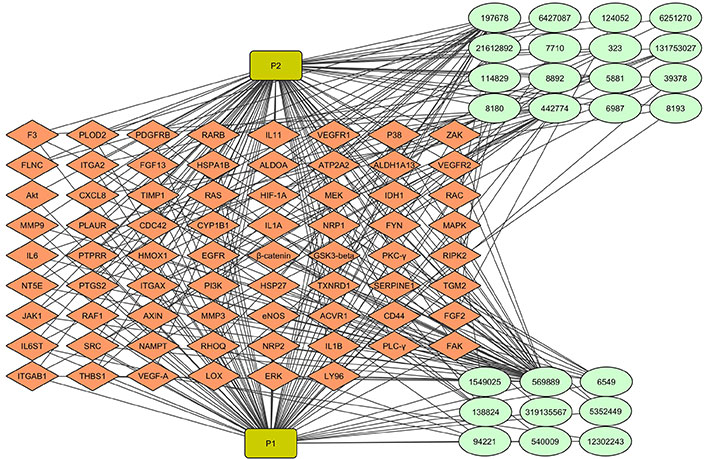
Multiple phytochemicals from Medhya Rasayana plants targeting multiple proteins in angiogenesis. 25 phytochemicals from Celastrus paniculatus and Glycyrrhiza glabra interact with proteins produced by 70 differentially expressed genes (DEGs) in anti-vascular endothelial growth factor (VEGF) resistant glioblastoma (GBM). Light green colored round shaped node represents phytocompounds. Green rectangle node represents plants. Diamond-shaped orange node represents target proteins. The network consists of 97 nodes and 285 edges. P1: Celastrus paniculatus; P2: Glycyrrhiza glabra. IL11: interleukin 11; VEGFR1: VEGF receptor 1; FGF13: fibroblast growth factor 13; Akt: protein kinase B; CXCL8: C-X-C motif chemokine ligand 8; HIF-1A: hypoxia-inducible factor-1A; IDH1: isocitrate dehydrogenase 1; RAC: Ras-related C3 botulinum toxin substrate; MMP9: matrix metalloprotease 9; MAPK: mitogen-activated protein kinase; GSK3-beta: glycogen synthase kinase 3-beta; PKC-γ: protein kinase C-γ; PI3K: phosphoinositide 3-kinase; JAK1: Janus kinase 1; RHOQ: Ras homolog family member Q; PLC-γ: phospholipase C-γ; FAK: focal adhesion kinase; ERK: extracellular-signal-regulated kinases; F3: coagulation factor III; PLOD2: procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2; PDGFRB: platelet-derived growth factor receptor beta; RARB: retinoic acid receptor beta; FLNC: filamin-C; ITGA2: integrin alpha 2; HSPA1B: heat shock protein family A (Hsp70) member 1B; ALDOA: aldolase A; ATP2A2: ATPase, sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2; ALDH1A13: aldehyde dehydrogenase 1 A13; TIMP1: tissue inhibitor of metalloproteinases 1; RAS: rat sarcoma; MEK: MAPK kinase; PLAUR: plasminogen activator, urokinase receptor; CDC42: cell division control protein 42 homolog; CYP1B1: cytochrome P450 1B1; NRP1: neuropilin 1; FYN: proto-oncogene tyrosine-protein kinase; PTPRR: protein tyrosine phosphatase receptor type R; HMOX1: heme oxygenase 1; EGFR: epidermal growth factor receptor; RIPK2: receptor-interacting serine/threonine-protein kinase 2; NT5E: 5’-nucleotidase ecto; PTGS2: prostaglandin-endoperoxide synthase 2; TXNRD1: thioredoxin reductase 1; SERPINE1: plasminogen activator inhibitor 1; TGM2: transglutaminase 2; eNOS: endothelial nitric oxide synthase 3; ACVR1: activin A receptor type-1; IL6ST: IL6 cytokine family signal transducer; NAMPT: nicotinamide phosphoribosyltransferase; THBS1: thrombospondin 1; LOX: lysyl oxidase; LY96: lymphocyte antigen 96
In cancer, adjuvant therapy is an additional treatment given to patients either along with or after the main treatment procedure. The primary goal of adjuvant therapy is to improve disease-free and overall survival by targeting the micrometastatic and residual disease. The advantage of adjuvant therapy includes an increased chance of disease-free survival, a decreased chance of recurrence, or added life-years. Natural product/traditional medicine-based adjuvant therapy is an important therapeutic strategy. Hence, the potential of using a formulation containing Medhya Rasayana plants, which has been previously used for treating neurological disorders, as an adjuvant therapy along with anti-VEGF therapy is a promising approach.
