Affiliation:
1Xenobiotics Research Unit, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
2Graduate Program in Animal Science, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
ORCID: https://orcid.org/0000-0002-5570-8036
Affiliation:
1Xenobiotics Research Unit, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
3Graduate Program in Dentistry, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
ORCID: https://orcid.org/0000-0001-8067-6630
Affiliation:
1Xenobiotics Research Unit, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
3Graduate Program in Dentistry, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
ORCID: https://orcid.org/0000-0001-6631-8618
Affiliation:
1Xenobiotics Research Unit, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
ORCID: https://orcid.org/0009-0009-2182-7684
Affiliation:
1Xenobiotics Research Unit, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
ORCID: https://orcid.org/0000-0002-8352-9078
Affiliation:
1Xenobiotics Research Unit, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
ORCID: https://orcid.org/0000-0003-4634-6624
Affiliation:
1Xenobiotics Research Unit, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
2Graduate Program in Animal Science, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
3Graduate Program in Dentistry, School of Medicine and Life Sciences, Pontifícia Universidade Católica do Paraná, Curitiba 80215-901, Brazil
Email: edvaldo.rosa@pucpr.br
ORCID: https://orcid.org/0000-0001-6087-4365
Explor Drug Sci. 2025;3:100884 DOI: https://doi.org/10.37349/eds.2025.100884
Received: July 30, 2024 Accepted: September 26, 2024 Published: January 14, 2025
Academic Editor: Alessandra Tolomelli, University of Bologna, Italy
The article belongs to the special issue Greening Drug Manufacturing for a Sustainable Healthcare
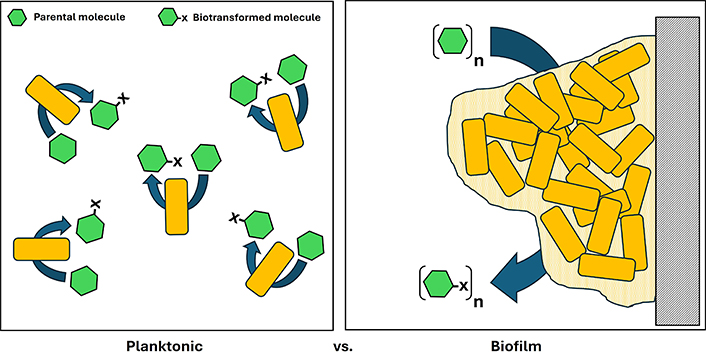
Microbial biotransformations are valuable tools from “green chemistry” and involve converting parental molecules into new daughter ones with unique physical, chemical, or pharmacological properties. These reactions are often carried out by cells grown under a planktonic phenotype. However, microbial cells grown under a phenotype of biofilm can improve biotransformation bioprocesses once they form more biomass per volume, are more resistant to extreme conditions (pH, temperature, and toxic substances), remain active for extended periods, are less prone to cell washouts, and reduce re-inoculation demands, leading to increased production rates due to their unique physiological features. In addition, experience has shown that biofilms may furnish a broader array of new daughter molecules. This review highlighted the benefits of using biofilms in microbial biotransformations to obtain a variety of bioactives.
High-value molecules such as pharmaceuticals, cosmeceuticals, and other fine chemicals had their needs increased in recent decades. To attend to such an increasing demand, academia and industry enrolled in a rat race toward discovering new molecules. Although large molecules such as recombinant proteins, monoclonal antibodies, antibody-drug conjugates, fusion proteins, and vaccines received significant attention, small molar mass molecules were in the sight of many companies [1].
The industrial production of small molecules that respond to more than 90% of therapeutic drugs involves their discovery, design, and development [2]. Biotransformations are energy-efficient, environmentally friendly processes that yield high-value molecules [3], attending to most of the Green Chemistry principles for sustainable pharmaceutical production [4].
When microbes carry out biotransformations in two-stage fermentations, significant amounts of final products can be achieved [5], even overcoming restrictions associated with microbial strains [6].
In microbial bioprocesses, cells can be presented in various phenotypes, free or immobilised, growing or resting [7]. As living organisms, they must transform the surrounding matter to produce biomass [8] and energy [9, 10] or to detoxify growth-limiting molecules [11]. All these targets are achieved by simple or intricate chemical reactions arranged in pathways. Such microbial cellular reactions involve metabolic reactions or biotransformations.
