Affiliation:
1Departamento de Farmacia, Facultad de ciencias, Universidad Nacional de Colombia, sede Bogotá, Bogotá 111321, Colombia
ORCID: https://orcid.org/0000-0002-7266-8769
Affiliation:
2Departamento de Microbiología, Universidad Pontificia Javeriana, Bogotá 110231, Colombia
ORCID: https://orcid.org/0000-0003-4734-3310
Affiliation:
2Departamento de Microbiología, Universidad Pontificia Javeriana, Bogotá 110231, Colombia
ORCID: https://orcid.org/0000-0003-1302-5429
Affiliation:
2Departamento de Microbiología, Universidad Pontificia Javeriana, Bogotá 110231, Colombia
ORCID: https://orcid.org/0000-0003-4788-8150
Affiliation:
3Instituto de Biotecnología, Facultad de ciencias, Universidad Nacional de Colombia, sede Bogotá, Bogotá 111321, Colombia
ORCID: https://orcid.org/0000-0001-7545-8129
Affiliation:
4Departamento de Química, Facultad de ciencias, Universidad Nacional de Colombia, sede Bogotá, Bogotá 111321, Colombia
ORCID: https://orcid.org/0000-0003-4636-4443
Affiliation:
5Multidisciplinary Centre for Studies in Biotechnology, Universidad Michoacana de San Nicolas de Hidalgo, Morelia Michoacán 58893, México
ORCID: https://orcid.org/0000-0002-3269-9202
Affiliation:
1Departamento de Farmacia, Facultad de ciencias, Universidad Nacional de Colombia, sede Bogotá, Bogotá 111321, Colombia
ORCID: https://orcid.org/0000-0002-4003-1157
Affiliation:
4Departamento de Química, Facultad de ciencias, Universidad Nacional de Colombia, sede Bogotá, Bogotá 111321, Colombia
ORCID: https://orcid.org/0000-0001-6915-8488
Affiliation:
1Departamento de Farmacia, Facultad de ciencias, Universidad Nacional de Colombia, sede Bogotá, Bogotá 111321, Colombia
Email: jaegarciaca@unal.edu.co
ORCID: https://orcid.org/0000-0001-6882-4397
Explor Drug Sci. 2025;3:100891 DOI: https://doi.org/10.37349/eds.2025.100891
Received: September 16, 2024 Accepted: December 20, 2024 Published: February 26, 2025
Academic Editor: Fernando Albericio, University of KwaZulu-Natal, South Africa, Universidad de Barcelona, Spain
The article belongs to the special issue Bioactive Peptides: Pioneering Innovations in Latin American Research
Aim: To identify peptides derived from bovine lactoferricin (LfcinB) as potential therapeutics for colon cancer treatment. We systematically modified dimeric peptides to enhance their selectivity against colon cancer cells and reduce toxicity. We examined the effects of specific changes, such as substituting L-arginine (Arg) with L-ornithine (Orn) and/or D-Arg, on cytotoxic activity in colon cancer cells, as well as activity in prostate and cervical cancer cell lines. Additionally, we assessed the type of cell death induced and the in vivo toxicity of the dimeric peptides.
Methods: The peptides were synthesized by manual solid-phase peptide synthesis, purified by reverse phase-solid phase extraction (RP-SPE), and characterized by RP-high performance liquid chromatography (RP-HPLC), and mass spectrometry (MS). Their cytotoxic effect on cancer and non-cancerous cells was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The most promising dimeric peptide underwent scale-up synthesis to yield approximately 1 g. The type of induced cell death was analyzed through cytometry assays, while preliminarily toxicity studies were conducted in Galleria mellonella, zebrafish, and CD1 mice.
Results: Our findings demonstrated that dimeric peptides containing L-Orn or D-Arg residues exhibited potent and selective cytotoxic effects against colon cancer cells (Caco-2 and HT-29), prostate cancer cells (DU-145), and cervical adenocarcinoma (HeLa). Notably, these modified peptides showed minimal toxicity in human erythrocytes, HEK 293 cells or fibroblasts, and Galleria mellonella larvae. Peptide 3: (R-Orn-WQWRFKKLG)2-K-Ahx, emerged as particularly promising, preserving its integrity and anticancer activity during scaled-up synthesis. Furthermore, peptide 3 induced behavioral changes and sedation in CD1 mice and showed significantly lower toxicity in zebrafish.
Conclusions: The results suggested that specific modifications of Arg/Orn residues in dimeric peptides enhance their cytotoxicity against colon cancer cells and reduce in vivo toxicity. These modified peptides hold promise as safe and effective therapeutic candidates, potentially expanding the treatment options available for cancer.
There is an increasing need for novel anticancer agents as drug resistance arises, leading to difficulties in curing patients with cancer [1], this panorama worsens as cancer is the current leading cause of death worldwide [2]. Among this burden, in the United States in 2022 colon cancer was expected to be the third most common and lethal cancer in both sexes, affecting 8% of the population [3, 4].
Currently, colon cancer treatment involves surgery, however, the success of this approximation highly depends on the time to surgery after diagnosis [5], and in more advanced cases it is usually associated with pharmacological treatment, such as FOLFOX, FOLFIRI, bevacizumab, or CAPEOX. Unfortunately, these treatments involve high toxicity, and for metastatic cancers, the challenge of survival remains active [6]. Therefore, the development of new treatments is considered a priority [6].
Among cancer treatment alternatives, positively charged peptides known as anticancer peptides (ACPs) have taken an important place in research as they are associated with low toxicity, are less likely to generate resistance, have high specificity for cancer cells, as well as some of them have been associated with immunomodulatory, anti-metastatic, and anti-angiogenic properties [7, 8]. In fact, there are different peptides in colon cancer studies, however reaching clinical studies is still challenging [9, 10].
The mechanism of action of these ACPs is associated with electrostatic interaction mediated by cationic residues of peptides and negatively charged molecules on the cancer cell membrane. Likewise, cancer cells have high fluidity in their membrane, and cell disruption is favored, which is carried out by hydrophobic residues of ACPs, which allows the formation of pores in the membrane or cell lysis or induces membrane permeability that leads to the entry of the peptide to exert other effects such as the promotion of apoptotic or necrotic processes [11]. In fact, this mechanism, which does not depend on cancer cell type, also indicates the possibility of broad-spectrum effects, which is favorable for drug discovery processes. Additionally, as their mechanism of action does not depend on the speed of cell replication, as is the case with current chemotherapy, these ACPs can act on slow-growing cancers [12, 13].
Bovine lactoferricin (LfcinB) has demonstrated cytotoxic activity against the HT-29 colon cancer cell line by activating p53, inducing apoptosis, and inhibiting angiogenesis [14]. Previously, we have identified monomeric (linear) and dimeric (bivalent) peptides derived from LfcinB with selective cytotoxic effects against oral, breast, and colon cancer [15–17]. Dimeric peptide (20RRWQWRFKKLG30)2-K-Ahx (here named 26[F]) containing the sequence fragment 20–30 of LfcinB, showed cytotoxic activity against breast cancer cells (MCF-7 and MDA-MB-231), colon cancer cells (HCT-116 and Caco-2) through apoptotic mechanisms mainly [15–17]. We also showed that the linear peptide LfcinB (21–25)Pal (RWQWRWQWR) and some of its derivatives exhibited greater potency against colon cancer compared to LfcinB and its parent protein [15, 16]. These cationic peptides contain residues of arginine (Arg) and lysine (Lys) which are recognized as key amino acids in the interaction of the peptide with the negatively charged cancer cells surface. Previous studies have focused on understanding the role of these cationic residues in the cell membrane [18]. Arg is a highly hydrophilic amino acid with the capacity to form electrostatic and extensive hydrogen bonds with the phosphate groups and water that allow perturbations on the cell membrane, promoting membrane perturbations while retaining its charge in the membrane [19–21]. Arg-peptides have been associated with cellular uptake [20], however, have also been correlated with higher toxicity when compared to Lys-containing analogous peptides [22]. Conversely, peptides with Lys form fewer hydrogen bonds and exert less strength in their interactions, do not retain their charge in the membrane, and thus lack ease in crossing membranes [23, 24].
The lead-like optimization of peptides, aimed at enhancing their potency, selectivity, and bioavailability, is a commonly used strategy [23, 25–29]. This optimization involves punctual modifications, such as altering the charge origin, incorporating various derivatives of unnatural amino acids, or utilizing their D-amino acids counterparts [24–26]. Within those, Arg/Lys changes have shown increased selectivity for cancer cells over normal cells. Additionally, the inclusion of ornithine (Orn) has been linked to enhanced anticancer activity against Jurkat cells, along with greater proteolytic stability [23]. Our previous research has shown that Arg/Orn modifications could improve the cytotoxic activity of linear LfcinB-derived peptides against the Caco-2 colon cancer cell line [15, 16].
Here, we demonstrated the impact of systematically substituting Arg with Orn, L-Arg with D-Arg, N-acetylation, N-biotinylation, and/or dimerization on the cytotoxic effects, first against colon cancer and non-cancer cells. Some of the active peptides were also tested against prostate cancer cells (DU-145) and cervical adenocarcinoma cells. Finally, we determined the in vivo toxicity in Galleria mellonella, CD1 mice, and zebrafish. Our findings indicated that the potency against cancer cell lines could be enhanced or maintained with specific alterations to the amino acid sequence, while concurrently reducing in vivo toxicity.
