Abstract
Aim:
The aim of this research was to generate new peptide molecules with cytotoxic activity against cervical cancer that can become effective in mitigating the impact of the disease and preventing its progression. The design is based on the hybrid peptide formation strategy that allows new chemical entities to be obtained from the union of fragments of different bioactive peptides. Specifically, we worked by combining the RWQWRWQWR sequence derived from bovine lactoferricin with different functional peptides such as anticancer peptides, cervical cancer cell-targeting peptides, and cell-penetrating peptides.
Methods:
Hybrid peptides and precursors were synthesized by solid-phase peptide synthesis using the Fmoc/tBu strategy, purified via reverse phase (RP)-solid phase extraction, and characterized by RP-high performance liquid chromatography (RP-HPLC) chromatography and mass spectrometry. In vitro cytotoxicity of hybrid peptides in human cervical cancer cells lines HeLa and Ca Ski was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Results:
All hybrid peptides were obtained with high purity and the experimental mass corresponds with the theoretical mass. Some hybrid peptides exhibited significant, fast, and selective cytotoxic activity against the cancerous cells evaluated, specifically those containing sequences of anticancer peptides and cell-penetrating peptides. The cytotoxic effect exerted by the monomeric and dimeric hybrid peptides depended on the concentration of the peptide, which allowed the determination of the IC50 values and the selectivity index (SI).
Conclusions:
We obtained hybrid peptides with the core sequence RWQWRWQWR that are active against HeLa and Ca Ski cell lines. The combination of the RWQWRWQWR sequence with short anticancer peptides and cell-penetrating peptides allowed the creation of hybrid peptides with improved cytotoxic potency against cervical cancer. Hybrid peptides constitute a novel, viable, and useful strategy for the design and identification of peptide drugs with anticancer activity.
Keywords
Hybrid peptides, cervical cancer cells, cytotoxicity, bovine lactoferricinIntroduction
Cervical cancer is one of the four most frequent types of cancer in adult women, for 2022, an approximate incidence of 660,000 new cases and around 350,000 deaths were reported in the world due to this disease [1]. Low and middle-income countries have the highest incidence rates, mainly economic and social factors such as lack of access to health services. However, all countries are working to eliminate cervical cancer as a public health problem by 2030, through a strategy defined by the World Health Organization (WHO) that seeks to achieve an incidence rate of fewer than 4 cases per 100,000 women-years through three pillars: prevention, effective detection, and treatment of precancerous lesions [2, 3]. According to WHO projections, in Colombia, the morbidity of cervical cancer will continue to increase from 4,600 to 6,300 cases and from 2,400 to 3,700 deaths in 2040 [4]. The development of this type of cancer can be prevented mainly by avoiding human papillomavirus (HPV) infection, however, if infection has occurred, early detection and treatment of precancerous lesions prevent them from turning into cancer [5]. Treatments available for cervical cancer include surgery, chemotherapy, and/or radiation therapy. Frequently chemotherapeutics (alone or in combination) are used for the treatment of advanced and recurrent cervical lesions. Cisplatin, 5-fluorouracil (5-FU), carboplatin, paclitaxel, gemcitabine, irinotecan, mitomycin, and bevacizumab are frequently used in conventional treatments [6]. However, their clinical use is limited by the emergence of tumor resistance, low survival rate, and side effects [7]. Therefore, it is necessary to search for new, more effective, and selective drugs that can be obtained at low cost to guarantee greater coverage. Therapeutic peptides can be an alternative thanks to the advantages they have shown in terms of potency, spectrum of action, mechanism of action, side effects, selectivity, specificity, and ease of synthesis [8, 9]. Particularly, peptides with anticancer activity have become a clinical option for the diagnosis and treatment of different types of cancer due to their high specificity and effectiveness [10]. This is how there are currently approved and marketed anticancer therapy peptides such as buserelin, tebentafusp, plitidepsin, dactinomycin, leuprorelina, goserelin, cetrorelix, and triptorelin, with applications in melanoma, breast cancer, prostate and other types of cancer [10, 11]. These peptides act through different mechanisms such as apoptosis of cancer cells, directing the specific immune response against cancer, hormonal signaling, and the cellular transcription process [11]. These molecules can be obtained through solid-phase peptide synthesis (SPPS), which allows for achieving peptide diversity through sequence design and modification, such as the synthesis of hybrid peptides, which consists of joining two different or more sequences of bioactive peptides in one molecule, with the aim of improving its properties, generating and/or enhancing biological activity, improving selectivity, and even reducing toxicity [12]. This method of construction of hybrid peptides has been applied mainly in the generation of compounds with antibacterial activity from the use of antimicrobial peptides, to enhance the activity, broaden the spectrum of action, and/or reduce the toxicity of the peptides. It has also been used to generate peptides with antitumor [13] and anticancer activity [14], as well as for other applications such as the treatment of metabolic disorders [15], natriuretic and diuretics [16], to promote wound healing [17], antifungals [18], anti-inflammatory agents [19], among others.
For the construction of the hybrid peptides, two or more families of bioactive peptides can be used. In this case, we combined a sequence derived from bovine lactoferricin (LfcinB) and the sequence of a functional peptide. The sequence derived from LfcinB (RWQWRWQWR) was the core and was combined with (i) anticancer peptides (ACPs), (ii) cervical cancer cell-targeting peptides (CTPs), and (iii) cell-penetrating peptides (CPPs). The sequence RWQWRWQWR is a palindromic peptide cataloged as an ACP because it has shown anticancer activity in vitro against human cell lines derived from breast cancer [20, 21], oral squamous-cell carcinoma [22], and colon cancer [23, 24]. The proposed mechanism of action of LfcinB and derived peptides has been related to their amphipathicity due to the content and distribution of arginine (Arg) and Trp residues in the sequence. The peptide-cell interaction has been associated with an initial interaction of an electrostatic nature between the Arg residues and the negatively charged components of the cell surface, then the tryptophan residues interact with the lipid bilayer of the cell membrane causing its disruption or peptide internalization [20]. The ACPs used for the design of the hybrid peptides were the RRWQWR and RLLRRLLR sequences, the first also derived from LfcinB and the second one derived from Buforin IIb. Buforin is a peptide derived from histone H2A and has shown anticancer potential in several types of cancer and the activity has been associated with peptide internalization act on intracellular targets [25]. The sequences of CTP and CPP used in this study were selected from peptide libraries. These functional peptides are sequences with specific biological activities that present diverse mechanisms of action useful in the design of hybrid peptides. To establish whether the design of hybrid peptides containing the RWQWRWQWR sequence is a useful strategy to enhance the selective cytotoxic effect in cervical cancer cell lines, this sequence was chemically combined with other functional sequences of ACPs, CTPs, and CPPs. All hybrid peptides were synthesized using manual SPPS Fmoc/tBu strategy, purified, and characterized, and the cytotoxicity was determined in vitro against the human cervical cancer cells lines HeLa and Ca Ski, subsequently.
Materials and methods
Materials and reagents
Rink amide resin, Fmoc amino acids, 6-chloro-1-hydroxybenzotriazole (6-Cl-HOBt), and N,N-dicyclohexylcarbodiimide (DCC) were purchased from AAPPTec (Louisville, KY, USA). Methanol, diethyl ether, N,N-dimethylformamide (DMF), absolute ethanol, dichloromethane (DCM), acetonitrile (ACN), isopropylalcohol (IPA), trifluoroacetic acid (TFA), 1,2-ethanedithiol (EDT), triisopropylsilane (TIPS), SPE Supelclean columns were obtained from Merck (Darmstadt, Germany). 4-methylpiperidine, pyridine, Triton-X, potassium cyanide (KCN), pyridine, phenol, ninhydrin, ethylenediaminetetraacetic acid (EDTA), RPMI-1640 culture medium, and trypsin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from Gibco (Waltham, MA, USA).