Metabolic reactions are multi-step coupled reactions that convert a particular molecule into some others to produce energy and biomass (primary metabolism) or additional advantageous molecules (secondary metabolites) [12]. The production of acetone using Clostridium acetobutylicum or C. beijerinkii [13], the growth of the edible mycelium of Rhizopus microsporus var. oligosporus [14], and bacterial enzymes used for therapeutical (e.g., L-asparaginase and nattokinase) [15–17] or nutraceutical (e.g., phytases) [18] purposes are examples of metabolic end-points of sequential reactions from primary and secondary metabolisms.
In turn, biotransformations are one-step reactions (eventually, few-step reactions) that modify a specific molecule that is not a growth substrate, neither involved in energy or biomass production nor secondary metabolites by a biological agent [19]. Indeed, the reactions involved in biotransformation phenomena attempt to detoxify xenobiotics so those already grown living cells are exposed [20, 21]. Figure 1 shows the dynamics of metabolic and biotransformation reactions considering the growth curve of a microorganism. Notice that cells produce energy and biomass immediately required for cell divisions during previous phases. Secondary metabolites (e.g., pigments, antibiotics, etc.) are molecules produced early in the log phase and prolonged throughout the following phases, conferring some advantages to environmental challenges. Biotransformations are carried out only after reaching or quasi-reaching stationary growth phase when the central metabolism is reduced, and the production of biotransformed molecules (daughter molecules) may demand several days to get a beneficial amount. Metabolically engineered strains have changed such a scenario with considerably reduced overall production time [22].
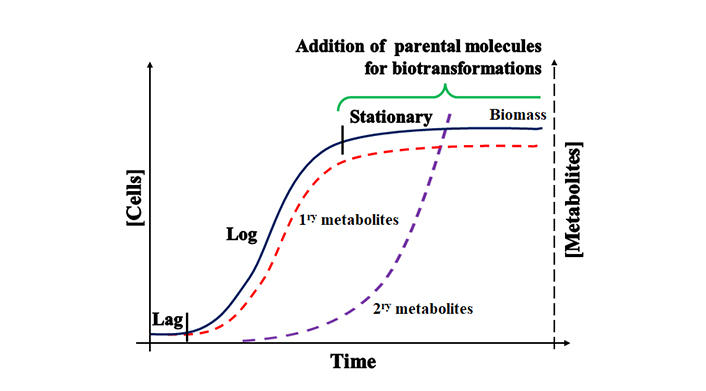
Microbial growth curve presenting when primary, secondary, and biotransformed xenobiotics are produced
Note. Adapted with permission from [23], © 2021 Springer Nature Switzerland AG
Biotransformation reactions are an interesting way to obtain high-value molecules, such as pharmaceuticals and fine chemicals, under mild operational and environment-friendly conditions [24]. Redox reactions such as hydroxylations, dihydroxylations, and epoxidations with significant demand for oxygen in the late logarithmic-to-stationary transient phase are the most commonly occurring during xenobiotic biotransformations [25, 26].
In some circumstances, using whole microbial cells to carry out biotransformations seems more efficient and cost-effective than using isolated enzymes. This is because the latter requires expensive cofactors to be recycled and renewed biocatalysts necessary for extended periods. Additionally, changes in environmental conditions, such as pH, can have a negative impact on enzymatic activity [27].
Using living microbial cells for biotransformations has many benefits, such as a high surface-volume ratio, fast processing times, fast rate of metabolic transformation, and easy control over the process [28]. Not only does it eliminate the need for multiple enzyme extraction and purification steps, but it also has positive effects on activity and stability [29].
Numerous articles have explored the potential of microorganisms to convert parental molecules (xenobiotics) into therapeutically or fine chemical high-valued daughter molecules, proving that this is a reliable manner to obtain such molecules in easy-to-control conditions [30–36].
Nevertheless, bioprocesses using whole cells may involve free-living planktonic cells, cells immobilised in a matrix by passive entrapment, and cells self-entrapped in biofilms. Cell morphology, adherence mechanisms, surface properties, and bioreactor flow patterns drive microbial growth to planktonic or sessile lifestyles [37].