Rink amide resin (Lot# 9952717), Fmoc (9-Fluorenylmethoxycarbonyl)-Orn(Boc)-OH (Lot# 9953167), Fmoc-Gln(Trt)-OH (Lot# 9955159), Fmoc-Gly-Oh (Lot# 9950869), Fmoc-Phe-OH (Lot# 9850879), 6-chloro-1-hydroxybenzotriazole (6-Cl-HOBt) (Lot# 9930287) were purchased from AAPPTec (Louisville, KY, USA). Fmoc-Lys(Boc)-OH (Lot# 9951014), Fmoc-Leu-OH (Lot# S7687911904), Fmoc-Arg(Pbf)-OH (Lot# S7830367003), Fmoc-Trp(Boc)-OH (Lot# S8159450), N,N-dicyclohexylcarbodiimide (DCC) (Lot# K41630554), methanol, diethyl ether, N,N-dimethylformamide (DMF), absolute ethanol, dichloromethane (DCM), acetonitrile (ACN), isopropylalcohol (IPA), trifluoroacetic acid (TFA), 1,2-ethanedithiol (EDT), triisopropylsilane (TIPS), and SPE (solid phase extraction) Supelclean columns were obtained from Merck (Darmstadt, Germany). 4-methylpiperidine, pyridine, Triton-X, potassium cyanide (KCN), pyridine, phenol, ninhydrin, EDTA (ethylenediaminetetraacetic acid), RPMI (Roswell Park Memorial Institute)-1640 culture medium, and trypsin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from Gibco (Waltham, MA, USA). The HT-29 (HTB-38TM), Caco-2 (HTB-37TM), HEK 293 (CRL-1573TM), DU-145 (HTB-81TM), and HeLa (CCL2TM) cell lines were purchased from ATCC (American Type Culture Collection) (Manassas, VA). Primary culture of human fibroblasts was provided by the Cellular and Molecular Physiology Laboratory of the National University of Colombia.
The designed peptides were synthesized using manual solid-phase peptide synthesis (SPPS-Fmoc/tBu) as previously reported [16]. Briefly, rink amide resin (0.46 meq/g) was swelled with DMF for 2 h, followed by two cycles of deprotections through treatment with 2.5% 4-methylpiperidine in DMF and then, washed with DMF and DCM. For the coupling reaction, five equivalents of excess were used, Fmoc-amino acids were pre-activated with DCC and 6-Cl-HOBt (1:1:1) in DMF at room temperature (RT) for 10 min; coupling and deprotection steps were confirmed by the Kaiser test [30]. Synthesized peptides were cleaved with a cocktail containing TFA/water/TIPS/EDT (93/2/2.5/2.5, v/v/v/v). The reaction was stirred for 8 h at RT, then the resin-peptide was filtered, and the solution was collected. Crude peptides were precipitated and washed by treatment with cold ethyl ether and finally dried at RT.
Peptide 3 batches synthesis. The synthesis followed a similar procedure to the one previously described, with slight modifications. The coupling reaction was performed by dissolving Fmoc-amino acid/DCC/6-Cl-HOBt [1:1:1 molar ratio, with a 5-fold excess (batch 02) or 3-fold excess (batch 03) relative to the resin substitution]. The reaction mixture was dissolved in 4 mL (batch 02) or 6 mL (batch 03) of DCM:DMF (1:1, v/v).
The molecules were dissolved in mobile phase A (0.05% TFA in H2O) and loaded onto SPE columns (SUPELCO LC-18 with 5.0 g resin). SPE columns were activated prior to use with ACN (0.05% TFA) and equilibrated with water (0.05% TFA), a gradient was used for their elution and the collected fractions were analyzed using RP-HPLC (reverse phase-high performance liquid chromatography) and if contained the pure products were lyophilized.
RP-HPLC was performed on a Merck Chromolith® C18 (50 × 4.6 mm) column using an Agilent 1200 liquid chromatograph (Omaha, NE, USA) with a UV-Vis detector (210 nm). For peptide analysis, a linear gradient was applied from 5% to 50% solvent B (0.05% TFA in ACN) in solvent A (0.05% TFA in water) for 8 min at a flow rate of 2.0 mL/min at RT. 10 μL of crude or pure samples were injected with an approximate concentration of 1.0 mg/mL. Expected masses were confirmed using LC-MS (liquid chromatography-mass spectrometry); peptides (10 μg/mL) were analyzed on a Bruker Im-pact II LC Q-TOF MS spectrometer equipped with an ESI (electrospray ionization) source operated in positive mode at the conditions: late end offset 500 V, capillary voltage 4,500 V, drying temperature 220°C, and nitrogen flow 8 L/min.
The Fourier transform infrared (FT-IR) spectra were obtained in a SHIMADZU IRAffinity-1S spectrophotometer, by the KBr pellet method. The peptide was mixed with KBr standard (1:300, w/w) and macerated in an agate mortar until a uniform mixture was obtained. Then the mixture was placed in the pill dispenser and a manual press was used to obtain the pellet. The background reading was performed according to the supplier’s instructions and then the pellet was analyzed in transmittance mode, measuring range 400–4,000 cm–1, resolution 8 and 64 scans.
Peptide (10 mg/700 µL) was dissolved in D2O and analyzed in a Bruker Avance NMR (nuclear magnetic resonance) spectrometer (Bruker de 400 MHz, console Avance NEO 400). In the 1H-NMR experiment, a proton frequency of 400.13 Hz and at a temperature of 23°C was used. Each 1H NMR spectrum was acquired with a width of 20 ppm, 64 scans, and a 2.0 s relaxation period.
For Caco-2 cells, the medium used was Dulbecco’s modified Eagle’s medium (DMEM)/nutrient mixture F-12 Ham. For HEK 293, fibroblast cell lines, the medium used was RPMI-1640, and for HT-29, the medium used was DMEM. For all cell lines, the medium was supplemented with 10% FBS and 1.5 g/L NaHCO3 and NaOH was added up to pH 7.4, amphotericin (200 μg/mL) and 1% penicillin and streptomycin were added in DMEM medium. All mediums were filtered through a 0.22 μm membrane. The cells were incubated at 37°C and 5% CO2 in a humid atmosphere.
Cytotoxic assays were performed as previously described [31]. Briefly, 1 × 104 cells per well (HT-29, Caco-2, HEK 293, DU-145, HeLa, or fibroblasts) were seeded and synchronized in 96-well plates for 24 h. Then, the medium was changed to a peptide solution in the same medium ranging from 6.25 to 200 μg/mL for 2, 24, or 48 h at 37°C. To test cell viability 10 μL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution (5 mg/mL) was added to each well and incubated for 4 h at 37°C, then the medium was removed and 100 μL of DMSO (dimethyl sulfoxide) added to dissolve formazan crystals at 37°C for 30 min, later absorbance at 570 nm was read on Bio-Rad 680 microplate reader. IC50 values were determined using GraphPad Prism 8 statistical analysis software.
Cancer cells were observed using phase contrast microscopy. Imaging was performed with the microscopes (SUNNY OPTICAL, model ICX41, and MoticTM, model AE31E).
The HT-29 cells were seeded and synchronized in 24-well plates at a concentration of 1 × 105 cells per well. The culture medium was then replaced with 400 μL of a solution containing the peptide to be evaluated at a final concentration corresponding to the IC50. Subsequently, the cells were incubated for 2 h. Afterward, the cells were trypsinized for 5 min and transferred to 1.5 mL conical tubes. Following centrifugation at 2,500 rpm, the pellet was dissolved at a concentration of 1 × 105 cells/mL. Next, 100 μL of cell suspension was mixed with 100 μL of the Muse® Annexin V & Dead Cell reagent (Luminex, Lot# B83464). The cells were incubated in the dark at RT for 20 min, and analysis was conducted using the Guava® Muse® Cell Analyzer (Luminex, model MUSE 0500-3115B). For the determination of caspase activity, the cell pellet was dissolved at a concentration of 1 × 105 cells/mL in the buffer 1× from the Muse® MultiCaspase Kit (Luminex, Lot# B82303), and 50 μL of cells were taken and mixed with 5 μL of the multi-caspase working solution, which was incubated for 30 min at 37°C. Subsequently, 150 μL of the 7-AAD working solution was added. Finally, the samples were analyzed using the Guava® Muse® Cell Analyzer.
Hemolysis assays were performed as previously described [32]. The blood was donated by healthy volunteers with blood type O+ 5 mL of peripheral blood in EDTA was centrifuged at 500 rpm for 15 min; the erythrocytes were separated and washed three times with 0.9% saline solution. Then 100 µL of peptide (final concentrations of 6.2 to 200 µg/mL) was mixed with 100 µL of erythrocytes (2% hematocrit) and incubated at 37°C for 2 h. It was then centrifuged at 2,500 rpm for 5 min and the absorbance of the supernatant was measured at 450 nm. Two controls were used; positive control: distilled water and negative control: saline solution 0.9%. Hemolytic activity was determined as the concentration of the peptide to cause 10% hemolysis. Experiments were approved by the institutional ethics committee of the National University of Colombia (code number 05-2019).
A toxicity assay was assessed in a Galleria mellonella model [33, 34]. Briefly, the larvae were obtained from Productos Biológicos Perkins Ltda (Palmira, Valle del Cauca, Colombia). Larvae (n = 10, per group) in the last larval stadium weighing 140–260 mg. The larvae were rinsed with sterile deionized water. The control group was inoculated with 10 μL of saline solution or each peptide. The larvae were maintained in Petri dishes and incubated in darkness at 37°C. The dead larvae were detected by a color change (dead individuals become dark brown) and lack of movement. Survival was monitored every 24 h over a period of 10 days.
Wild-type TABS AB zebrafish were bred in the laboratory facilities, with animals randomly selected. Feeding, breeding, and reproduction procedures were approved by the Institutional Committee for Animal Care and Use of the University of the Andes (CICUAL). The environmental conditions, egg water, were maintained at 28°C, with an electrical conductivity of 650 µS/cm, pH 7.5. Additionally, the procedure followed the Organization for Economic Cooperation and Development (OECD) guidelines for testing chemicals. Tests were conducted in sterile 6-well cell culture plates, and peptide treatment was administered chronically for 72 h, with media changes during exposure. This study utilized three-day post-fertilization (dpf) embryos, following a completely randomized block design. The experiment was conducted at high and low peptide concentrations. Initially, 10 larvae were used for high concentrations. Based on the results obtained, three replicates were subsequently performed, each with 15 larvae for low concentrations. The negative control consisted of egg water, the growth medium for the embryos. This assay was carried out by the Neuroscience and Circadian Rhythms Laboratory of the Faculty of Medicine at the University of the Andes (paid service; ethical committee code 24-01-2020).