Solid-phase peptide synthesis
All peptides were obtained by manual SPPS Fmoc/tBu strategy on rink amide resin (0.46 meq/g). Fmoc group removal was carried out by treatment with 5% 4-methylpiperidine in DMF with constant stirring and at room temperature (RT) for 10 min (2×). Subsequently, the resin was washed with DMF and DCM. The coupling reaction was carried out by dissolving Fmoc-amino acid/DCC/6-Cl-HOBt (1:1:1, equiv and 5 excess respects to resin substitution) in DMF and the reaction mixture was stirred for 15 min at RT, after the reaction mixture was mixed with resin or resin-peptide and stirred for 2 h at RT. The elimination of the Fmoc group and the incorporation of each amino acid were monitored by the Kaiser test. The deprotection of the amino acid side chain and the separation of the peptides from the resin was carried out by treatment with cleavage solution containing TFA/water/EDT/TIPS (92.5:2.5:2.5:2.5, v/v). Peptides were precipitated by treatment with cold diethyl ether, and the precipitate was washed 5 times with diethyl ether.
Reverse phase solid-phase extraction purification
All the peptides were purified using solid-phase extraction (SPE) on Supelclean columns (SPE Tube 17%, 5 g, 45 μm, 60 Å) following the methodology reported by Insuasty Cepeda et al. [26]. SPE columns were activated prior to use with methanol, followed by solvent B (ACN containing 0.1% TFA), and were equilibrated with solvent A (water containing 0.1% TFA). 100 mg of crude peptide was dissolved in 1 mL of A and the solution was added to the column and eluted using a gradient of B. The collected fractions were analyzed by reverse phase-high performance liquid chromatography (RP-HPLC) chromatography and the fractions containing the pure peptide were mixed and then lyophilized [26].
RP-HPLC analysis
RP-HPLC analysis was performed on a ChromolithTM C18 (50 × 4.6 mm) column using a Hitachi liquid chromatograph (PrimaideTM, Hitachi High-Tech) with UV/Vis and photodiode array (PDA) detector. For the analysis a linear gradient was employed from 5% to 50% solvent B in solvent A in 8 min (method 1). The flow rate was 2.0 mL/min at RT, detection was at 210 nm and volume injection was 10 µL. The most hydrophilic peptides were analyzed using a modified linear gradient from 1% to 11% solvent B in 5 min (method 2).
Mass spectrometry analysis
Mass spectrometry was performed on a high resolution mass spectrometry (HRMS) Q ExactiveTM Hybrid Quadrupole-OrbitrapTM Mass Spectrometer (Thermo Scientific™) equipped with electrospray ionization (ESI) in positive mode. The chromatographic conditions were: Kinetex C18 column (1.7 µm 100 Å), RT, and a flow rate of 0.250 mL/min. The mobile phase was water as solvent A and ACN as solvent B, each containing 0.1% formic acid. Solvent A linear gradient was employed from 5% to 50% solvent B in 8 min. ESI source conditions: mode Full MS, end plate offset 500 V, capillary 4,500 V, nebulizer 1.8 bar, dry gas nitrogen 8.0 L/min, and dry temperature 320°C. Scan mode AutoMS/MS with a spectral range of 200−3,000 m/z, a spectra rate of 2 Hz, and a collision energy of 5.0 eV were used. The theoretical mass of the peptides was calculated using ChemDraw software (version 8.0.1).
Cell culture
HeLa (ATCC CRM-CCL-2) and Ca Ski cells (ATCC CRL-1550) were cultured in RPMI-1640 medium supplemented with 10% FBS. L929 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, D5523, Sigma-Aldrich Co.) supplemented with 10% FBS. Media were filtered through a 0.22 μm membrane. The cell culture was incubated at 37°C with 5% CO2.
Cytotoxicity assays
Cytotoxicity assays were performed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously described in Rodriguez Guerra et al. [27]. Briefly, HeLa, Ca Ski, or L929 cells (1 × 104 cells per well) were seeded in 96-well plates. After cell adherence, the medium was replaced by not supplemented medium to allow cell syncing for 12 h. Then the culture medium was removed, and the cells were treated with each peptide (6.25 to 200 µg/mL) dissolved in medium for 2 h at 37°C. Then 10 μL of MTT solution (5 mg/mL) was added to each well and the plates were incubated for 4 h at 37°C. Crystals were dissolved in 100 μL of DMSO, and absorbance was recorded at 590 nm on a Bio-Rad microplate reader (Model 680, iMark). Negative control: cells incubated with medium, (n = 3) and positive control: H2O2. The viability curves and IC50 value were determined using GraphPad Prism 8 statistical analysis software.
Hemolytic activity assay
The hemolytic activity assay was carried out following the methodology reported by Aguirre-Guataqui et al. [28] with some modifications, as follows. 5 mL of heparinized peripheral blood was centrifuged at 1,000 g for 15 min. The erythrocyte fraction was resuspended in 10 mL of sterile saline solution and washed twice by centrifugation under the same conditions. Then 100 μL of erythrocytes (4% hematocrit) were incubated with 100 μL of peptide (concentrations ranging from 6.2 to 200 μg/mL) and incubated for 2 h at 37°C. It was then centrifuged at 1,000 g for 5 min, and the absorbance of the supernatant was measured at 450 nm. Saline solution was used as the negative control, while Triton X-100 was used as the positive control [29].
Microscopy
Cells were observed by means of phase contrast microscopy with the Leica DMiTM (DMi1, Leica-microsystems) and MoticTM (AE31E, MoticEurope, S.L.U.) microscope at 20°C.
Results
The hybrid peptides were designed with the sequence RWQWRWQWR conjugated with different sequences of bioactive peptides: (i.) ACPs, (ii.) cervical cancer CTPs, and (iii.) CPPs. Sequences from each group of bioactive peptides that allowed us to obtain the hybrid peptides hereinafter called precursor peptides, were synthesized using SPPS Fmoc/tBu strategy, purified, and characterized by HPLC and mass spectrometry. The results in Table 1 show that these precursor peptides, with lengths between 6 and 12 amino acids, were successfully obtained with purities greater than 86% determined by RP-HPLC and their identity corresponds to that expected. In the same way, the precursor peptides also were obtained with the selected spacer, 6-amino hexanoic acid (Ahx). The results for these sequences also show that these were obtained satisfactorily.