The most common bioprocesses for obtaining fine chemicals and pharmaceuticals are submerged fermentations (SmF) with planktonic bacterial, fungal, actinomycetal, or algal cells [38, 39]. Bioprocesses to get metabolites from primary and secondary metabolism tend to use planktonic cells due to the immediate consumption of nutrients. On the other hand, when cells are in an immobilised matrix, such nutrient consumption occurs slower [30], demanding more time to accomplish.
The advantages of using immobilised over planktonic cells include easy removal and reuse of the biocatalyst, decreased operational costs, the ability to use continuous flow reactors, increased biocatalyst concentration, and higher reagent flow compared to batch reactors. Additionally, whole cells avoid producing secondary reactions that could diminish the reaction yield in a non-growing state. Regarding production, a yield comparison between immobilised vs. planktonic cells showed higher production rates of the first for some compounds [40]. All these benefits become more attractive for biotransformation ends.
Cell immobilisation refers to confining cells within a designated space while maintaining their catalytic activity and viability. Mass transfer of substrates or reaction products is often the rate-controlling contributing factor, and immobilisation may also cause reaction inhibition by initial or end products [41].
Various methods for immobilising whole cells include passive adsorption onto solid surfaces, covalent binding to solid surfaces, crosslinking between the cell and polymers, and whole-cell trapping by membranes [42], as presented in Figure 2. A common characteristic of all those methods is that the cells are “put” in contact with the immobilising surface and become attached by simple surface interactions. In none of them, microbial cells act to favour such binding by producing a colonisation factor that enables the active establishment of an adherent microbial community.
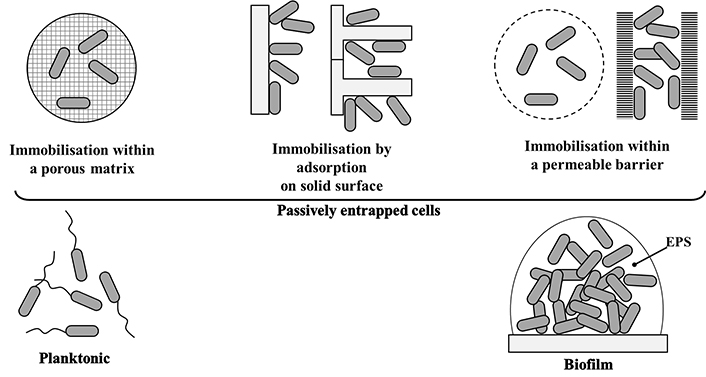
Different manners to immobilise microbial cells. EPS: extracellular polymeric substance
Note. Adapted with permission from [42], © 2001 Springer Nature Switzerland AG
When whole cells adhere and grow entrapped within an extracellular polymeric substance (EPS), the resulting community is known as a biofilm [43]. Figure 3 presents some features of bacterial and fungal biofilms. In both cases, microbial cells are surrounded by a variable mixture of substances (the EPS), such as insoluble polysaccharides, proteins, nucleic acids, and a significant amount of solvating water.