Eight male CD1 mice (8 weeks old, weighing 33–35 g) were housed in cages containing four animals each. Among them, three mice received an intraperitoneal injection of the chosen optimized peptide at a final dose of 140 mg/kg, while the remaining three received a dose of 70 mg/kg. One mouse per group served as a control and was inoculated with saline solution. The behavioral test was conducted and the recovery of the animals was monitored for 8 days. At the end of the experiment, animals were under physical euthanasia (cervical dislocation). These experiments were conducted in accordance with resolution 08430 of 1993 from the Ministry of Health and Law 84 of December 27, 1989. Additionally, they followed the principles and norms established by the ethics committee for the care and use of animals, experiments were approved by the institutional ethics committee of the National University of Colombia (Code number 03-2018).
Statistical analysis was performed using the GraphPad Prism software (Version 8). A one-way ANOVA (analysis of variance) was used for comparisons within a single treatment group, while two-way ANOVA was applied for comparisons between different treatment groups, followed by Tukey’s multiple comparisons test. Significance was determined at p < 0.05.
In previous studies, we showed that specific point changes at position 26 of the dimeric peptide 26[F] sequence significantly enhanced its cytotoxic effect against breast cancer cell lines [17, 35]. In this study, we modified the 26[F] peptide sequence (RRWQWRFKKLG)2-K-Ahx, to identify dimeric peptides with improved cytotoxicity against colon cancer cells. The peptides were synthesized using the 6-aminohexanoic residue (Ahx) as a spacer and Fmoc-Lys(Fmoc)-OH, which has both the alpha and epsilon-amino groups protected by Fmoc, allowing the simultaneous growing of the peptide chain in both arms. Modifications included substituting L-Arg with L-Orn (denoted as Orn), and/or L-Arg with D-Arg (denoted as dR) (Table 1).
Dimeric peptides characterization by RP-HPLC and LC-MS
| Code | Sequence | RP-HPLC | Monoisotopic mass | ||
|---|---|---|---|---|---|
| tR (min) | Purity (%) | Theoretical | Experimental | ||
| 26[F] | (RRWQWRFKKLG)2-K-Ahx | 5.9 | 99 | 3,341.964 | 3,341.9736 |
| 1 | (dRRWQWRFKKLG)2-K-Ahx | 6.0 | 88 | 3,341.964 | 3,342.2998 |
| 2 | (Orn-RWQWRFKKLG)2-K-Ahx | 5.9 | 84 | 3,257.995 | 3,258.7513 |
| 3 | (R-Orn-WQWRFKKLG)2-K-Ahx | 5.8 | 90 | 3,257.995 | 3,257.9099 |
| 4 | (RRWQW-Orn-FKKLG)2-K-Ahx | 5.8 | 99 | 3,257.995 | 3,257.7685 |
| 5 | (dR-Orn-WQWRFKKLG)2-K-Ahx | 5.9 | 84 | 3,257.995 | 3,256.8235 |
| 6 | (dRRWQW-Orn-FKKLG)2-K-Ahx | 5.9 | 86 | 3,257.995 | 3,257.8924 |
| 7 | (Ahx-RRWQWRFKKLG)2-K-Ahx | 5.9 | 83 | 3,568.132 | 3,568.1213 |
| 8 | (Biotin-Ahx-RRWQWRFKKLG)2-K-Ahx | 6.1 | 90 | 4,021.452 | 4,020.2752 |
| 9 | (CH3CO-Ahx-RRWQWRFKKLG)2-K-Ahx | 6.3 | 86 | 3,652.142 | 3,652.1590 |
| 10 | (RWQWRWQWR)2-K-Ahx | 7.2 | 99 | 3,195.670 | 3,196.6716 |
| 11 | (Ahx-RWQWRWQWR)2-K-Ahx | 7.1 | 99 | 3,422.838 | 3,422.8376 |
| 12 | (RWQWRWQW-Orn)2-K-Ahx | 7.2 | 97 | 3,111.642 | 3,111.6287 |
| 13 | (CH3CO-RWQWRWQWR)2-K-Ahx | 7.4 | 91 | 3,280.713 | 3,280.6926 |
LC-MS: liquid chromatography-mass spectrometry; RP-HPLC: reverse phase-high performance liquid chromatography; tR: retention time
Peptides were synthesized manually using the SPPS-Fmoc/tBu strategy, with all syntheses conducted in the same reactor when feasible (Figure S1). Purification was performed via RP-SPE (reverse phase-SPE), as previously reported by Cepeda et al. [36]. The purified peptides were highly water-soluble (> 30 mg/mL), and their chromatographic profiles revealed a dominant species with a chromatographic purity range of 83–99%. In all cases, the experimental mass matched the theoretical mass (Table 1, Figure 1, Figures S2–S15). The synthetic process remained consistent across all peptides, with the incorporation of Fmoc-L-Orn(Boc)-OH and Fmoc-D-Arg(Pbf)-OH proceeding similarly to other amino acids. The retention time (tR) of the modified peptides was comparable to the original peptide (26[F]), indicating that their hydrophobicity was not significantly affected (peptides 1–9).
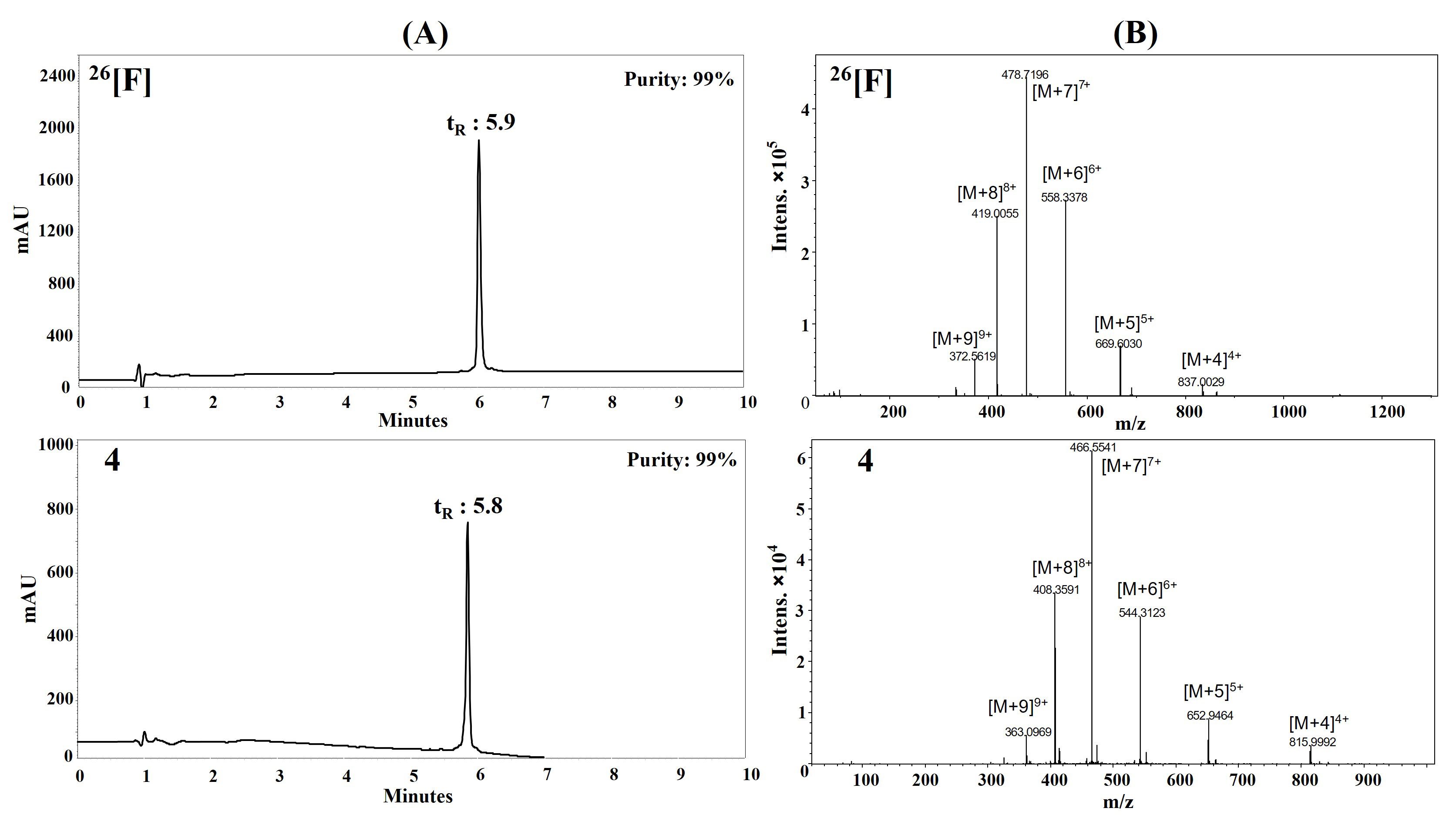
Peptide characterization via RP-HPLC and LC-MS. (A) Chromatographic profiles of peptides 26[F] and 4 display the main peaks with tR = 5.9 min and tR = 5.8 min respectively; (B) the MS spectra show the m/z ratio of the multicharged species ranging from [M+4]4+ to [M+9]9+ for both peptides. LC-MS: liquid chromatography-mass spectrometry; RP-HPLC: reverse phase-high performance liquid chromatography; tR: retention time
The anticancer activity of the modified peptides was evaluated against colon cancer cell lines (Caco-2 and HT-29) (Table 2). Most peptides exhibited anticancer activity against the tested colon cancer cell lines. As peptide concentration increased, cell viability decreased, allowing the determination of IC50 values (the peptide concentration required to reduce cell viability by 50%) for most peptides. Notably, anticancer activity against Caco-2 cells was highest at peptide concentrations of 100 and 200 μg/mL, IC50 values ranging from 10–45 μM.