Characterization and cytotoxic effect of precursor peptides in HeLa and Ca Ski cells after 2 h treatment at 37°C
| Functional peptide | Sequence | RP-HPLC | ESI-MS (M) | IC50 (μM) | |||
|---|---|---|---|---|---|---|---|
| tR (min) | Purity (%) | Theorical | Experimental | HeLa | Ca Ski | ||
| Anticancer peptides (ACP) | RWQWRWQWR | 6.6 | 98.1 | 1,485.76 | 1,485.78 | 47.6 | > 134.5 |
| Ahx-RWQWRWQWR | 6.5 | 97.6 | 1,598.85 | 1,598.88 | 103.3 | > 125.0 | |
| RWQWRWQWR-Ahx | 7.1 | 98.6 | 1,598.85 | 1,598.86 | > 125.0 | > 125.0 | |
| RRWQWR | 5.3 | 99.3 | 985.55 | 985.58 | > 202.0 | > 202.0 | |
| Ahx-RRWQWR | 5.4 | 97.8 | 1,098.63 | 1,098.65 | > 181.9 | > 181.9 | |
| RLLRRLLR | 5.1 | 97.8 | 1,093.77 | 1,093.79 | > 182.7 | > 182.7 | |
| Ahx-RLLRRLLR | 5.9 | 97.3 | 1,206.85 | 1,206.87 | > 165.6 | > 165.6 | |
| Cervical cancer cell-targeting peptides (CTP) | QQLPSSSTSTYP | 4.0 | 90.7 | 1,293.62 | 1,293.65 | > 154.5 | > 154.5 |
| Ahx-QQLPSSSTSTYP | 4.1 | 96.5 | 1,406.70 | 1,406.72 | > 142.1 | > 142.1 | |
| GDALFSVPLEVY | 7.1 | 98.7 | 1,307.68 | 1,308.20 | > 152.8 | > 152.8 | |
| Ahx-GDALFSVPLEVY | 7.2 | 98.2 | 1,420.76 | 1,420.78 | > 140.7 | > 140.7 | |
| QVNGLGERSQQM | 3.8 | 86.2 | 1,344.66 | 1,344.67 | > 148.6 | > 148.6 | |
| Ahx-QVNGLGERSQQM | 4.0 | 93.1 | 1,457.74 | 1,457.76 | > 137.1 | > 137.1 | |
| KQNLAEG | 3.8a | 97.2 | 757.41 | 757.42 | > 263.9 | > 263.9 | |
| Ahx-KQNLAEG | 4.4a | 97.0 | 870.49 | 870.52 | > 229.6 | > 229.6 | |
| Cell-penetrating peptides (CPP) | YGRKKRPQRRR | 3.8a | 99.2 | 1,498.92 | 1,498.92 | > 133.4 | > 133.4 |
| Ahx-YGRKKRPQRRR | 4.4a | 94.5 | 1,612.00 | 1,612.04 | > 124.0 | > 124.0 | |
| RRRRRRRR | 4.0a | 96.7 | 1,265.84 | 1,265.84 | > 157.9 | > 157.9 | |
| Ahx-RRRRRRRR | 4.2a | 95.6 | 1,378.92 | 1,378.94 | > 145.0 | > 145.0 | |
| RKKRRQRRR | 3.7a | 93.8 | 1,337.88 | 1,337.90 | > 149.4 | > 149.4 | |
| Ahx-RKKRRQRRR | 3.8a | 98.2 | 1,450.97 | 1,450.98 | > 137.8 | > 137.8 | |
| KLALKLALK | 5.6 | 92.4 | 995.72 | 995.73 | > 200.7 | > 200.7 | |
| Ahx-KLALKLALK | 5.8 | 95.5 | 1,108.81 | 1,108.81 | > 180.3 | > 180.3 | |
a: RP-HPLC modified linear gradient (method 2). tR: retention time; Ahx: 6-amino hexanoic acid; RP-HPLC: reverse phase-high performance liquid chromatography; ESI-MS: electrospray ionization-mass spectrometry. All peptides were obtained with amide function at the C-terminal end
The hydrophobicity of the peptides can be estimated through the retention time (tR) in RP-HPLC analysis (Table 1). These precursor peptides have tR between 3.8 and 7.2 minutes (method 2) and those containing the spacer showed higher tR independent of the position in the sequence, possibly due to the increased hydrophobicity caused by the hydrocarbon chain of the Ahx. Peptides containing the sequences KQNLAEG, YGRKKRPQRRR, RRRRRRRR, and RKKRRQRRR showed a lower tR due to their high hydrophilicity conferred by hydrophilic amino acids [lysine (Lys), Arg, glutamic acid (Glu)], these peptides were analyzed using a modified linear gradient (method 2). All precursor peptides were evaluated for cytotoxic activity against the human cervical cancer cell lines HeLa and Ca Ski by MTT assay, finding that most of the peptides did not show cytotoxic activity against the cell lines at the concentrations evaluated, as shown in Table 1. The precursor peptides containing the palindromic sequence RWQWRWQWR (IC50 = 47.6 µM) and Ahx-RWQWRWQWR (IC50 = 103.3 µM) showed cytotoxic effect only in HeLa cervical cancer cells. These sequences did not exhibit cytotoxicity on Ca Ski cells. The addition of the spacer to the N-terminal end of the RWQWRWQWR peptide influenced the toxicity, suggesting that incorporation of the spacer at the N-terminal end decreased the activity; and the addition of the spacer at the C-terminal causes a loss of cytotoxicity.
The other precursors did not show cytotoxicity against HeLa and Ca Ski cell lines. Likewise, the selected CPP was expected to have a cytotoxic effect on cervical cancer cell lines, but this was not the case; these sequences did not show cytotoxic activity at the concentrations evaluated. In general, it is also observed that the incorporation of the spacer (Ahx) into the precursor peptide sequences does not have a significant effect on the cytotoxic activity of most peptides, as shown in Table 1.
