Affiliation:
1Department of Clinical Biochemistry, University Hospitals Birmingham NHS Foundation Trust, B75 7RR Birmingham, UK
ORCID: https://orcid.org/0000-0002-7972-4794
Affiliation:
2School of Pharmacy & Bioengineering, Keele University, ST5 5BG Newcastle-under-Lyme, UK
ORCID: https://orcid.org/0000-0002-0980-6348
Affiliation:
3School of Health and Life Sciences, Aston University, B4 7ET Birmingham, UK
ORCID: https://orcid.org/0000-0003-2073-3001
Affiliation:
4Department of Mechanical and Aerospace Engineering, Brunel University London, UB8 3PH London, UK
ORCID: https://orcid.org/0000-0002-9289-3154
Affiliation:
1Department of Clinical Biochemistry, University Hospitals Birmingham NHS Foundation Trust, B75 7RR Birmingham, UK
2School of Pharmacy & Bioengineering, Keele University, ST5 5BG Newcastle-under-Lyme, UK
4Department of Mechanical and Aerospace Engineering, Brunel University London, UB8 3PH London, UK
5Department of Clinical Biochemistry, University Hospitals of North Midlands NHS Foundation Trust, ST4 6QG Stoke-on-Trent, UK
Email: sud.ramachandran@uhb.nhs.uk
ORCID: https://orcid.org/0000-0003-2299-4133
Explor Endocr Metab Dis. 2024;1:83–100 DOI: https://doi.org/10.37349/eemd.2024.00010
Received: October 13, 2023 Accepted: February 06, 2024 Published: June 28, 2024
Academic Editor: Marijn Speeckaert, Universitair Ziekenhuis Ghent, Belgium
The article belongs to the special issue The Fountain of Youth: Decoding the Hormonal Regulation of Aging
Adult-onset testosterone deficiency (TD) in men is diagnosed by the finding of low serum testosterone levels and recognised, associated symptoms. The condition has high prevalence in men over 50 years of age, particularly those with type 2 diabetes (T2DM). Accumulating data show adult-onset TD is associated with increased mortality risk. We review the literature and consider the evidence suggesting testosterone therapy (TTh) reduces mortality, especially in men with T2DM. We previously reported that in the Burntwood Lichfield Atherstone Sutton Coldfield Tamworth (BLAST) study screened cohort of men with adult-onset TD and T2DM adult-onset TD was associated with increased mortality with TTh decreasing this higher mortality. The data hinted that the effect was greater in older men. We confirmed this observation with statistical analyses to study the effect of age on the association between adult-onset TD and mortality; Cox regression analysis demonstrated that the reduced risk (hazard ratio: 0.61, 95% CI: 0.38–0.96) following TTh was restricted to men above the median age of 65.89 years. Finally, we speculate on putative mechanisms that may mediate these associations. Heterogeneity in men with adult-onset TD is expected in view of its definition of low testosterone levels together with associated clinical phenotypes that are not always directly related. Many of these classifying phenotypes are associated with increased mortality. Thus, it is perhaps possible that mechanism(s) of all-cause mortality reduction following TTh is via the impact on these associated phenotypes such as the metabolic syndrome (MetS), hyperglycaemia, hypertension, dyslipidaemia, low haematocrit, sex hormone binding levels, erectile dysfunction, etc. We propose that further research studying the effect of TTh takes heterogeneity into account.
In 2022, Zhao and Crimmins speculated on possible biological, behavioural and environmental reasons for the world-wide disparity between male and female lifespans [1, 2]. They described an age-related pathway of morbidity/mortality at a population level (updating the model proposed by Crimmins et al. in 2019) [2, 3]. The process is viewed as proceeding sequentially in the following order; molecular/ageing, physiological deterioration, diseases/conditions, frailty/disability/functioning loss and finally death [2, 3]. In view of testosterone levels diminishing with age in adult-onset testosterone deficiency (TD), we examine how mortality, the final stage of the above sequence, is affected by diminishing hormone levels and testosterone therapy (TTh). This review is based on data from basic science, longitudinal and randomised controlled trials (RCT) and reviews familiar to our research group or selected from PubMed (US National Library of Medicine) and described in Table 1. To study the impact of age on the associations between adult-onset TD and all-cause mortality, we further analysed data from the Burntwood Lichfield Atherstone Sutton Coldfield Tamworth (BLAST) study screened cohort previously carried out by our group.