Cytotoxic effect of the dimeric peptides against colon cancer cells and non-cancer cells (cells were treated for 2 h at 37°C)
| Code | Sequence | IC50 (μM) | Selectivity index | Haemolysis (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HT-29 | Caco-2 | HEK 293 | Fibroblasts | SI1 | SI2 | SI3 | |||
| 26[F] | (RRWQWRFKKLG)2-K-Ahx | 12 | 18* | 22 | 34 | 2.8 | 1.8 | > 5.1 | 6 |
| 1 | (dRRWQWRFKKLG)2-K-Ahx | > 59 | 45 | nd | nd | nd | nd | nd | nd |
| 2 | (Orn-RWQWRFKKLG)2-K-Ahx | 18 | 38 | 25 | 20 | 1.1 | 1.4 | > 3.3 | 3 |
| 3 | (R-Orn-WQWRFKKLG)2-K-Ahx | 30 | 12 | 41 | 17 | 0.6 | 1.4 | > 2.0 | 5 |
| 4 | (RRWQW-Orn-FKKLG)2-K-Ahx | 49 | 10 | > 61 | > 61 | > 1.2 | > 1.2 | > 1.2 | 5 |
| 5 | (dR-Orn-WQWRFKKLG)2-K-Ahx | 20 | 11 | 14 | 9 | 0.5 | 0.7 | > 3.1 | 5 |
| 6 | (dRRWQW-Orn-FKKLG)2-K-Ahx | 35 | 16 | 25 | 23 | 0.7 | 0.7 | > 1.7 | 7 |
| 7 | (Ahx-RRWQWRFKKLG)2-K-Ahx | 19 | 25 | 19 | 29 | 1.5 | 1.0 | > 3.0 | 5 |
| 8 | (Biotin-Ahx-RRWQWRFKKLG)2-K-Ahx | 17 | 20 | 16 | 49 | 2.9 | 0.9 | > 2.9 | 6 |
| 9 | (CH3CO-Ahx-RRWQWRFKKLG)2-K-Ahx | 24 | nd | nd | 19 | 0.8 | nd | > 2.3 | 9 |
SI of peptides is described as the ratio IC50 non-cancer cells/IC50 cancer cells.
When HT-29 cells were treated with the dimeric peptides for 2 h at 200 µg/mL, they showed significant morphological changes compared to untreated cells. The peptide treatment induced loss of extensions, cell shrinkage, condensed cytoplasm, increased vesicles, irregular cell border, and no significant evidence of cellular membrane disruption (Figure 2).
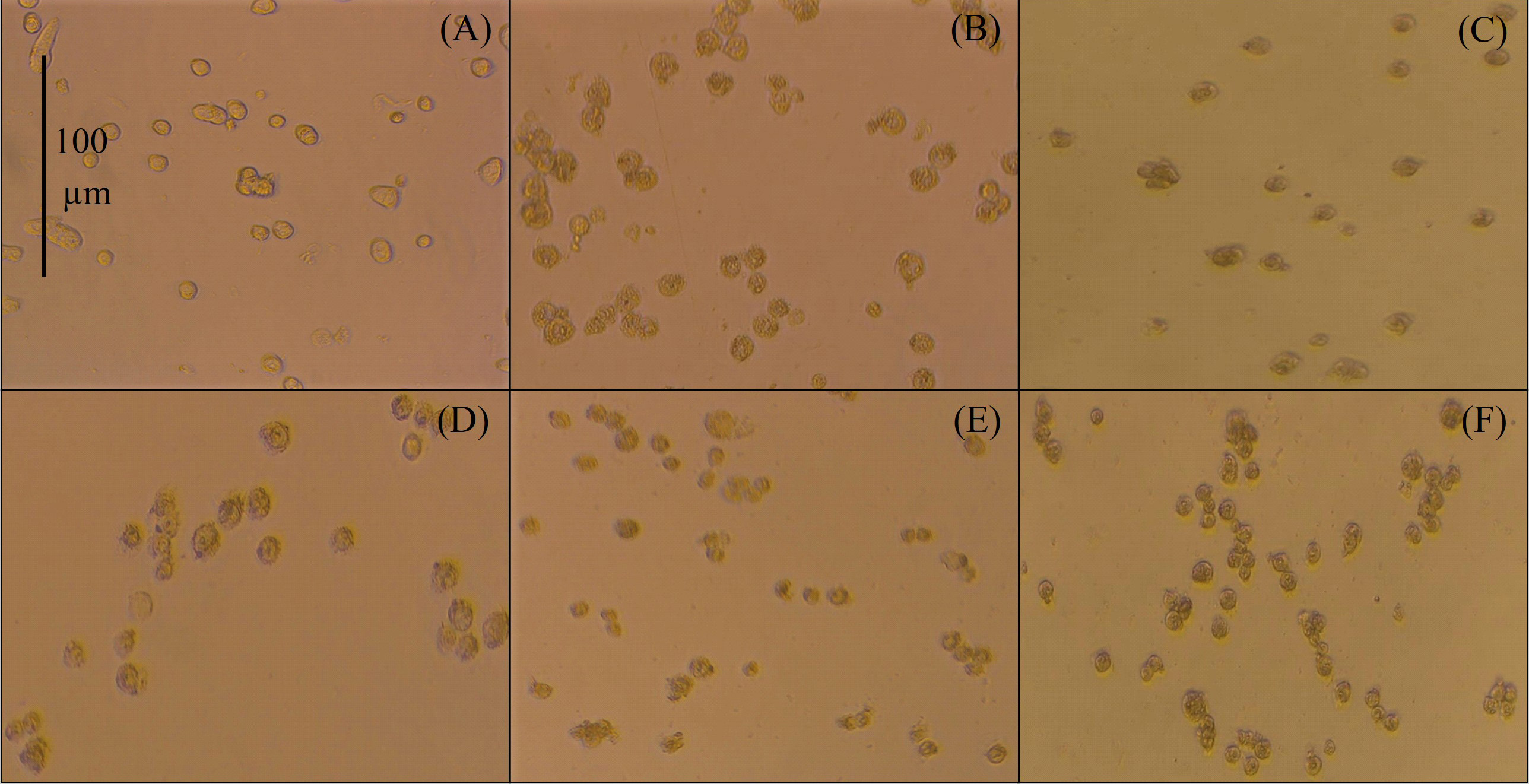
Micrographs by contrast microscopy (using an AxioCam ICc1 camera). 20× of HT-29 cells after 2 h treatment with peptide at 200 µg/mL. (A) Untreated cells; (B) peptide 26[F]; (C) peptide 4; (D) peptide 3; (E) peptide 5; (F) peptide 6
Considering the results of cytotoxic assays of peptide 26[F] and its derivatives against HT-29 and Caco-2 cells, we decided to dimerize the sequences RWQWRWQWR, Ahx-RWQWRWQWR, RWQWRWQW-Orn, and CH3CO-RWQWRWQWR [15], with the aim of confirming the increased cytotoxic effect thanks to dimerization (peptides 10–13). Peptides were synthesized and as previously mentioned, the synthetic process allowed us to obtain high-purity peptides (Table 1). The cytotoxic effect of these peptides was also tested against the less sensitive colon cancer cell line HT-29 (Table 3).
Cytotoxic effect of the dimeric peptides 10–13 against colon cancer cells HT-29 (cells were treated for 2 h at 37°C)
| Code | Sequence | IC50 (μM) | Selectivity index | Haemolysis (%) | ||
|---|---|---|---|---|---|---|
| HT-29 | Fibroblasts | SI1 | SI3 | |||
| 10 | (RWQWRWQWR)2-K-Ahx | 34 | 34 | 1.0 | > 1.9 | 2 |
| 11 | (Ahx-RWQWRWQWR)2-K-Ahx | 49 | 19 | 0.4 | > 1.2 | 1 |
| 12 | (RWQWRWQW-Orn)2-K-Ahx | 12 | 4 | 0.3 | > 5.1 | 1 |
| 13 | (CH3CO-RWQWRWQWR)2-K-Ahx | 12 | > 61 | > 5.0 | > 5.0 | 3 |
Haemolysis (%) is reported at peptide concentration of 200 μg/mL.
The dimeric peptides 10–13 demonstrated cytotoxic effect against HT-29 cells, with IC50 values ranging from 12 to 34 µM. Peptides 12 and 13 exhibited the highest cytotoxicity against HT-29 cells.
To assess the selectivity of dimeric peptides for cancer cells, we performed a cell viability assay on non-cancerous primary fibroblast and HEK 293 cells and assessed hemolytic activity (Tables 2 and 3). The cytotoxic effect of peptide 1 on HEK 293, fibroblasts, and red blood cells (RBCs) was not determined, as this peptide showed no cytotoxicity against HT-29 cells. Peptides 26[F], 2, 4, and 7 showed SI1, SI2, and SI3 values greater than 1, indicating high selectivity for cancer cells. Peptide 13 showed even greater selectivity for HT-29 cancer cells (SI > 5), whereas peptides 10–12 significantly reduced fibroblast cell viability. None of the evaluated peptides caused hemolysis exceeding 7% at 200 μg/mL, and hemolysis at IC50 concentrations (as determined in MTT assays for Caco-2 and HT-29 cells) was minimal, suggesting that peptides at or near IC50 concentrations are non-toxic to human red blood cells.