Conclusions
The review presents novel approaches to improving cancer therapeutics with drug resistance by applying the multi-target potential of natural plant-based products to combat resistance. Angiogenesis is crucial for tumor growth, and hence anti-angiogenic agents have been used for cancer treatment. Several anti-angiogenic drugs targeting VEGF and VEGFRs such as bevacizumab, sunitinib, etc. have been approved for clinical use in cancer therapeutics. However, anti-angiogenic therapeutic strategies based on VEGF might not be sufficient to reduce tumor growth due to the development of anti-VEGF resistance. This is mainly due to the development of compensatory mechanisms for neovascularization, particularly due to the existence of alternative/bypass signaling pathways other than VEGF signaling. Moreover, the VEGF signaling pathway cross-talks with other multiple signaling pathways, and several signaling molecules converge to modulate angiogenesis. So, in order to improve anti-angiogenic therapeutics, it is crucial to develop a multi-target therapeutic strategy other than the currently employed single target approach. Development of a comprehensive VEGF-A/VEGFR2 signaling pathway map identified signaling modules and several junction points, where other pathways can converge.
Natural plant-based products are polyherbal formulations that contain several phytochemicals. THL is an ayurvedic polyherbal formulation that possesses anti-angiogenic properties with multiple phytochemicals targeting multiple proteins involved in the angiogenic process. Such a multi-ligand multi-target treatment strategy is very important to enhance anti-angiogenic therapeutics.
In pathological conditions, instead of targeting all proteins, it is necessary to identify specific proteins that contribute to the disease pathology. A possible strategy is to identify the DEGs in disease conditions by transcriptome analyses and identify the hub genes and associated proteins by computational approaches. These multiple proteins can be synergistically targeted by polyherbal formulations containing multiple phytochemicals. This approach has been employed to target GBM, one of the most lethal tumors resistant to anti-VEGF therapy. Anti-VEGF resistant GBM patients showed differences in gene expression patterns during the development of resistance. Here, we identified hub genes and proteins in anti-VEGF resistant GBM and the multiple phytochemicals of Medhya Rasayana that target these multiple proteins. This phytochemical formulation may be used as an adjuvant therapy along with anti-VEGF therapy in anti-VEGF resistant GBM patients based on the use of these formulations for centuries for other conditions. These in silico findings support the need for pre-clinical and clinical studies to evaluate the use of natural products as adjuvant therapy in drug resistant GBM conditions. Clinical trials must be conducted to evaluate the safety and efficacy of natural product-based therapeutics in glioma patients. GBM therapeutics can be developed by exploring the potential of combination therapies using natural products along with chemotherapy and immunotherapy, which may improve outcomes for GBM patients [70].
The identification of DEGs and multi-ligand multi-target approach is very relevant in cancer conditions with drug resistance. Polyherbal formulations can be used as adjuvant therapy along with conventional therapy in these conditions though bioavailability of desired molecules could be an issue. However, to implement this approach, transcriptome sequencing needs to be done for each patient to identify gene expression and DEGs. This is a major limitation of this approach as the sequencing is too expensive but it needs to be done for personalized targeted therapy as DEGs differ among patients. Generally, the multi-ligand multi-target approach represents a personalized strategy for cancer therapy to overcome resistance and improve patient outcomes.
Abbreviations
| Akt: | protein kinase B |
| Ang-1: | angiopoietin-1 |
| DEGs: | differentially expressed genes |
| EC: | endothelial cell |
| FGF: | fibroblast growth factor |
| GBM: | glioblastoma |
| IL8: | interleukin 8 |
| NF-kB: | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PI3K: | phosphoinositide 3-kinase |
| PPIs: | protein-protein interactions |
| THL: | Triphala churna |
| TMZ: | temozolomide |
| VEGF: | vascular endothelial growth factor |
| VEGFR2: | vascular endothelial growth factor receptor 2 |
Declarations
Acknowledgments
Financial assistance from the following organizations is greatly acknowledged. Government of Kerala (CMNPF fellowship) for assisting SJS; KSCSTE (Kerala State Council for Science, Technology, and Environment) for supporting KRA, CSA, and APA; University of Kerala for assisting PS, Ashuthosh Mukherjee fellowship to PRS. Financial support provided by State Inter-University Centre of Excellence in Bioinformatics (SIUCEB), Govt. of Kerala is also acknowledged.
Author contributions
SJS: Conceptualization, Data curation, Writing—original draft, Writing—review & editing, Funding acquisition. KRA and PRS: Conceptualization, Data curation, Writing—original draft, Writing—review & editing. CSA, PS, and APA: Conceptualization, Data curation. ASN: Writing—review & editing, Funding acquisition. OVO: Writing—review & editing.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The raw data, other than those published, supporting the conclusions of this manuscript will be made available by the authors, on request, to any qualified researcher.
Funding
The study is supported by Government of Kerala (KSHEC - CMNPF fellowship); KSCSTE (Kerala State Council for Science, Technology, and Environment); University of Kerala; Indian Science Congress Association (ISCA), Kolkata (Ashuthosh Mukherjee fellowship); State Inter-University Centre of Excellence in Bioinformatics (SIUCEB), Govt. of Kerala. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2024.