Regarding hybrid peptides, the sequence RWQWRWQWR was joined with precursor sequences at the N- or C-terminal end and the sequences were connected in all cases with the same spacer (Ahx). Hybrid peptides were also synthesized using the manual SPPS method (Figure 1), allowing peptides with lengths between 16 and 22 amino acids to be obtained without problems. These peptides were also purified by the RP-SPE method and characterized by RP-HPLC and mass spectrometry as shown in Table 2. All hybrid peptides were satisfactorily obtained, with purity greater than 91% determined by HPLC and their molecular weight corresponds to that expected (supplementary material Figures S1–S4).
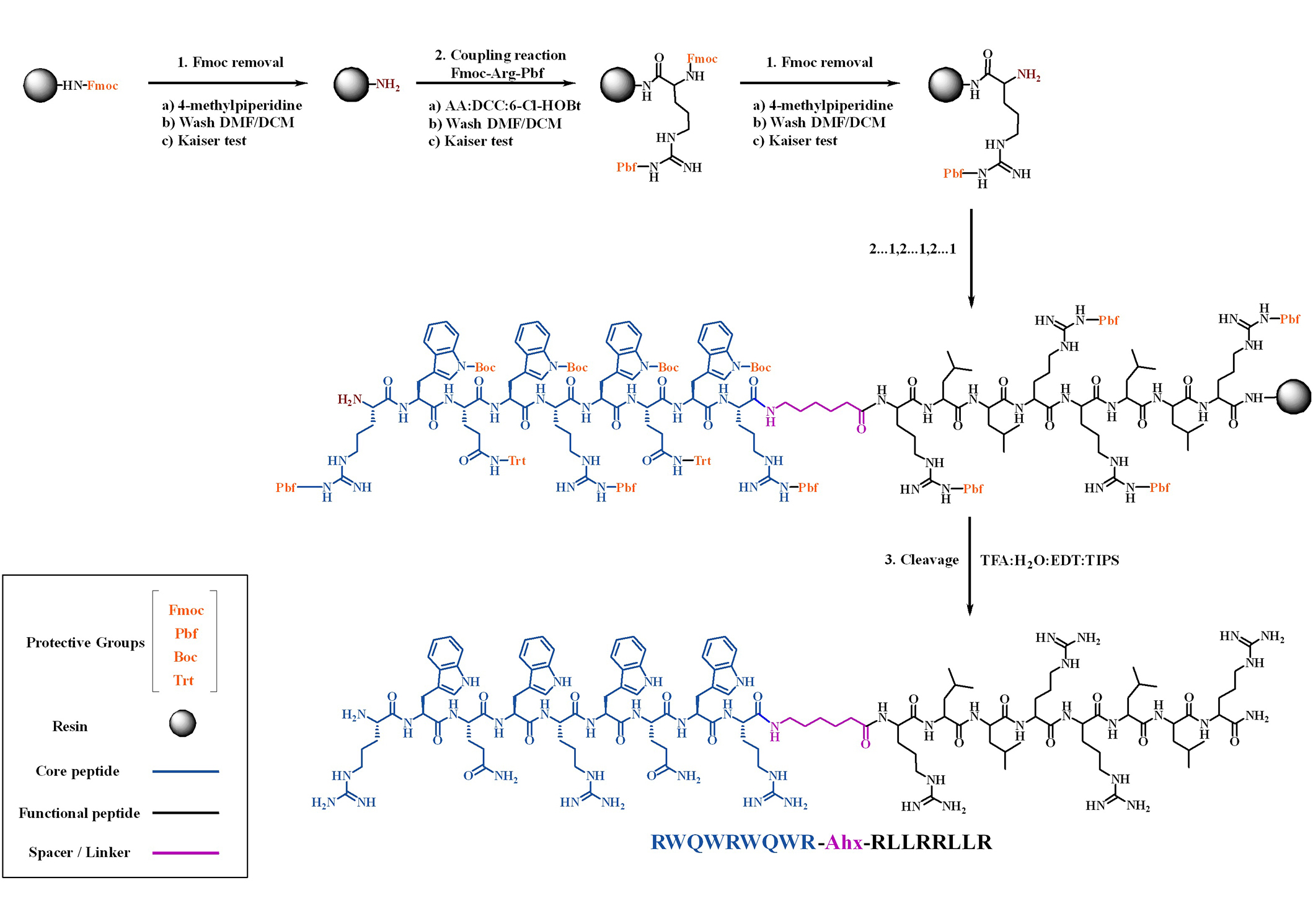
Scheme of the SPPS Fmoc/tBu strategy used to obtain hybrid peptides. Ahx: 6-aminohexanoic acid (spacer); DMF: N,N-dimethylformamide; DCM: dichloromethane; TFA: trifluoroacetic acid; EDT: 1,2-ethanedithiol; TIPS: triisopropylsilane; 6-Cl-HOBt: 6-chloro-1-hydroxybenzotriazole
Characterization and cytotoxic effect of hybrid peptides in HeLa, Ca Ski, and L929 cells after 2 h treatment at 37°C
| Code | Sequence | RP-HPLC | ESI-MS (M) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|---|---|
| tR (min) | Purity (%) | Theorical | Experimental | HeLa | Ca Ski | L929 | ||
| 1 | RWQWRWQWR-Ahx-RRWQWR | 7.0 | 96.1 | 2,567.37 | 2,567.40 | 21.6 | 28.6 | 40.2 |
| 2 | RRWQWR-Ahx-RWQWRWQWR | 6.9 | 96.3 | 2,567.37 | 2,567.37 | 26.7 | 48.9 | 55.5 |
| 3 | RWQWRWQWR-Ahx-RLLRRLLR | 7.8 | 97.8 | 2,675.59 | 2,675.61 | 13.3 | 16.8 | 30.7 |
| 4 | RLLRRLLR-Ahx-RWQWRWQWR | 7.0 | 97.4 | 2,675.59 | 2,675.60 | 16.0 | 22.4 | 42.4 |
| 5 | RWQWRWQWR-Ahx-QQLPSSSTSTYP | 7.0 | 97.7 | 2,875.44 | 2,875.46 | > 69.5 | ND | ND |
| 6 | RWQWRWQWR-Ahx-GDALFSVPLEVY | 8.3 | 99.3 | 2,889.50 | 2,889.55 | > 69.2 | ND | ND |
| 7 | RWQWRWQWR-Ahx-QVNGLGERSQQM | 7.0 | 98.2 | 2,926.48 | 2,926.52 | > 68.3 | ND | ND |
| 8 | RWQWRWQWR-Ahx-KQNLAEG | 6.8 | 99.2 | 2,339.23 | 2,339.25 | > 85.4 | ND | ND |
| 9 | RWQWRWQWR-Ahx-YGRKKRPQRRR | 6.5 | 99.1 | 3,080.74 | 3,080.76 | 19.4 | 34.3 | 23.6 |
| 10 | RWQWRWQWR-Ahx-RRRRRRRR | 6.4 | 98.5 | 2,847.66 | 2,847.70 | 12.8 | 55.5 | 22.3 |
| 11 | RWQWRWQWR-Ahx-RKKRRQRRR | 6.3 | 98.3 | 2,919.70 | 2,919.72 | 17.2 | 67.7 | 25.0 |
| 12 | RKKRRQRRR-Ahx-RWQWRWQWR | 6.4 | 97.3 | 2,919.70 | 2,919.68 | 17.1 | 16.4 | 20.2 |
| 13 | RWQWRWQWR-Ahx-KLALKLALK | 7.5 | 98.5 | 2,577.54 | 2,577.60 | 12.0 | 71.7 | > 77.5 |
| 14 | KLALKLALK-Ahx-RWQWRWQWR | 7.2 | 99.2 | 2,577.54 | 2,577.54 | 15.1 | 34.6 | 30.9 |
| 15 | KWQWKWQWK-Ahx-RRWQWR | 6.8 | 91.8 | 2,483.35 | 2,483.38 | 14.6 | 19.6 | 6.7 |
| 16 | RWQWRWQWR-Ahx-KKWQWK | 6.9 | 98.4 | 2,483.35 | 2,483.38 | 15.7 | 74.6 | > 80.5 |
| 17 | KWQWKWQWK-Ahx-KKWQWK | 6.7 | 98.2 | 2,399.33 | 2,399.36 | 23.9 | 35.1 | 16.5 |
| 18 | (RWQWRWQWR)2K-Ahx-RRWQWR | 7.3 | 93.1 | 4,164.2 | 4,164.24 | 9.6 | 36.0 | 35.6 |
ND: not determined; Ahx: 6-aminohexanoic acid (magenta); RP-HPLC: reverse phase-high performance liquid chromatography; ESI-MS: electrospray ionization-mass spectrometry
Figure 2 shows the characterization of the hybrid peptide 3, its chemical structure, chromatographic profile, and ESI-MS mass spectra. The chromatographic profile presents a main signal corresponding to the peptide (tR = 7.8 min), with high chromatographic purity. In the mass spectrum, the multicharged species are observed and the magnification shows the isotopic distribution of the [M+3H]3+ species, the experimental mass corresponds with the expected one.
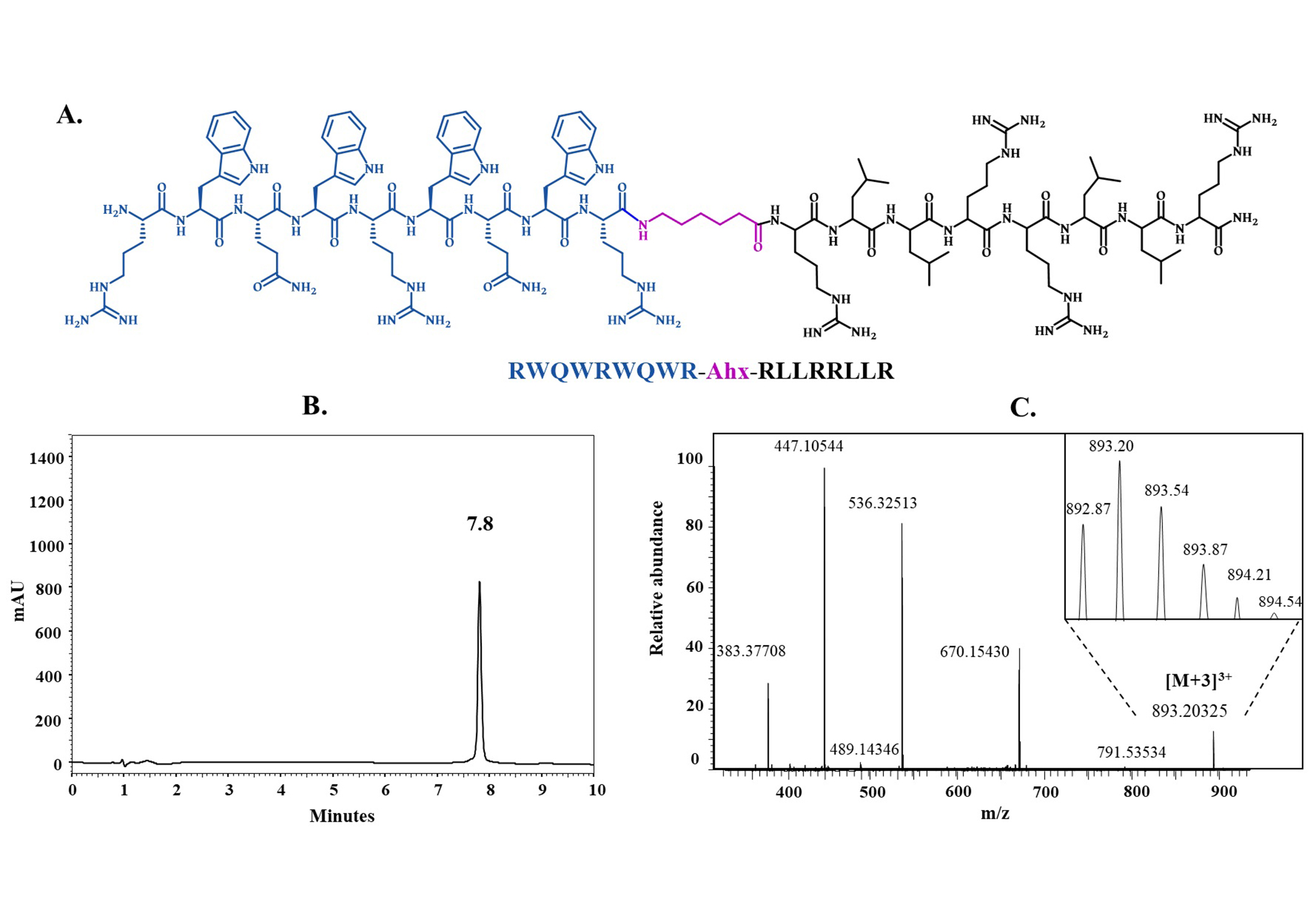
Characterization of the hybrid peptide RWQWRWQWR-Ahx-RLLRRLLR (3). (A) Chemical structure; (B) chromatographic profile (RP-HPLC); (C) ESI-MS. Ahx: 6-aminohexanoic acid (magenta); RP-HPLC: reverse phase-high performance liquid chromatography; ESI-MS: electrospray ionization-mass spectrometry
Table 2 shows that the retention times are different for each hybrid peptide even though they have a common nucleus which is the sequence RWQWRWQWR. Likewise, it is highlighted that the retention times of the hybrid peptide linked at the C-terminal end in all cases are different from that of the corresponding hybrid peptide linked at the N-terminal end, even though the sequences are identical and only change its position (peptides 1, 2, 3, 4, 11, 12, 13, and 14).