Given that cytotoxic effects were observed within 2 h of treatment, dimeric peptides (26[F], 2–6, 8, 10, 12, 13) were further evaluated by treating HT-29 cells for 24 h, considering the experimentally determined doubling time of 22.4 h. Results confirmed that cytotoxic activity persisted over time, with some peptides showing a slight reduction in IC50 values after prolonged exposure (Table 4).
Cytotoxic effect of the dimeric peptides in HT-29 cells treated by 2 or 24 h
| Code | Sequence | IC50 (µM) | |
|---|---|---|---|
| 2 h | 24 h | ||
| 26[F] | (RRWQWRFKKLG)2-K-Ahx | 12 | 26 |
| 2 | (Orn-RWQWRFKKLG)2-K-Ahx | 18 | 22 |
| 3 | (R-Orn-WQWRFKKLG)2-K-Ahx | 30 | 10 |
| 4 | (RRWQW-Orn-FKKLG)2-K-Ahx | 49 | 29 |
| 5 | (dR-Orn-WQWRFKKLG)2-K-Ahx | 20 | 23 |
| 6 | (dRRWQW-Orn-FKKLG)2-K-Ahx | 35 | 19 |
| 8 | (Biotin-Ahx-RRWQWRFKKLG)2-K-Ahx | 19 | 22 |
| 10 | (RWQWRWQWR)2-K-Ahx | 34 | 7 |
| 12 | (RWQWRWQW-Orn)2-K-Ahx | 12 | 6 |
| 13 | (CH3CO-RWQWRWQWR)2-K-Ahx | 12 | 13 |
The cytotoxic effect of peptides 3, 4, 6, and 10 on HT-29 cells increased after 24 h of treatment compared to the 2-hour treatment. In contrast, the cytotoxic effect of peptide 26[F] was slightly decreased after 24 h, while peptides 2, 5, 8, and 13 showed consistent cytotoxicity at both 2-hour and 24-hour treatment intervals.
The cytotoxic effect of peptide 4 on HT-29 cells was evaluated at 2, 24, and 48 h of treatment. The effect was greater at 24 h (IC50 = 23 μM) and 48 h (IC50 = 27 μM) compared to 2 h (IC50 = 43 μM) (Figure 3). At the maximum peptide concentration (200 µg/mL), cell viability decreased by 50%, 20%, and 10% respectively. A similar trend was observed for peptides 26[F], 4, 10, 12, and 13.
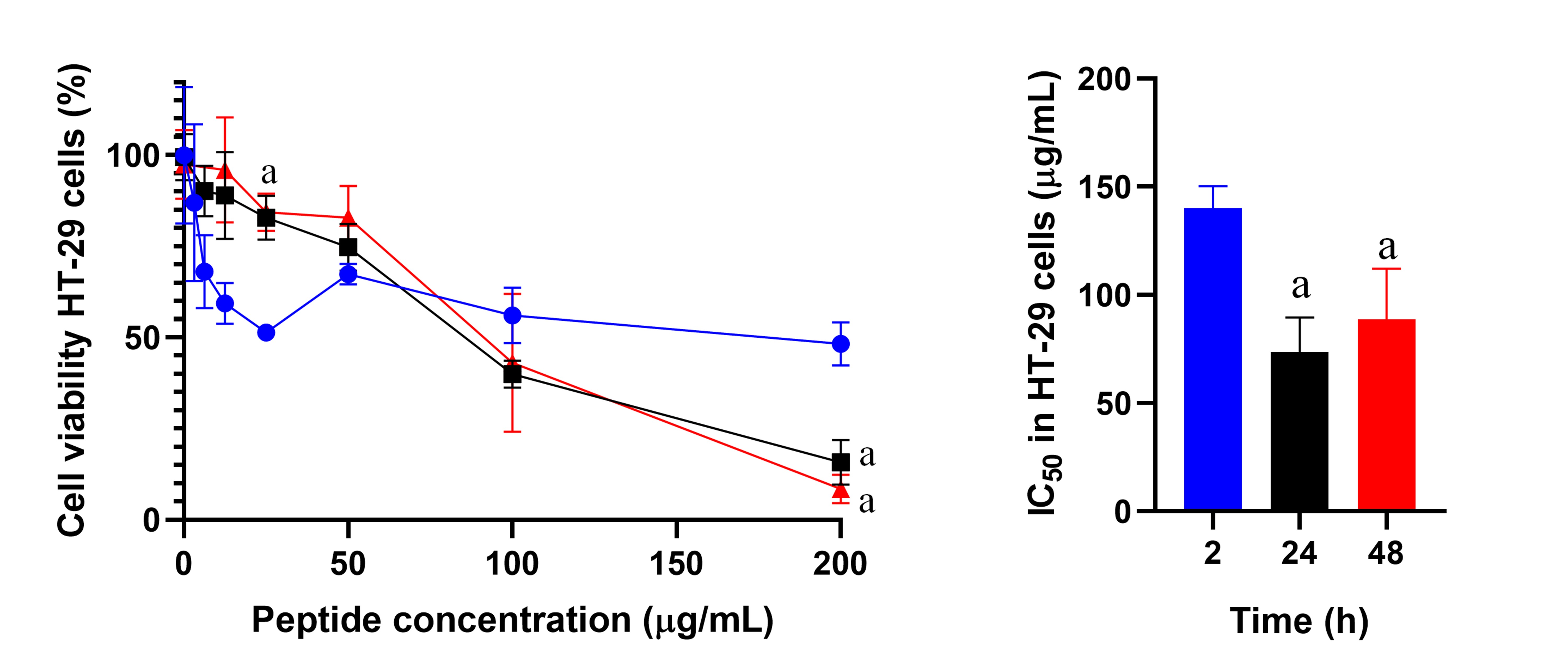
Cell viability of HT-29 cells treated with peptide 4. The cells were treated with peptide 4 (6.25 to 200 µg/mL) for 2 (blue), 24 (black), or 48 h (red). IC50 values were: 2 h (43 μM ± 3 μM), 24 h (23 μM ± 5 μM), and 48 h (27 μM ± 7 μM). Data are expressed as the mean ± SD (n = 3). An ANOVA test was performed followed by Tukey’s multiple comparisons test; statistically significant differences were obtained compared to the 2 h assay of each peptide (the term “a” represents a value of p < 0.05). SD: standard deviation
Building on previous findings about LfcinB-derived peptides against breast, oral, and prostate cancer [15–17, 37], we tested some of the optimized peptides against DU-145 (prostate cancer) and HeLa (cervical cancer) cell lines (Table 5). Peptides 26[F], 3, 4, and 8 were for their high purity, significant cytotoxicity in Caco-2 and HT-29 cancer cells, and SI values. The aim was to determine if these peptides had a broad-spectrum effect on other cancer cell types. Peptides 26[F], 3, and 8 exhibited cytotoxic effects across HT-29, Caco-2, DU-145, and HeLa cells, marking the first report of LfcinB-derived peptides exhibiting cytotoxic effects on prostate cancer cells. Peptide 26[F] was the most selective against HT-29, DU-145, and HeLa cells, while peptide 3 showed consistent cytotoxicity across all tested cancer cells (IC50 = 30 µM) (Table 5).
Cytotoxic effect of the dimeric peptides against DU-145, HeLa, and HT-29 cell lines (2 h)
| Code | Sequence | IC50 (µM) | ||
|---|---|---|---|---|
| HT-29 | DU-145 | HeLa | ||
| 26[F] | (RRWQWRFKKLG)2-K-Ahx | 12 | 17 | 7 |
| 3 | (R-Orn-WQWRFKKLG)2-K-Ahx | 30 | 29 | 29 |
| 4 | (RRWQW-Orn-FKKLG)2-K-Ahx | 49 | > 62 | > 62 |
| 8 | (Biotin-Ahx-RRWQWRFKKLG)2-K-Ahx | 17 | 29 | 14 |
Based on the above results, peptides 26[F], 3, and 8 are considered as the promising candidates. Due to its cost-effective and straightforward synthesis, peptide 3 was selected for scale-up to obtain the quantities needed for in vivo assays. The synthesis was performed using a manual SPPS-Fmoc/tBu strategy, as described in the methodology, following good manufacturing practices (GMP). Three batches were synthesized: batch 01 (100 mg resin), batch 02 (200 mg resin), and batch 03 (700 mg resin). The process was optimized to minimize reagent and solvent usage under controlled conditions (Table S1).
All products were thoroughly characterized using RP-HPLC, LC-MS, FT-IR, and NMR. The chromatographic purity of all batches exceeded 90%, with batches 02 and 03 reaching 95% and 98% purity, respectively (Figure S16). Mass spectrometry confirmed that the experimentally obtained masses matched the theoretical masses, verifying the identity of the synthesized peptides (Figures S17 and S18). In addition to mass spectrometry analysis, the FT-IR spectra of the 02 and 03 batches were obtained. The spectra were superimposed for comparison (Figure S19).
Additionally, ¹H-NMR was used for batch characterization. The ¹H-NMR spectra of the peptide 3 batches showed overlapping signals, with identical chemical shifts, multiplicity, and relative signal intensity (Figures S20–S22). This confirms the consistency of peptide synthesis across batches, supporting their use for further experiments.
To evaluate whether scaling up the synthesis of peptide 3 impacted its cytotoxicity against HT-29 cells, MTT assays were conducted on all three batches. The results indicated similar cytotoxic activity across batches, with IC50 values of 10 µM for batch 01, 9 µM for batch 02, and 6 µM for batch 03 (Figure 4).