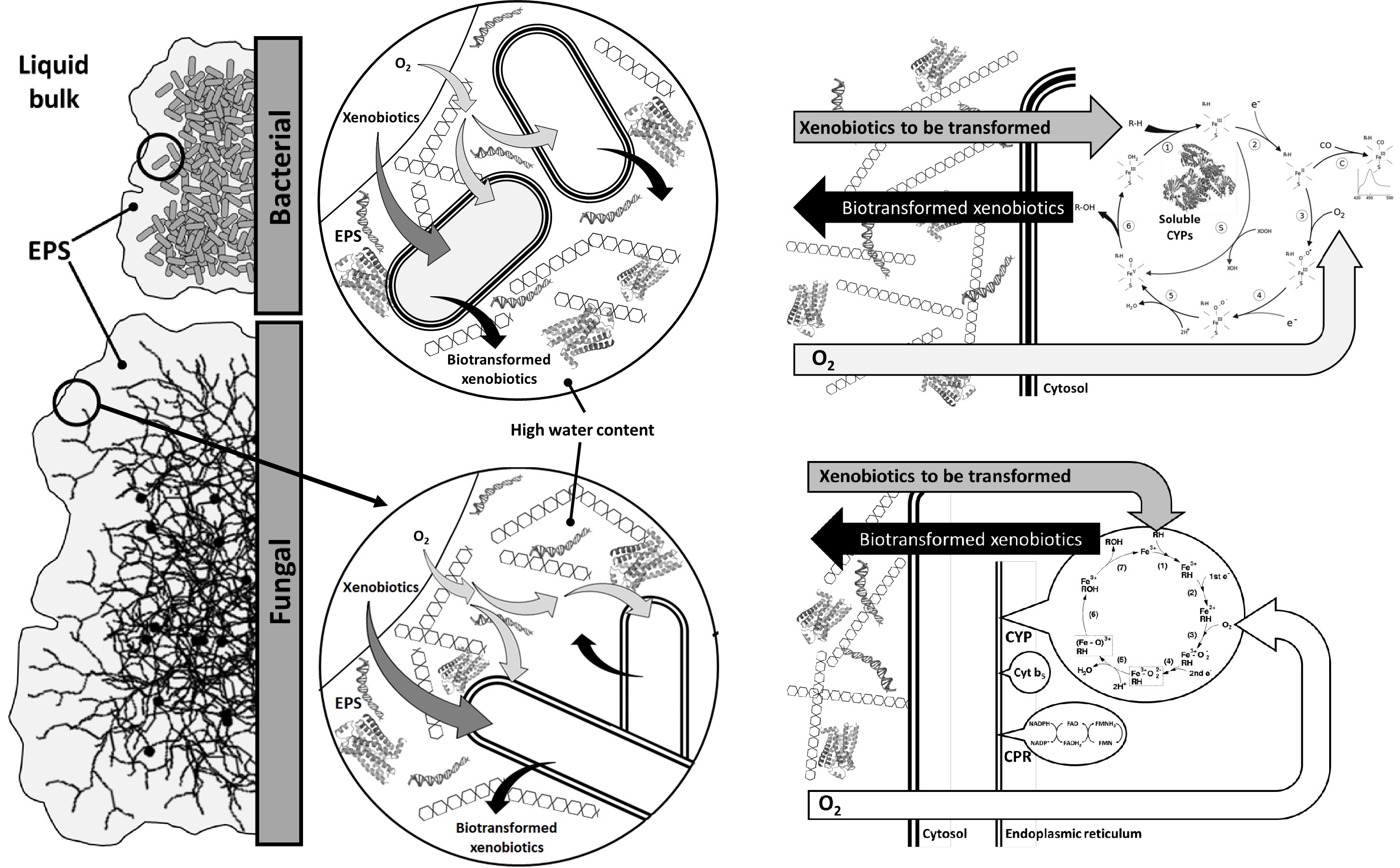
Steps of microbial biotransformation of xenobiotics in microbial (fungal and bacterial) biofilms. EPS: extracellular polymeric substance; CYPs: cytochrome P450; CPR: cytochrome P450 reductases
Unlike planktonic and passively immobilised cells, EPS protects biofilm-grown cells against extreme pH, temperature, and toxic substances [44, 45]. Also, these protected cells remain active for extended periods [46]. These characteristics make biofilms more effective in overcoming common challenges in biotrans-formations, including the toxicity of substrates and products and shorter biocatalytic stability when under planktonic phenotype [47]. Other positive points in favour of biofilms are the ability to allow the obtaining five to ten-fold more biomass per unit volume of the bioreactor, reduced risks of cell washout at high dilution rates in continuous processes, and the elimination of the need for re-inoculation during repeated batches were presented as determinants for the best results obtained with this growth phenotype [48, 49].
Regarding the last supposed advantage, although some studies indicated that the production of bio-products and bioremediation could be carried out using pre-formed biofilms by renewing nutrients [50–52], even with fluctuation of yields amongst batches [53], to our knowledge, such revitalisations for new biotransformations were not addressed yet. It is likely that during the late stationary phase in which biotransformation reactions generally occur, the culture would experience inhibition, thus reducing productivity.