The cytotoxic activity of the hybrid peptides was subsequently evaluated against the human cervical cancer cell lines HeLa and Ca Ski for 2 hours. The results in Table 2 show that the hybrid peptides joining RWQWRWQWR with ACPs (peptides 1–4, 15, 17, and 18) or CPPs (peptides 9–14) generated peptides with a significantly cytotoxic effect in the two cervical cancer cell lines. Regarding the hybrid peptides containing CTP, it was observed that these did not show any cytotoxic effect at the concentrations evaluated in HeLa cells, for this reason, assays on Ca Ski and L929 cells with these peptides was not continued.
Cytotoxicity assays were also performed for 24 hours for hybrid peptides 1, 3, 4, 15, 16, 17, and 18 on HeLa cells. The IC50 results and the viability curves show that there are no statistically significant differences between the results at 2 hours and 24 hours for these hybrid peptides and that the curves present a similar behavior at both times (supplementary material, Table S1 and Figure S5). The tests carried out at different incubation times (2 and 24 hours) allowed the study time to be selected since a fast cytotoxic effect (2 hours) of these hybrid peptides on cancer cell lines is notable and therefore it was the time selected to carry out the cytotoxicity assays.
Hybrid peptides 1, 2, 3, and 4 were generated joining the RWQWRWQWR core with the shorter sequences RRWQWR and RLLRRLLR. The resulting peptides showed cytotoxicity in cervical cancer cells, which allows us to infer that adding the sequence of an ACP (RRWQWR or RLLRRLLR) at either end of this short peptide generates cytotoxic hybrid peptides against HeLa and Ca Ski cells. The cytotoxic effect of the hybrid peptide analogues 1 and 2, (as also for 3 and 4) against both cervical cancer cells evaluated, was similar, suggesting that the anticancer activity of these chimeras is independent of whether the precursor sequences are at the N- or C-terminal end. In similar way, analogues hybrid peptides 11 and 12 (IC50 = 17.2 and 17.1 µM, respectively) and 13 and 14 (IC50 = 12.0 and 15.1 µM, respectively) showed similar cytotoxic effect in Hela cells while that hybrid peptides containing the palindromic sequence RWQWRWQWR in the C-terminal end (peptides 12 and 14) showed higher cytotoxic effect against Ca Ski cells than those counterparts (peptides 11 and 13 respectively), suggesting that anticancer activity of hybrid peptides is dependent of the position of palindromic sequence RWQWRWQWR in the peptidyl chain. All hybrid peptides, except peptide 12, present higher IC50 values for the Ca Ski cell line than for HeLa, showing that Ca Ski cells are more resistant.
On the other hand, three additional hybrid peptides were designed where the residues of Arg was replaced by Lys in the sequence RWQWRWQWR or RRWQWR, allowing peptides 15, 16, and 17 to be obtained (Table 2). The results show that the three peptides had cytotoxic activity against the HeLa and Ca Ski cells, however, the lowest IC50 value was obtained by the peptide 15 (IC50 = 14.6 and 19.6 µM, respectively) where all Arg residues of the palindromic sequence were replaced by Lys. In turn, peptide 17 which for both precursor sequences all Arg residues were changed by Lys, also showed cytotoxic activity against the two cell lines HeLa and Ca Ski cells (IC50 = 23.9 and 35.1 µM, respectively), suggesting that it is possible to replace the Arg with Lys in this entire sequence without drastically affecting the activity. Similarly, the cytotoxicity of these hybrid peptides was greater in HeLa cells than in Ca Ski cells.
The hybrid dimeric-monomeric peptide 18 was also designed and obtained, in which the palindromic sequence was dimerized. This peptide also showed cytotoxic activity against the two cervical cancer cells lines evaluated; however, its activity was not much better than the other linear sequences. Again, the Ca Ski cell line was more resistant.
Subsequently, the hybrid peptides were evaluated against the L929 cell line, a mouse fibroblast cell line useful in toxicity studies. The IC50 results shown in Table 2 allow us to observe that all hybrid peptides also affect fibroblasts, however, the magnitude of the effect is different for each peptide. Therefore, the selectivity index (SI) was determined with the objective of determining which sequences have the best selectivity. This was determined through the rate between the IC50 values in L929 fibroblast cells or the HC50 values in red blood cells (RBC) (hemolysis assay) and the IC50 values in cancer cells [30] (Table 3).
Cytotoxic effect after 2 h treatment at 37°C and selectivity of hybrid peptides
| Code | Hemolysis (%)* | RBCs HC50 (μM) | Selectivity index (SI) | |||
|---|---|---|---|---|---|---|
| L929/HeLa | L929/Ca Ski | RBCs/HeLa | RBCs/Ca Ski | |||
| 1 | 6.3% | > 77.9 | 1.9 | 1.4 | > 3.6 | > 2.7 |
| 2 | 9.5% | > 77.9 | 2.1 | 1.1 | > 2.9 | > 1.6 |
| 3 | 25.1% | 59.2 | 2.3 | 1.8 | 4.4 | 3.5 |
| 4 | 16.8% | > 74.7 | 2.6 | 1.9 | > 4.7 | > 3.3 |
| 9 | 3.5% | > 64.9 | 1.2 | 0.7 | > 3.3 | > 1.9 |
| 10 | 4.2% | > 70.2 | 1.7 | 0.4 | > 5.5 | > 1.3 |
| 11 | 4.0% | > 68.5 | 1.5 | 0.4 | > 4.0 | > 1.0 |
| 12 | 4.4% | > 68.5 | 1.2 | 1.2 | > 4.0 | > 4.2 |
| 13 | 33.1% | 60.4 | > 6.4 | > 1.1 | 5.0 | 0.8 |
| 14 | 30.6% | 41.8 | 2.0 | 0.9 | 2.8 | 1.2 |
| 15 | 1.3% | > 80.5 | 0.5 | 0.3 | > 5.5 | > 4.1 |
| 16 | 1.2% | > 80.5 | > 5.1 | > 1.1 | > 5.1 | > 1.1 |
| 17 | 1.8% | > 83.3 | 0.7 | 0.5 | > 3.5 | > 2.4 |
| 18 | 35.2% | > 48.0 | 3.7 | 1.0 | > 5.0 | > 1.3 |
* Percentage of hemolysis at the IC50 determined in MTT assays in HeLa cells. RBCs: red blood cells
HeLa cells are derived from cervical human adenocarcinoma and typically grow in an adherent fashion in vitro (supplementary material, Figure S6). HeLa cells form a monolayer resembling an epithelial morphology with some cells showing cytoplasmatic elongations. Also, some viable floating cells undergoing mitosis can be seen. At a 50% confluence, some discrete patches are visualized which are typical of epithelial-like cells (supplementary material, Figure S6). The growth profiles of HeLa cells were evaluated using three initial cell densities of 500, 2,000, and 4,000 cells/well. The results showed that the doubling time is in accordance with those reported (supplementary material, Figure S7) [31].
The results showed that all peptides were more selective for the tested cancer cells than for RBCs, (SI > 1) while peptides 1–4, 12, 13, 16, and 18 were more selective for HeLa and Ca Ski cells than for L929 fibroblasts. Peptides 9–11, and 14 were more selective for HeLa cells than for L929 fibroblasts and conversely peptides 15 and 17 were not selective for the cervical cancer cells tested. SI values were higher in the hybrid peptides conjugated with ACP (SI > 1), highlighting peptides 1, 3, and 4, with SI values between 1.4 and 2.6 and peptide 16 was the more selective for cervical cancer cells.