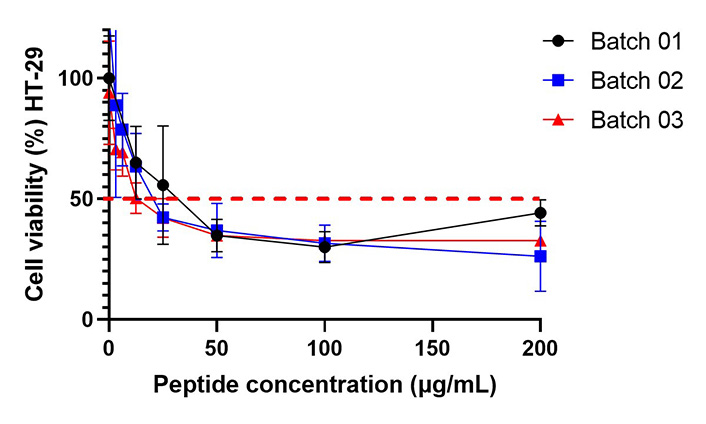
Cell viability of HT-29 cells treated with batches 01 (IC50 = 10 µM), 02 (IC50 = 9 µM), and 03 (IC50 = 6 µM) of peptide 3. Determination was performed using the MTT cell viability assay at 2 h. The dashed red line indicates the point at which cell viability is 50%. The data are expressed as mean ± SD (n = 3). An ANOVA test followed by Tukey’s multiple comparison test was conducted, and no statistically significant differences were obtained among the different treatments. MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SD: standard deviation
To determine the type of cell death induced by the peptides in HT-29 cells, flow cytometry analysis was performed using annexin V staining alongside a cell death marker. The analysis revealed that peptide-induced cell death primarily occurred through early and late apoptosis, with minimal necrosis observed (Figure 5).
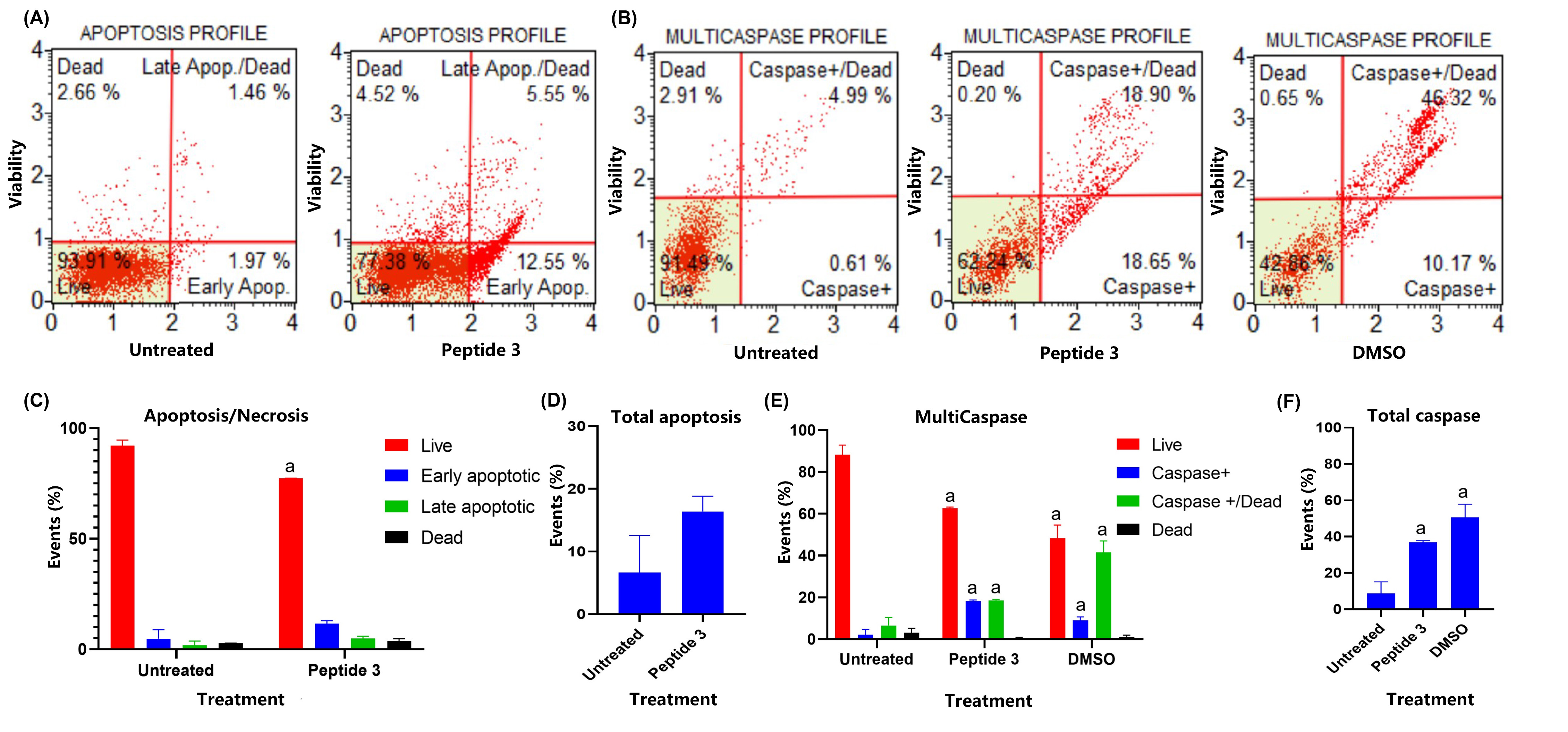
Evaluation of the effect on the HT-29 cell line upon treatment with peptide 3 using the Muse™ Annexin V Kit and the Muse® MultiCaspase Kit. Cells were treated with peptides at the IC50 concentration for 2 h; then cells were treated with Muse™ Annexin V Kit (A) and the Muse® MultiCaspase Kit (B) respectively. Representation of the cell populations, each bar is a representation of the three replicates of each determination (C and E). (D) shows the percentage of cells undergoing apoptosis. (F) shows the percentage of cells with caspase activity. Statistical analysis of the data was performed using ANOVA. The term “a” represents a value of p < 0.001, indicating statistically significant differences compared to the control group (untreated)
In vivo toxicity was evaluated following the 3Rs (reduce, refine, replace) principles [38, 39], starting with an invertebrate model (Galleria mellonella) at a maximum dose of 100 mg/kg. This was followed by acute toxicity testing using a zebrafish model and the Irwin test on mice. The toxicity of peptides 26[F], 3, and 4 was assessed in Galleria mellonella (wax moth) larvae at three concentrations (20 mg/kg, 40 mg/kg, and 100 mg/kg). Larvae survival was tracked for 10 days, showing that survival rates remained above 50% at all concentrations, indicating that the lethal dose (LD50) was not reached within the tested range (Figure 6).
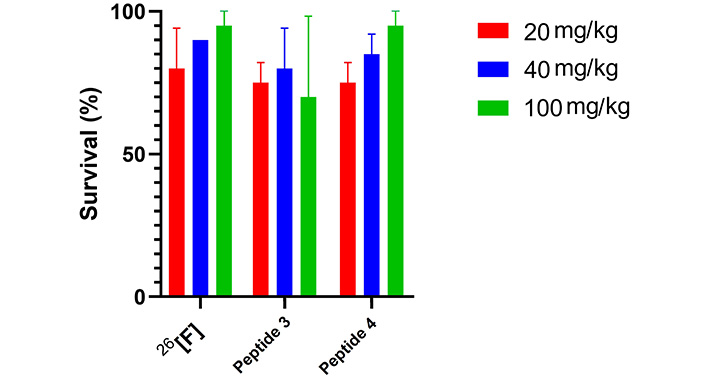
Galleria mellonella acute toxicity assay (n = 2). Survival (%) after 10 days of peptide administration (20, 40, and 100 mg/kg), the survival control was 95%. There are no significant differences among the evaluated groups
We then extended our toxicity assessment to zebrafish larvae at 3 dpf. Larvae were exposed to peptide 3 in the culture medium for 72 h using diffusion administration. Survival rates remained above 96% at concentrations ranging from 0.5 to 15 μg/mL. However, at 20 μg/mL, survival dropped to 80%, and at 25–30 μg/mL, 100% mortality occurred within 24 h of exposure. At even higher concentrations (40–50 μg/mL), rapid mortality was observed within the first 30 min of treatment. The accelerated movement made it impossible to assess other effects, such as head or tail deformation (Figure S23). Most larvae displayed normal morphology, but some showed nonspecific toxic effects, such as twisted tails and pericardial or yolk sac edema, which are common in toxicity evaluations and usually occur at low concentrations. Their predictive value for toxicity in mammalian models is still unclear [40]. The LC50 for peptide 3 was estimated to be 20–25 μg/mL. Comparatively, peptide 3 demonstrated lower toxicity than its derivative, peptide 26[F] [35], as shown in Figure 7.
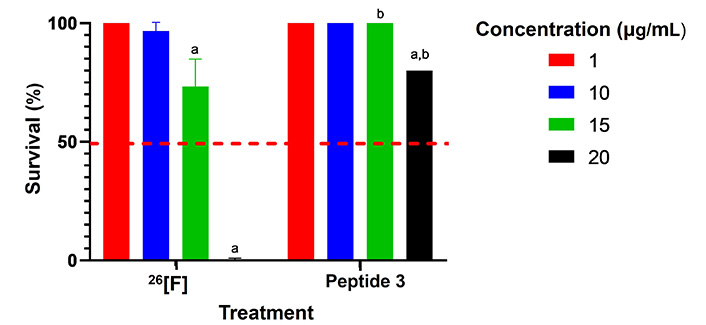
Comparison of survival of zebrafish larvae at different concentrations for peptide 26[F] and peptide 3. In all cases, survival was 100% for the controls and the survival of individuals treated with peptides was greater than 50% (red line). An ANOVA test followed by Tukey’s multiple comparisons test was conducted, yielding statistically significant differences among the different treatments compared to control (p < 0.05) and between peptides (p < 0.0001); the letter “a” mean p < 0.05; the letter “b” mean p < 0.0001
To preliminarily assess the effect of optimized peptide 3 on mice and following the reduction principle, the Irwin test was conducted with two groups of 4 male CD1 mice. In each group, 3 mice were injected with peptide 3 at different doses, and one mouse was injected with saline solution. Group 1 was injected with a dose of 70 mg/kg, and group 2 with a dose of 140 mg/kg. The choice of doses was based on previous reports for the parent peptide 26[F] [35]. Similarly, the injection was performed intraperitoneally, as this route has high bioavailability, allowing a rapid systemic effect.