Downstream processes involve capturing and purifying a target molecule, accounting for anywhere from 50% to over 90% of the overall production cost [54]. As biofilm cells are entrapped within the matrix, cell separations are unnecessary, reducing expenses in continuous and even batch biotransformation processes.
Figure 3 shows the path of xenobiotics from the bulk fluid to the bacterial cytosol or the fungal smooth endoplasmic reticulum and the inverse return to liquid bulk and to EPS, where a significant amount of biotransformed molecules become entrapped. At first sight, this retention in EPS may appear to be a trouble once separations in liquid bulk are relatively easy to carry out [55]. However, as most EPS is formed by solvating water, it may act as a concentrator of biotransformed molecules, demanding no complex procedures of filtration or centrifugation to recover such molecules.
Much experience has been acquired with planktonic-growing cells, but only a few studies have evaluated the bioconversion ability of biofilms, probably due to methodological constraints [56].
Table 1 briefly compiles manuscripts published in the last ten years (2013–2023) involving biotransformations conducted using fungal and bacterial biofilms. Others have already compiled older published papers of the same nature [57–59]. The present compilation solely focused on immobilised microbial communities for high-value production. It did not cover topics such as biodegradation, bioremediation, effluent treatment, biofuel production, and host/microbiota-related issues concerning microbial biotransformation and biofilms.
Compilation of ten years (2013–2023) of published studies concerning microbial biotransformations conducted by biofilms to produce high-value molecules
| Group | Strain | Parental molecule(s) | Daughter molecule(s) | Use | Scaffold | Reference |
|---|---|---|---|---|---|---|
| Fungi | Candida viswanathii NBRC10321 | Citronellal | Citronellic acid | Ingredient used in perfumes | Microspheres of PMMA | [60] |
| Cunninghamella echinulata ATCC9244 | Hesperetin | Hesperetin 7-O-glycoside | Arome of sweet orange | Stainless steel wool | [61] | |
| Cunninghamella echinulata ATCC9244 | LQFM-021 | N-glycosylated LQFM-021 | Phosphodiesterase-3 inhibitor | Stainless steel mesh | [62] | |
| Cunninghamella elegans DSM1908 | Diclofenac | 4’-Hydroxydiclofenac | Cox-2 inhibitor | Stainless steel springs | [63] | |
| Cunninghamella elegans DSM1908 | Diclofenac | 4’-Hydroxydiclofenac | Cox-2 inhibitor | Stainless steel mesh | [57] | |
| Cunninghamella elegans DSM1908 | Flurbiprofen | 4-Hydroxyflurbiprofen | Standart for purity assays | Stainless steel springs | [64] | |
| Lasiodiplodia (Botryodiplodia) theobromae 1368 | (+)-Valencene | (+)-Nootkatone | Arome of grapefruit | Permeable silicon rubber | [65] | |
| Mucor circinelloides URM4182 | Babassu oil lipides | Ethyl esters (various) | Diverse applications | Polyurethane foams | [66] | |
| Pichia kluyveri NBRC1165 | Citronellol | Citronellyl acetate | Mosquito repellent | Microspheres of PMMA | [60] | |
| Yarrowia lipolytica W29 (ATCC 20460) | Methylricinoleate | 3-Hydroxy-γ-decalactone | Aromatic compound | Groupons of PMMA | [67] | |
| Bacteria | Lactobacillus brevis RK03 | Monosodium glutamate | γ-Aminobutyric acid (GABA) | Anti-seizure and anti-anxiety drug | Groupons of HEMA/PEGDA | [68] |
| Bacillus subtilis BS-7 | Ferulic acid | Vanilin | Aromatic compound | Carbon fiber textiles | [69] | |
| Bacillus subtilis BS-7 S | Ferulic acid | Vanilin | Aromatic compound | Active carbon fiber | [70] | |
| Escherichia coli BL21 | 5-Nitrononane-2,8-dione | (R)-syn/anti-hydroketone | Diverse applications | PDMS | [71] | |
| Escherichia coli BL21(DE3) | Indole | Tryptophan | Amino acid | Cellulose sponge | [72] | |
| Escherichia coli MG1655 | 5-Haloindole | 5-Halotryptophan | Pharmaceutical intermediary | Glass slides with poly-L-lysine | [73] | |