Cell viability graphs of the hybrid peptides 1, 3, and 4 (supplementary material, Figure S8) show that the peptides affect both cancer cells and fibroblasts, however, the effect on fibroblasts is smaller. It is shown that peptide cytotoxicity is fast (2h), dependent on the concentration of the hybrid peptide and that cell viability is less than 20% at the maximum concentration of 200 µg/mL. Peptides 3 and 4 had the greatest cytotoxic effect against HeLa cells treated with the peptide at 100 µg/mL and this effect was like those treated with 200 µg/mL. HeLa cervical cancer cells were more sensitive to the treatments than Ca Ski cells (range 50–200 µg/mL) and the highest selectivity was observed when cells were treated with the peptide at 100 µg/mL, while the lowest selectivity was at 200 µg/mL.
Figure 3 shows microphotographs of HeLa, Ca Ski, and L929 cells untreated and treated with some hybrid peptides, an alteration is observed in the morphology of these cells such as shrinking, rounding, loss extensions, and characteristic of cell damage induced by apoptotic processes [32]. Untreated cells showed the normal morphological pattern of HeLa, Ca Ski, and L929 cells. Similar alterations were observed with the other hybrid peptides (supplementary material, Figures S9 and S10).
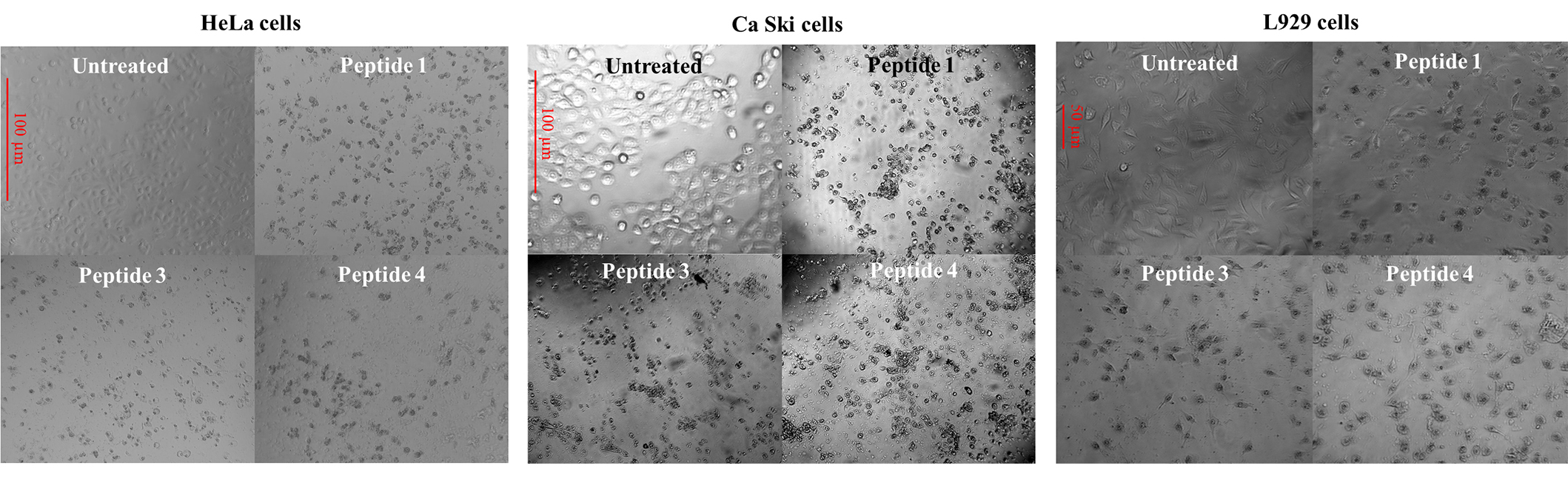
Microphotographs by contrast microscopy (200×) of the HeLa, Ca Ski, and L929 cells untreated and treated with hybrid peptides 1 (RWQWRWQWR-Ahx-RRWQWR), 3 (RWQWRWQWR-Ahx-RLLRRLLR), 4 (RLLRRLLR-Ahx-RWQWRWQWR), at 200 μg/mL, after 2 h treatment at 37°C. Ahx: 6-aminohexanoic acid
The hemolysis assay was also performed with the hybrid peptides, as a first step to evaluate its toxicity in vitro, and it was found that peptides 3 (25.1%), 4 (16.8%), 13 (33.1%), 14 (30.6%), and 18 (35.2%) were hemolytic (more than 10%) at the IC50 determined by MTT in HeLa cells (Table 3). Many of the hybrid peptides display a hemolysis percentage of less than 10% (supplementary material, Figure S11); in peptides 1 and 2, hemolysis was 6.3% and 9.5%, respectively, indicating that the position of the precursor sequences did not significantly affect hemolytic activity. Peptides 3 and 4 were hemolytic compared to peptides 1 and 2, suggesting that the precursor sequence RLLRRLLR might be responsible for hemolysis. Peptides 9–12 showed hemolysis between 3.5–4.4%, suggesting that the incorporation of CPP sequences rich in Arg residues (YGRKKRPQRRR and RRRRRRRRR) did not increase hemolytic activity. In peptides 15–17, in which Arg residues were replaced by Lys, hemolysis was decreased with respect to the original peptide 1 (6.3%), without compromising cytotoxic activity. However, for the dimeric peptide, an increase of almost 6 times in the hemolysis was observed with respect to the linear peptide.
On the other hand, the MTT assay was performed with the physical mixing of the precursors; the precursors were mixed at the same concentration covering the range of 0–200 µg/mL, and their cell viability was evaluated in the HeLa cell line. The results show that the physical combination of the precursor peptides showed a lower cytotoxic effect than the hybrid peptides (Table 4 and Figure S12, supplementary material), regardless of whether they have the spacer or not, which contrasts with the cytotoxic effect observed in the hybrid peptides that have these same sequences chemically linked.
Cytotoxic effect of the physical mixing of precursor peptides in HeLa cells after 2 h treatment at 37°C
| Mix | Physical mixing of precursors peptides | HeLa IC50 (μg/mL) |
|---|---|---|
| A | RWQWRWQWR + RLLRRLLR | > 200 |
| B | RWQWRWQWR-Ahx + RLLRRLLR | > 200 |
| C | RWQWRWQWR + Ahx-RLLRRLLR | > 200 |
| D | RWQWRWQWR + RRWQWR | > 200 |
| E | RWQWRWQWR + Ahx-RRWQWR | > 200 |
| F | RWQWRWQWR-Ahx + RRWQWR | > 200 |
| G | KWQWKWQWK + RRWQWR | > 200 |
| H | KWQWKWQWK-Ahx + RRWQWR | > 200 |
| I | KWQWKWQWK + Ahx-RRWQWR | > 200 |
Ahx: 6-aminohexanoic acid
Figure 4 shows the cytotoxic effect of the physical mixing of precursors that make up hybrid peptide 1. It is observed that the effect on cell viability is not the same when the precursors are mixed at any of the concentrations. The different mixtures present a similar cytotoxic behavior between them but in no case is the effect similar to that produced by hybrid peptide 1. A similar behavior of the precursor peptide mixtures is presented for hybrid peptides 3 and 15 (supplementary material, Figure S12).