Immediate effects after administration (< 5 min) were noted in both groups and were mostly associated with local irritation and/or pain from administration. Observed symptoms included contortions and a decrease in spontaneous activity. Over time (15 min), additional effects emerged, predominantly neuromuscular impairment characterized by abnormal posture, reduced eyelid opening, and diminished reactivity to handling. In group 2, these effects became more pronounced, with a complete loss of spontaneous activity at 30 min. Notably, subjects in group 1 managed to recover, showing no signs of toxicity by 30 min (Table 6). A dose-dependent effect was observed, with the most severe effects occurring between 30 and 90 min after administration for group 2. During this period, autonomic effects were noted, impacting respiratory capacity and resulting in the death of two individuals.
Results for the Irwin test. Mice administered with peptide 3 at a dose of 70 mg/kg (group 1; n = 3) or 140 mg/kg (group 2; n = 3)
| Item | Group 1 | Group 2 | ||||
|---|---|---|---|---|---|---|
| < 5 min | 15 min | > 30 min | < 5 min | 15 min | > 30 min | |
| Autonomic | ||||||
| Salivation | ||||||
| Lachrymation | ||||||
| Piloerection | ↑ | |||||
| Excessive urine | ||||||
| Diarrhea | ||||||
| Abnormal breathing | ↓↓ | ↓↓↓ | ||||
| Neuromuscular | ||||||
| Abnormal posture | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓↓ | |
| Muscular tone | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓↓ | |
| Tremors | ||||||
| Seizures | ||||||
| Catalepsy | ||||||
| Straub’s tail | ||||||
| Exophthalmos | ||||||
| Motor-sensory | ||||||
| Touch response | ↓ | ↓ | ↓↓ | ↓↓↓ | ↓↓↓ | |
| Pinna reflex | ||||||
| Tensile response | ↓ | ↓ | ↓ | ↓↓ | ↓↓↓ | |
| Behavioral | ||||||
| Sedation | ↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑↑ | |
| Reactivity to handling | ↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓↓ | |
| Spontaneous activity | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓↓ | |
| Scratching | ↑↑↑ | ↑↑↑ | ||||
| Dead | ||||||
In the Irwing test, differences were observed in the evaluated items compared to the control group (injection with saline solution) (n = 2). The arrow ↑ indicated an increase in the effect and ↓ a decrease. The intensity of the effect was evaluated by the number of arrows present (for example: ↓: mild intensity; ↓↓: moderate intensity; ↓↓↓: high intensity). The colors represent the number of individuals in each group that exhibited the behavior; pink (none), deep pink (one), red (two), and deep red (three)
In this work, dimeric peptides derived from (RRWQWRFKKLG)2-K-Ahx were synthesized, purified, and characterized and their cytotoxic effect on HT-29 and Caco-2 colon cancer lines were evaluated. We modified different parts of the peptide sequence, substituting L-Arg with L-Orn and/or D-Arg, to assess their synthetic viability and potential for enhanced cytotoxic activity against colon cancer cells. The peptides were synthesized using the SPPS-Fmoc/tBu methodology with rink amide resin, optimizing the process to produce multiple peptides from a single reactor. Initially, a reactor was produced (WQWRFKKLG)2-K-Ahx-resin, which was then divided among four reactors for the synthesis of peptides 26[F], 1, 2, and 3. This approach reduced the consumption of Fmoc-amino acids and solvents and shortened the synthesis time.
The dimeric peptides were purified, achieving chromatographic purities between 83–99%. Peptides with purities above 85% are suitable for biochemical and semi-quantitative applications such as enzyme-substrate interactions and biological activity testing [41]. Therefore, we evaluated the cytotoxic effects of all peptides against HT-29 and Caco-2 colon cancer cells.
All dimeric peptides showed cytotoxic effects in both cell lines (HT-29 and Caco-2) except peptide 1 (Table 2). Peptides 3, 4, 5, and 6 had a greater cytotoxic effect against Caco-2 cells than the parent peptide 26[F] (IC50 = 18 μM), indicating that the substitution of L-Arg with L-Orn and/or D-Arg enhanced the cytotoxic effect against these colon cancer cells. The cytotoxic effect of peptides 1, 3, 4, 5, and 6 was more significant in Caco-2 cells than in HT-29 cells (Table 2). Substitution of Arg at the N-terminal end, with L-Orn or D-Arg (peptides 1 and 2), reduced the cytotoxic effect on Caco-2 cells compared with that observed for peptide 26[F], suggesting that this position may not be suitable for modification. Peptides containing the L-Orn residue at positions 21 or 25 (peptides 3, 4, 5, and 6) displayed greater cytotoxic effect against Caco-2 cells than peptides 1 and 2, indicating that replacing L-Arg with L-Orn at these positions is crucial for enhancing the anticancer activity. In addition, the N-terminal capping did not lead to loss of activity as previously reported for linear peptides [15], and N-biotinylation (peptide 8) may serve as a useful tool for further mechanistic studies. The IC50 values of the dimeric peptides 26[F], 3–8 ranged from 10 to 25 μM, suggesting that these molecules have a potent and rapid cytotoxic effect on Caco-2 cells and hold potential for cancer treatment development.
Peptide 26[F] (IC50 = 12 µM) showed the strongest cytotoxic effect against HT-29 cells, while peptides 2 (IC50 = 18 µM), 5 (IC50 = 20 µM), and 8 (IC50 = 17 µM) also showed significant cytotoxic. Unlike the results observed in Caco-2, the substitution of L-Arg at positions 20 or 21 by L-Orn did not increase cytotoxic activity in HT-29 cells, suggesting that these amino acids are relevant to interact with Caco-2 cells. Peptides 3–6 were more cytotoxic to Caco-2 cells while peptides 2, 7, and 8 were more toxic to HT-29 cells. This suggests that the cell-peptide interaction may be mediated by (i) non-specific interactions such as electrostatic attraction between positively charged peptide side chains and negatively charged cell surface molecules that may be common to both cell lines; and/or (ii) receptor-ligand interactions that may be cell-specific.
HT-29 cells treated with peptides exhibited morphological changes, including loss of extensions, cell shrinkage, condensed cytoplasm, increased vesicles, irregular cell border, and no significant evidence of cellular membrane disruption (Figure 2). These morphological changes were consistent across treatments, suggesting that the cells were affected by the dimeric peptides in a similar manner. These changes are associated with the apoptotic process, suggesting that the cytotoxic effect of the peptides in these cells could be primarily mediated by apoptosis. Furthermore, the cell damage was also observed with other types of cancer cells that were treated with linear, dimeric, or hybrid LfcinB-derived peptides, suggesting a consistent mechanism of action for these peptides across cancer lines [15–17].
In all cases, the dimerization of linear peptides led to a decrease in IC50 ranging from 40–70% (peptides 10–12), compared to our previous reports for linear peptides RWQWRWQWR (IC50 = 100 μM), Ahx-RWQWRWQWR (IC50 = 119 μM), and RWQWRWQW-Orn (IC50 = 109 μM) , this behavior has been linked to the need for ACPs to aggregate in order to exert their activity, with dimerization promoting membrane interactions [42, 43]. Interestingly, acetylation of dimeric peptide further improved its cytotoxic effect, whereas acetylation of the linear form caused a complete loss of activity [16]. This finding suggests that dimeric molecules are more resistant to activity loss compared to their linear counterparts.
In summary, the dimeric peptides 26[F], 2–13 demonstrated cytotoxic effects on the tested colon cancer cell lines. The cytotoxic effect was rapid, concentration-dependent, and induced morphological changes in the cells. HT-29 cells were less sensitive to the peptides, which is why additional assays were performed on this cell line.
The cytotoxic effect of the peptides on non-cancer cells HEK 293, fibroblasts, and RBCs was determined. Peptides 26[F], 2–4 were more cytotoxic to colon cancer cells than to HEK 293 cells, with an SI greater than 1. Whereas peptides 26[F], 2, 4, 7, 8, and 13 showed lower cytotoxicity toward fibroblasts compared to HT-29 cells, and peptides 4 and 13 had no cytotoxic effect on fibroblasts at the tested concentrations. SI values greater than 1 indicated that the peptides exhibit higher selectivity for colon cancer cells compared to non-cancerous cells, whereas SI values less than 1 indicated that the peptides affected non-cancerous cells more than cancer cells. Previous reports suggested that cationic peptides preferentially interact with cancer cells via electrostatic interactions with negatively charged molecules on the surface of cancer cells. Modified peptides 5, 6, 9, 11, and 12 had SI values below 1, suggesting that net charge is not the sole requirement influencing cell-peptide interaction and point mutations of Arg/Orn not necessarily increase selectivity. Peptides 26[F], 2, 3, and 4 displayed greater selectivity for cancer cells than peptides 5 or 6, despite having the same net positive charge (+12). Furthermore, the sequences of peptides 3 and 4 (SI > 1) only differ from that of peptides 5 and 6 (SI < 1) in the enantiomeric configuration of the N-terminal Arg. The dimeric peptides 4 and 13 were not toxic to non-cancer cells and had the highest SI being more selective than the unmodified peptide 26[F]. Our results suggested that the cytotoxic activity of the peptides in HEK 293 cells and fibroblasts was not significantly affected by the sequence changes and the differences in the SI value are more related to the activity on cancer cells. Moreover, all peptides had a hemolytic effect of less than 7% at 200 µg/mL, indicating that these peptides are safe within the tested concentration range (6.25–200 µg/mL).