| Pseudomonas diminuta ATCC19146 | Ethylene glycol | Glycolic acid | Anti-aging agent | Stainless steel structured packing | [74] | |
| Pseudomonas putida GS1 | (R)-(+)-Limonene | (R)-(+)-Perillic acid | Antineoplastic agent | Silicon membrane | [75] | |
| Pseudomonas taiwanensis VLB120 B83 T7 | Glucose | (S)-3-Hydroxyisobutyric acid | Intermediate in the metabolism of valine | Silicon tubing | [75] | |
| Rhodococcus hoagii NBRC3730 | 2-Octanol | 2-Octanone | Ingredient used in perfumes | Microspheres of PMMA | [76] | |
| Associations | Synechocystis sp. PCC 6803 plus | Cyclohexane | Cyclohexanol | Plasticizer agent | Borosilicate glass | [77] |
| Pseudomonas sp. VLB120 | [78] |
LQFM-021: 5-(1-(3-Fluorophenyl)-1H-pyrazol-4-yl)-2H-tetrazole; PMMA: methyl-polymethacrylate; HEMA/PEGDA: hydroxyethyl methacrylate/polyethylene glycol diacrylate; PDMS: polydimethylsiloxane
It is noticeable that the studies have been centred on a few microbial species. In the case of fungi, the genera employed belong to divisions Ascomycota (Candida, Lasiodiplodia, Pichia, and Yarrowia) or Zygomycota (Cunninghamella and Mucor). Biofilm-driven bacterial biotransformations were carried out by Bacillus subtilis, Escherichia coli, Lactobacillus brevis, Pseudomonas spp., and Rhodococcus hoagii.
Biotransformations involved one [57, 60–65, 69–73, 77, 78], two [66, 68], three [74, 75], or multiple reactional steps [67, 76].
Scaffolds used for biofilm formation varied from glass without any treatment [77, 78] to diverse presentations of stainless steel [57, 59–63, 73] and intricated polymers such as methyl-polymethacrylate (PMMA) [60, 67, 69], silicon [65, 71, 75, 76], and polyurethane foams [66].
Although relatively little information is available regarding biofilms and biotransformations to obtain high-value goods, the combinations of microbial species/strains, xenobiotics, biofilm scaffolds, and types of bioreactors generate many possibilities to prospect.
When selecting a candidate for biofilm-based industrial biotransformations, the microorganism must fill in specific criteria. The organism must be non-pathogenic, genetically stable, able to quickly form a well-attached biofilm in an inexpensive cultivation medium, and not have an excessive formation of the EPS, which can decrease the catalytic rate [24].
Once the biofilm is formed, it should be highly porous to allow for the diffusion of nutrients, dissolved oxygen, and biotransformation substrates and products [46, 57, 79]. Failure to do so can lead to considerable physiological heterogeneity, negatively impacting conversion rates [80].
Various biofilm bioreactors have been designed for microbial biotransformations. Most biocatalytic biofilms demand immersion, quasi-immersion, or temporary immersion in liquid bulks where xenobiotics are dissolved; thus, bioreactors designed for solid-state fermentation (SSF) are not practical for obtaining ingredients for the biopharma industry. SSF is valuable for obtaining enzymes, antioxidants, biofuel, and secondary metabolites [81], but not for strictu senso biotransformations.
High-quality reviews regarding biofilm bioreactors have been published [80, 82–84]. Special attention must be paid to Rosche et al. [24] review covering biofilm reactors for biotransformations. Those authors depict the various features of many bioreactors, presenting their pros and cons and their applicability to produce high-value molecules.
The surface characteristics of supports over which biofilms will be formed drive the success of the bioprocess. Fungal biofilms grown on certain surfaces, such as corrugated stainless-steel structures [85–87] and stainless-steel pads [88], can enhance production. Similarly, bacteria form biofilms on surfaces like cellulose acetate, hydrophilic and hydrophobic polyvinylidene difluoride, and polycarbonate membranes [89]. These surfaces are often arranged in fixed-bed bioreactors or similar, which experience less shear stress than traditional stirred-tank reactors.