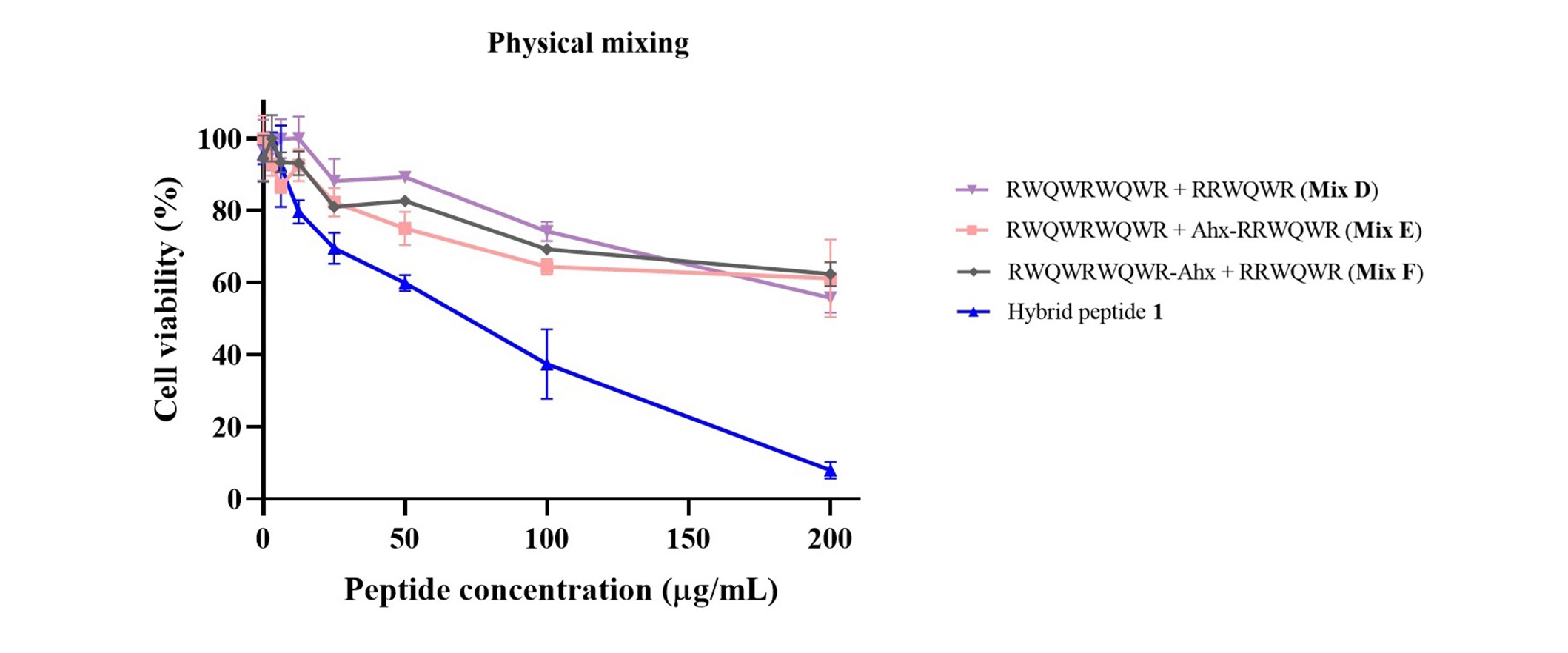
Cytotoxic effect of the physical mixing of the precursor peptides that make up the hybrid peptide 1 against the HeLa cell line, after 2 h treatment at 37°C. The data are expressed as the mean ± SE (n = 3). Two-way ANOVA and Sidak’s multiple comparisons test were used, p ≤ 0.05, showing statistically significant differences between hybrid peptide 1 and each mixture (D, E, and F) from 12.5 µg/mL to 200 µg/mL. Ahx: 6-aminohexanoic acid; SE: standard error
Discussion
The hybrid peptides were designed inspired by the RWQWRWQWR sequence, this core was conjugated with different bioactive peptides classified as (i.) ACPs, (ii.) cervical cancer CTPs, and (iii.) CPPs. The palindromic peptide RWQWRWQWR derived from LfcinB was chosen in the design of the hybrid peptides since it has shown activity against oral squamous-cell carcinoma cell lines [22], breast cancer cell lines MDA-MB-231, MCF-7 [20], and MDA-MB-468 [21] and colon cancer cell lines Caco-2 and HT29 [23, 24], which is why it was considered useful for the design of the hybrid peptides. We sought to improve its activity through conjugation with other peptide sequences.
The hybrid peptides were constructed by joining two sequences which can exert their cytotoxic effect on cancer cells by different mechanisms. The sequences RRWQWR and RLLRRLLR were selected, the first one is a sequence of six amino acids derived from LfcinB. It has been reported that this peptide did not show cytotoxicity in breast cancer, leukemia, and gastric cancer cells since it does not have the ability to destabilize cell membranes. However, when this peptide was encapsulated in liposomes and internalized in leukemia cancer cells, induced cell death was mediated mainly by apoptosis [33]. Likewise, it has been reported that this short sequence lacks cytotoxic activity against oral cancer, breast cancer, human T-leukemia, and B-lymphoma cells [22, 34, 35] and our results also show that precursor peptides containing RRWQWR sequence lack cytotoxicity against HeLa and Ca Ski cell lines (Table 1). In a similar way, precursor peptides containing RLLRRLLR sequence did not affect the cancer cells evaluated.
Regarding CTP, this family was included in the study to improve the selectivity of the hybrid peptide by specifically targeting the peptide to cancer cells causing minimal damage to healthy cells [36]. Four short peptide sequences were chosen QVNGLGERSQQM, GDALFSVPLEVY, KQNLAEG, and QQLPSSSTSTYP, these were included for their ability to bind to cells such as HeLa and SiHa [37–39]. CPPs can enter cells and generate their cytotoxic effect inside and were selected to facilitate the transport of the palindromic peptide and its internalization to generate the cytotoxic effect [40]. The selected sequences correspond to the cationic peptides YGRKKRPQRRR, RKKRRQRRR, RRRRRRRR, and KLALKLALK [41–44]. These molecules contain highly positive net charges for the amino acids Arg and Lys, which facilitate internalization into cells thanks to the interaction of charges between the guanidine group of Arg and the phosphate groups of the membrane that facilitate movement through it. Similar to what was observed with the other precursor peptides, CTPs did not exert a cytotoxic effect on HeLa and Ca Ski cells.
The SPPS allowed the obtaining of all the precursor peptides, therefore, it can be indicated that the synthetic methodology was useful and convenient to obtain a variety of short peptides with different functionalities. The retention time values in Table 1 allow us to infer that in all cases the sequences with the spacer are more hydrophobic compared to the sequences without the spacer, since Ahx is a hydrophobic amino acid that imparts this property to the final hybrid peptide.
The ACP sequences were expected to show cytotoxic activity since, for example, the peptide RWQWRWQWR has reported activity against oral, breast, and colon cancer cell lines [21, 23]; however, this peptide did not show activity against Ca Ski cell lines but showed activity against the HeLa cell line. This may be due to the characteristics of each cell line which may be relevant for peptide-cell interaction. In this sequence, the addition of the spacer to the N-terminus did not influence toxicity, but the inclusion of the spacer at the C-terminus resulted in the loss of peptide activity. This may indicate that the amino acid sequence plays a critical role in the cytotoxicity of this peptide, especially the residues located in the C-terminal region.
The CTPs can selectively bind to these cells but do not have the ability to alter them nor do they have a toxic effect on the cells. This may be due to the inherent properties of the peptides, the length of the sequences, net charge, hydrophobicity, amphipathicity, and conformation that dramatically influence the activity. This group of peptides has a length between 7 and 13 amino acids and net charges of +1 and +2 which are apparently not sufficient to generate a cytotoxic effect. The CPPs did not show cytotoxicity, this sequences despite the high net charge they did not generate a toxic effect on the cells, perhaps due to the lack of amphipathicity and hydrophobicity of these molecules that prevent an adequate conformation for the interaction and thus an absence of effect.
Regarding hybrid peptides, the objective was to join two bioactive peptides to generate new molecules of a peptide nature that show cytotoxic activity, direct it to the therapeutic target and/or give it another way of acting on the cancer cell. The union of the sequences was made during the SPPS (Figure 1), this technique was suitable and effective to obtain short-length hybrid peptides. In all cases the peptide sequences were linked through the Ahx, this spacer was selected because it provides greater flexibility to the peptide chain, facilitates synthesis, increases retention time, and enables RP-HPLC analysis without significantly affecting solubility. In addition, the incorporation of Ahx can increase proteolytic stability and generate less steric hindrance, which would facilitate the interaction of each sequence with its target.
The retention times are different for each hybrid peptide even though they have a common nucleus which is the sequence RWQWRWQWR, this is due to the influence of the companion peptide which changes the properties of the final peptide. This allows us to infer that the cytotoxic properties of peptides not only depend on the length, net charge, and amino acid composition, but that the primary structure of the peptide also plays an important role.