The cytotoxic effect was observed within 2 h of treatment, peptides were further assessed by treating HT-29 cells for 24 h, based on the experimentally determined doubling time of the HT-29 cell line (22.4 h). The results demonstrated that the peptides cytotoxic activity persisted in HT-29 cells, and in some cases, the IC50 values slightly decreased. This suggests that these peptides act rapidly and maintain their activity over time (Table 4). This finding contrasts with molecules like cisplatin, bevacizumab, and irinotecan, which typically reduce cell viability after 48 or 72 h. Additionally, we explored the effect of peptides 26[F], 4, 10, 12, and 13 on cellular proliferation in HT-29 cells at 48 h. Interestingly, the IC50 values further decreased with extended treatment time, even beyond the expected growth period, suggesting that the peptide’s effects persist for 2 to 48 h (Figure 3). This behavior was also observed for LfcinB (21–25)Pal, 26[F] and related peptides, which showed similar cytotoxic effects on Caco-2, HTB-132, and MCF-7 cells, regardless of treatment duration (2, 24, or 48 h) [15, 17]. The extended cytotoxic effect of dimeric peptides, lasting up to 48 h, may be due to peptide stability in the culture medium or internalization causing irreversible damage to the cells, preventing recovery and proliferation (further investigation is needed, and this is beyond the scope of this study).
The cytotoxic effect of peptides 26[F], 3, 4, and 8 was evaluated in DU-145 and HeLa cells. Peptide 26[F] exhibited the most selective cytotoxic effect in HT-29, DU-145, and HeLa cells, as well as in breast cancer cells HTB-132 (IC50 = 13 µM) and MCF-7 (IC50 = 6 µM) [17], suggesting its potential for preclinical studies in broad-spectrum anticancer treatment development. Interestingly, peptide 4 completely lost its cytotoxicity across other cell lines (DU-145 and HeLa), indicating the difference in peptide-cell interactions among cancer types and suggesting its selectivity for colon cancer cells. Peptide 8 (Biotin-Ahx-RRWQWRFKKLG)2-K-Ahx had a selective cytotoxic effect against Caco-2, HT-29, DU-145, and HeLa cells, making it a candidate for use as a fluorescent probe to study cell-peptide interactions. Peptides 26[F], 3, and 8 showed selective and significant cytotoxic effects in Caco-2, HT-29, DU-145, and HeLa cells, with comparable IC50 values, suggesting these peptides are promising candidates for further studies in cancer treatment development. Peptide 3 was selected as a model for preliminary in vivo toxicity studies, requiring scale-up synthesis to produce two additional batches. Batches 02 and 03 were synthesized starting from 200 and 700 mg of resin.
The FT-IR spectra of batches 02 and 03 overlapped, and their functional group regions displayed characteristic signals for the amide I, II, and III regions. The fingerprint regions of both batches were identical in shape and intensity, confirming that they belonged to the same molecule. Batch comparisons using 1H-NMR provided a unique fingerprint for each amino acid sequence, further confirming the identity of the batches. The chromatographic profiles of batches 01, 02, and 03 showed similar tR, with purities above 90%, and the experimental mass matched the expected mass. Characterization of the synthesized peptide batches using various analytical techniques demonstrated that the synthesis scale-up produced enough product without compromising molecular identity or purity. The biological activity of all three batches against HT-29 cells showed similar results (Figure 4), indicating that scaling up the synthesis successfully yielded the required purity for preclinical testing.
Flow cytometry assays were performed to preliminarily determine the type of cell death induced by the dimeric peptides. The results suggested that peptide 3 induces apoptosis in HT-29 cells. This apoptotic behavior was confirmed by evaluating caspase expression levels, which significantly increased compared to untreated cells in all cases. These findings align with previously observed morphological changes in HT-29 cells treated with peptide 3. Additionally, previous reports have shown that lactoferrin (LF) and LfcinB induce apoptosis-mediated cell death in various cell lines [14, 44]. In HT-29 cells, LfcinB, and bovine LF (BLF) induced apoptosis, with transcriptome analysis indicating that LfcinB exerts antitumor activity through several signaling pathways, including p53, apoptosis, and angiopoietin signaling. LFB and LfcinB also upregulated caspase-8, p53, and p21, key proteins in tumor suppression [14, 15, 45]. Similar findings were obtained for peptide 26[F] against the Caco-2 cell line, suggesting that modifications influenced potency but not the type of induced cell death.
Toxicity assays in Galleria mellonella indicated that the peptide concentration required to reach LD50 is greater than 100 mg/kg, which is approximately 40 times the IC50 value. The peptides were not toxic at the concentrations tested, and based on the Loomies and Hayes classification system, they could be considered moderately toxic or less [39]. This larvae assay is believed to provide an accurate prediction of mammalian toxicity [46], reducing the exaggerated toxicity sometimes observed in cell culture studies, and helping to identify highly toxic compounds while reducing the use of vertebrate animals, adhering to the 3Rs principles [38, 39].
The zebrafish toxicity assays offer advantages such as the ability to conduct rapid screening tests at lower costs with a reduced demand for the test substance. Zebrafish are a complex vertebrate model with high reliability and similarities in molecular pathways and physiology to humans, making them useful as a toxicity screening tool before progressing to more comprehensive studies in higher animals [47]. The LC50 of peptide 3 was between 20–25 μg/mL, indicating lower toxicity in this model and establishing a concentration range for future efficacy studies.
Irwin’s test was conducted to evaluate the effects of peptide 3 after intraperitoneal administration in mice. High doses induced severe motor impairment, potentially due to adverse effects associated with high-dose administration. These findings are consistent with previous reports for peptide 26[F], where intraperitoneal administration caused reduced motor function, abdominal contractions, ataxia, and loss of coordination [48]. It is speculated that these effects may involve the central nervous system, as has been observed for lactoferrin [49–51], though this requires further investigation and is beyond the scope of this study.
In conclusion, this study identified dimeric peptides with significant and selective cytotoxic effects against colon cancer cell lines. The cytotoxic effect was rapid, concentration-dependent, and persisted for up to 48 h. Peptides 26[F], 3, and 8 exhibited broad-spectrum cytotoxicity against HT-29, Caco-2, HeLa, and DU-145 cells, suggesting their potential as candidates for expanding therapeutic options in cancer treatment. Peptide 3 induced apoptosis-mediated cell death, and its moderate toxicity indicates that it can be considered for preclinical studies in developing peptide-based anticancer drugs.
3Rs: reduce, refine, replace
6-Cl-HOBt: 6-chloro-1-hydroxybenzotriazole
ACN: acetonitrile
ACPs: anticancer peptides
ANOVA: analysis of variance
Arg: arginine
DCC: N,N-dicyclohexylcarbodiimide
DCM: dichloromethane
DMEM: Dulbecco’s modified Eagle’s medium
DMF: N,N-dimethylformamide
dpf: day post-fertilization
EDT: 1,2-ethanedithiol
EDTA: ethylenediaminetetraacetic acid
FBS: fetal bovine serum
Fmoc: 9-Fluorenylmethoxycarbonyl
FT-IR: Fourier transform infrared
LC-MS: liquid chromatography-mass spectrometry
LD50: lethal dose
LfcinB: bovine lactoferricin
Lys: lysine
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
NMR: nuclear magnetic resonance
Orn: ornitine
RBC: red blood cells
RP-HPLC: reverse phase-high performance liquid chromatography
RPMI: Roswell Park Memorial Institute
RT: room temperature
SPE: solid phase extraction
SPPS: solid-phase peptide synthesis
TFA: trifluoroacetic acid
TIPS: triisopropylsilane
tR: retention time
The supplementary figures and table for this article are available at: https://www.explorationpub.com/uploads/Article/file/100891_sup_1.pdf.
KJCM: Conceptualization, Methodology, Investigation, Formal analysis, Writing—original draft, Visualization, Writing—review & editing. JERC, YVC, and LFOG: Formal analysis, Resources, Writing—original draft. CMPG: Formal analysis, Resources, Writing—original draft. ACBC: Formal analysis, Resources. RFM: Conceptualization, Supervision, Funding acquisition. JELM: Conceptualization, Methodology, Writing—review & editing. ZJRM: Conceptualization, Methodology, Resources, Writing—review & editing, Funding acquisition. JEGC: Conceptualization, Methodology, Formal analysis, Resources, Writing—original draft, Visualization, Funding acquisition. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
For hemolytic activity (code 05-2019) and Irwin test (code 03-2018) were approved by the institutional ethics committee of the National University of Colombia. Zebrafish experiments were approved by the Institutional Committee for Animal Care and Use of the University of the Andes, code 24-01-2020.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This research was conducted with the financial support of MinCiencias with the projects: “Obtención de un prototipo peptídico promisorio para el desarrollo de un medicamento de amplio espectro para el tratamiento del cáncer de colon, cuello uterino y próstata”. Contract RC No. [845-2019]. “Desarrollo de un medicamento contra el cáncer de mama basado en un péptido polivalente derivado de la LfcinB: Estudio de la fase preclínica (fase cero), caracterización fisicoquímica de un lote del fármaco para estudios preclínicos”. RC No. [706-2018]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1693
Download: 30
Times Cited: 0
Constanza Cárdenas ... Fanny Guzmán
Andrea Barragán-Cárdenas ... Javier García-Castañeda
Natalia Ardila-Chantré ... Javier Eduardo García-Castañeda
Jésica A. Rodríguez ... Silvia A. Camperi
Lucero Ruiz-Mazón ... Mireya de la Garza
Vaezeh Fathi Vavsari, Saeed Balalaie
Vanesa Herlax
Daniela Perdomo-Joven ... Mauricio Urquiza-Martinez