Biofilms can improve the production of pharmaceuticals through biotransformation reactions. As presented in Table 1, some non-steroidal anti-inflammatory drugs (NSAID) are easily hydroxylated and dihydroxylated by Cunninghamella spp. biofilms formed onto stainless steel compression springs [57, 58], with bioconversion rates reaching up to 43%. Due to its extreme simplicity, such support does not allow the formation of large biofilms, limiting the biotransformation rates, but serves primary screening purposes. Scaffolds with an enlarged area improve the biofilm formation. However, surface dimensions and corrugation must be considered to obtain productive biofilms [90]. Corrugation increases the colonisable area and creates retentive niches in which biofilms become protected from shear forces [91].
Our group recently proposed a low-cost and highly efficient bioprocess that involves a hybrid fixed bed-airlift. This process allows fungal biofilms to form onto a stainless-steel screen baffle [57]. Once the cells have attached and early biofilm formation occurs (usually within 24 h), the bioreactor mechanically functions as an airlift bioreactor. The resulting inner baffle now contains metabolically active cells that biotransform xenobiotics, so it can also be considered a fixed-bed bioreactor. Our yield showed that 29.6% of biotransformed molecules were free in liquid bulk, while 61.3% were trapped in the biofilm EPS. Combined, these parcels generated approximately 91% of 4’-hydroxilated daughter molecules. Combining a well-aerated system and appropriate support for biofilm growth improves the biotransformation rates and the scalability of the process.
A promising strategy for enhancing biofilm cells’ productivity through genetic manipulation has been proposed [92]. While there are established protocols for obtaining recombinant proteins and fine chemicals using planktonic bacteria, yeasts, and fungi, there is limited research on biofilms for this purpose [80]. However, it is already known that genetically engineered cells within biofilms can produce daughter molecules efficiently and cost-effectively [93]. For instance, Escherichia coli biofilms expressing the tryptophan synthase trpBA gene can biotransform 5-haloindoles into 5-halotryptophans, which are pharmaceutical intermediates, at a conversion rate three to four times higher than planktonic cells [73].
Biofilm engineering is a comprehensive method that manipulates the growth and functions of biofilms to promote biocatalytic processes. This approach includes various aspects such as monitoring intercellular communications using quorum sensing (QS) and quorum quenching (QQ) signalling molecules, regulating second inter and intracellular messenger networks, and forming the biofilm matrix [58].
Another feature that is poorly investigated is the possibility of using nanoparticles. Depending on their size and net charge, they can penetrate the biofilm matrix composed of highly hydrated exopolysaccharides [94, 95] and deliver parental molecules to deep biofilm-resident cells. This could increase the biotransformation rate of biofilms.
Differences in bioreactor architectures, bioreactor feedings, biofilm scaffolds, the chemical nature of parental and daughter molecules, the type of microorganisms, oxygen demands, and others make comparisons among bioreactors challenging. The best combination of parameters must be stated for each particular necessity.
The benefits of biotransformations in biofilm reactors are evident. However, more laboratories will still need to dedicate themselves to the search for new molecules through microbial biotransformation. Most studies are still in the screening phase using conical flasks, with limitations for transferring experiences to the industrial sector [96]. More profound obstacles that must be overcome when translating lab experiments into industrial reality include population heterogeneity caused by substrate and oxygen diffusion, ensuring strain stability and purity across multiple operations, scaling up the process, and improving downstream processes [80].
After considering the statements above, we are confident that advances in biotransforming biofilms will occur by creating specialised bioreactors, identifying new producing and biotransforming species/strains, and manipulating microbial genomes.
EPS: extracellular polymeric substance
SSF: solid-state fermentation
NSO, RCPSR, and RCP had scholarships from the Coordination for the Improvement of Higher Education Personnel (CAPES-Brazil financial code 001). MGCP had a scholarship from the National Council for Scientific and Technological Development (CNPq-Brazil).
NSdO: Data curation, Writing—original draft. RCPdSR, RCdP, MGdCP, and RTR: Data curation. LFB and EARR: Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Leonel Pereira, João Cotas
Leonel Pereira ... Ana Valado
Mathivathani Kandiah ... Ominda Perera