Cytotoxicity assays show that the action of the peptides is concentration-dependent, causes severe morphological changes in the cells, is rapid (2 h) and is maintained up to 24 or 48 h, suggesting that the peptides are stable during this period (supplementary material, Table S1 and Figure S5). When HeLa cells were treated with the precursor mixture, less cytotoxic activity was observed compared to those treated with the RWQWRWQWR peptide. Considering the proposed mechanism of action for cationic peptides such as LfcinB involving electrostatic interactions between the positive charges of the peptide and the negative charges of the cell surface, the results suggest that the physically mixed peptides may interact with each other through hydrophobic interactions seeking greater stability in the aqueous medium and/or that the precursor and the palindromic peptide RWQWRWRWQWR compete for the negatively charged sites on the cell surface since this electrostatic interaction is nonspecific.
The results of the cytotoxic activity show that the hybrid peptides obtained from the conjugation of the RWQWRWQWR sequence and ACPs (peptides 1–4, 15, 17, and 18) or CPPs (peptides 9–14) generate peptides with cytotoxicity on both cervical cancer cells lines. These amphipathic cationic ACPs can bind to and destroy cancer cells, either through a direct or indirect mechanism of action. This result agrees with what was reported for a lactoferricin-lactoferrampin chimera that showed cytotoxic activity against endometrial cancer cells and cervical cancer [45].
The hybrid peptides obtained with the shorter sequence RRWQWR showed cytotoxic activity, this is consistent with reports showing that hybrid peptides containing the GRRRRSVQWCA and RRWQWR sequences linked by a Pro or Gly-Gly linker were cytotoxic to Jurkat cells [46]. In contrast, hybrid peptides (peptides 5–8) containing CTP sequences were not cytotoxic to the cancer cells tested, suggesting that these CTPs possibly recognize cancer cells and thus could enhance selectivity toward these specific targets, but such conjugation did not confer toxicity to the hybrid peptide and even inactivated the activity of the RWQWRWQWR sequence.
For their part, the hybrid peptides (peptides 9–14) obtained with CPPs showed cytotoxicity against the two cancer cell lines, which coincides with what was previously reported for the hybrid peptide MPLfcinB6 (RRRRRRRGGRRWQWR) conjugated with a polyarginine that presented cytotoxicity against leukemia T and B lymphoma cells caused mainly by damage to the cell membrane and associated with the production of reactive oxygen species (ROS) and permeabilization of the mitochondrial membrane [34].
This allows us to conclude that the conjugation of the palindromic sequence with ACP and CPP sequences confers cytotoxicity to the resulting peptides, so its use is considered novel, appropriate, and effective for this type of application. It was also shown that these sequences are not inherently cytotoxic and require conjugation to the palindromic peptide for the toxic effect. However, the exact mechanism of these hybrid peptides is unknown, since they depend on the physicochemical properties, concentration, charge, and contact time. It is suggested that the cellular uptake mechanism may include an endocytosis-mediated pathway, inverted micelle, and carpet-like mechanism. However, the first contact with the surface is electrostatic with negatively charged glycoproteins [47, 48].
The fact that peptides 1–4, 9–11, 13, 14, and 15-18 showed a greater cytotoxic effect against HeLa cells than against Ca Ski cells suggests that the potency of the cytotoxic effect of the hybrid peptides depends on the cancer cell type. This is in agreement with previous reports that showed that the palindromic peptide as well as some of their derivatives exhibited higher cytotoxic effects in cancer cells CAL27, Caco-2, HCT-116, MDA-MB-231, and MCF-7 than in SCC-15, HT-29, MDA-MB-231, and BT-474 cells [20–23]. This behavior could imply that the cell-peptide interaction is mediated by several processes; the first may be a nonspecific peptide-cell recognition governed by the electrostatic interaction between positively charged amino acids and negatively charged cell surface molecules that bring the peptide closer to the cell surface (interaction common in all cancer cells) [49]. Then, a specific cell-peptide interaction associated with the cytotoxic effect may occur and may be mediated by a cellular receptor which may be differentially expressed depending on the type of cancer cell.
Regarding the location of the sequences in the hybrid peptides, no significant effect on cytotoxicity was observed, allowing us to conclude that in this case, the two configurations allow similar results to be obtained. The hemolytic peptides 3, 4, 13, 14, and 18, have a high content of the amino acids Trp, Lys, and Arg that increase the hydrophobicity and charge of the peptide, thus increasing the hemolytic activity, mainly Trp due to its affinity for cholesterol present in the erythrocyte membrane [30, 50]. Peptides 13 and 14 were conjugated with CPP, therefore they have the ability to translocate to the erythrocyte membrane.
The objective of designing peptides where Arg was replaced by Lys, was to reduce the synthesis costs associated with the arginine content in the sequence while maintaining the activity. The results show that these modifications did not significantly affect the activity of the peptides, this may be because the substitution maintains the net charge of the final peptide and its configuration. Furthermore, decreasing the Arg residues in these peptides did not increase the effect against RBC. Our results agree with previous reports that showed that point changes of Arg by Lys or Ornithine in LfcinB-derived sequences increased or maintained antibacterial and/or anticancer activity [51]. The hybrid dimeric peptide 18 showed cytotoxic activity but it was not much better than the other linear sequences, therefore, doubling the valency of the peptides does not guarantee multiplying their activity. However, the inclusion of an additional chain of palindromic peptides in the molecule leads to an increase in the hemolytic effect.
The test of the physical mixture of peptides allows us to conclude that the chemical binding of precursor peptides is necessary to obtain hybrid peptides with enhanced cytotoxic effects on these cell lines. This may be due to the particular properties of the final peptide, such as its length, charge, amphipaticity, and hydrophobicity, since clearly hybrid peptides present a better balance in accordance with what has been described for ACPs.
In conclusion, in this study, we designed and synthesized hybrid peptides containing the RWQWRWQWR sequence conjugated to ACPs sequences, cervical cancer CTPs, and CPPs. The hybrid peptides were obtained with high purity and had enhanced cytotoxic effects against cervical cancer cell lines. Particularly, hybrid peptides obtained from ACP and CPP sequences showed concentration-dependent cytotoxic activity and rapidly reduced cell viability. Hybrid peptides 1, 3, 4, 16 stand out for their cytotoxicity in the two cervical cancer cell lines HeLa and Ca Ski and selectivity in fibroblasts and RBCs; and therefore, they are considered promising molecules for the development of new drugs for the treatment of cancer. Finally, it is considered that the formation of hybrid peptides was a very useful and recursive strategy for the design and identification of cytotoxic agents based on peptides that exhibit cytotoxicity against cell lines derived from cervical cancer.
Abbreviations
| ACN: | acetonitrile |
| ACPs: | anticancer peptides |
| Ahx: | amino hexanoic acid |
| Arg: | arginine |
| CPP: | cell-penetrating peptides |
| CTP: | cell-targeting peptides |
| DMF: | N,N-dimethylformamide |
| ESI: | electrospray ionization |
| FBS: | fetal bovine serum |
| LfcinB: | bovine lactoferricin |
| Lys: | lysine |
| MS: | mass spectroscopy |
| MTT: | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| RBC: | red blood cells |
| RP-HPLC: | reverse phase-high performance liquid chromatography |
| RP-SPE: | reverse phase-solid phase extraction |
| RT: | room temperature |
| SI: | selectivity index |
| SPPS: | solid-phase peptide synthesis |
| TFA: | trifluoroacetic acid |
Supplementary materials
The supplementary materials for this article are available at: https://www.explorationpub.com/uploads/Article/file/100864_sup_1.pdf.
Declarations
Author contributions
NAC: Conceptualization, Methodology, Investigation, Formal analysis, Writing—original draft, Visualization, Writing—review & editing. JEGC: Conceptualization, Methodology, Formal analysis, Resources, Writing—original draft, Visualization. CMPG: Formal analysis, Resources, Writing—original draft. YVC: Formal analysis, Resources, Writing—original draft. ACBC: Formal analysis, Resources, Writing—original draft. RFM: Conceptualization, Supervision. ZJRM: Conceptualization, Methodology, Resources, Writing—review & editing. JERM: Conceptualization, Methodology, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Funding
This research was conducted with the financial support of MinCiencias with the project: “Obtención de un prototipo peptídico promisorio para el desarrollo de un medicamento de amplio espectro para el tratamiento del cáncer de colon, cuello uterino y próstata”. Contract RC No. 845-2019. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2024.