A brief description of the principal studies describing factors associated with all-cause mortality in this review
| Factors associated with mortality | Evidence | Main finding | Reference |
|---|---|---|---|
| Serum TT | Holmboe et al. (MONICA10 study) | Lower (< 10th percentile) serum TT was associated with higher mortality | [9] |
| Pye et al. (EMAS) | Serum TT < 8 nmol/L & ≥ 3 symptoms was associated with higher mortality | [6] | |
| Araujo et al. (systematic review/meta-analysis) | Lowest tertile of serum TT was associated with higher mortality | [10] | |
| Muraleedharan et al. | Serum TT < 10.4 nmol/L in men with T2DM demonstrated higher mortality | [11] | |
| Hackett et al. (BLAST screened cohort) | Serum ≤ 12.0 nmol/L or FT ≤ 0.25 nmol/L in men with T2DM associated with higher mortality | [12, 13] | |
| TTh | Vigen et al. | Increased mortality, myocardial infarction and strokes in men (and 100 women) on TTh | [14] |
| Finkle et al. | Mortality was higher 3 months post-TTh compared to 12 months pre-TTh | [15] | |
| Basaria et al. (TOM trial) | Twenty-three men on TTh developed CVD related adverse events compared to 5 men on placebo | [16] | |
| Shores et al. | TTh in men with serum TT ≤ 8.7 nmol/L was associated with lower mortality | [20] | |
| Muraleedharan et al. | TTh in men with serum TT ≤ 8.7 nmol/L was associated with lower mortality | [11] | |
| Hackett et al. (BLAST screened cohort) | TTh in men with serum ≤ 12.0 nmol/L or FT ≤ 0.25 nmol/L and T2DM was associated with lower mortality | [12] | |
| Haider et al. | TTh in men with serum ≤ 12.0 nmol/L and T2DM was associated with lower mortality | [21] | |
| Hudson et al. (systematic review/meta-analysis) | TTh not associated with change in mortality risk compared to placebo over a mean follow-up of 9.5 months | [22] | |
| PDE5 inhibitors | Hackett et al. (BLAST screened cohort) | PDE5 inhibitor treatment in men with T2DM was associated with lower mortality | [12, 13] |
| Andersson et al. | PDE5 inhibitors in men with ED post first myocardial infarction was associated with lower mortality | [18] | |
| Anderson et al. | PDE5 inhibitor use was associated with lower mortality | [19] | |
| Kloner et al. | PDE5 inhibitor use in men with T2DM was associated with lower mortality | [53] | |
| SHBG | Tint et al. | Higher SHBG levels were associated with increased mortality | [34] |
| Ramachandran et al. (BLAST screened cohort) | Higher SHBG levels were associated with increased mortality | [35] | |
| HCT | Gagnon et al. | High HCT was associated with increased mortality | [40] |
| Boffetta et al. | Possible ‘U’ shaped relationship between HCT and mortality in men and women | [41] | |
| Locatelli et al. | Increase in HCT following erythropoietin therapy was associated with lower mortality | [42] | |
| Strange et al. | Lower mortality was seen in men with HCT between 50–52% following TTh compared to me with HCT ≤ 49% | [45] | |
| Ory et al. | HCT > 52% was associated with increased CVD and no significant increase in mortality | [46] | |
| ED | Dong et al. (meta-analysis) | ED was associated with CVD and all-cause mortality | [52] |
| CAG repeats | Heald et al. (EMAS) | A ‘U’ shaped association between CAG repeat numbers and mortality in men with serum TT < 14.2nmol/L | [89] |
BLAST: Burntwood Lichfield Atherstone Sutton Coldfield Tamworth; CVD: cardiovascular disease; ED: erectile dysfunction; EMAS: European Male Ageing Study; FT: free testosterone; HCT: haematocrit; PDE5: phosphodiesterase type 5; SHBG: sex hormone binding globulin; T2DM: type 2 diabetes; TTh: testosterone therapy
According to the British Society for Sexual Medicine 2023 guidelines, adult-onset TD in men is described by low serum testosterone [total testosterone (TT) < 12.1 nmol/L and/or free testosterone (FT) < 0.225 nmol/L] together with symptoms attributable (some of these are shown in Figure 1) to this low hormone concentration, after exclusion of primary hypogonadism [luteinising hormone (LH) and clinical presentation will indicate likelihood] and hypothalamic-pituitary-gonadal axis pathology (pituitary profile including LH and clinical presentation will suggest likelihood) [4]. Sex hormone binding globulin (SHBG) values are taken into account when diagnosing adult-onset TD as FT (which is usually calculated and requires SHBG in the algorithm) is included in the definition [4]. Prevalence of this syndrome is between 0.6–12% in men > 50 years of age and is substantially higher (40–70%) in men with type 2 diabetes (T2DM) [4–7]. The European Male Ageing Study (EMAS) demonstrated a gradual increase in prevalence of adult-onset TD using serum TT and FT thresholds of < 11 nmol/L and < 0.22 nmol/L respectively with advancing age; 0.1%, 0.6%, 3.2%, and 5.1% in men aged 40–49 years, 50–59 years, 60–69 years, and 70–79 years respectively [8]. In addition to the classifying symptoms, adult-onset TD is associated with comorbidities such as obesity, insulin resistance/T2DM, osteoporosis, anaemia as well as all-cause mortality [4].
Data from the Danish Monitoring Trends and Determinants of Cardiovascular Disease (MONICA10) study evaluated associations between mortality and changes in serum TT, FT, SHBG and LH in 1,167 men aged between 30–60 years over a mean follow-up period of 15.2 years, with mortality occurring in 36.1% (421 men) of the men [9]. Interestingly higher baseline serum TT and FT levels were associated with lower mortality during follow-up. The men were stratified into tertiles based on extent of change in levels of the serum TT and FT; < 10% (lowest tertile), 10–90% (reference category), and > 90% (highest tertile). Mortality in the lowest tertile was significantly higher than the reference category based on changes in serum TT [hazard ratio (HR): 1.60, 95% confidence interval (CI): 1.08–2.38] and FT (HR: 1.45, 95% CI: 1.09–1.92) [9].
Analysis from EMAS of 2,599 men aged 40–79 years in 2014 showed individuals with ≥ 3 hypogonadal symptoms and low TT levels (< 8 nmol/L) after adjustment for age, recruitment site, smoking status and general health predicted both cardiovascular disease (CVD) mortality (HR: 3.8, 95% CI: 1.3–10.8) and all-cause mortality (HR: 3.1, 95% CI: 1.7–5.7) over a median follow-up of 4.3 years [6]. Updated EMAS data on 1,788 men showed that various phenotypes defining sexual health (independent of serum TT and FT), were significantly associated with mortality over a mean follow-up of 12.6 years in multivariate statistical models using combinations of TT, FT and sexual symptoms as factorised independent variables, adjusted for recruitment site, body mass index, alcohol intake, smoking status, physical activity and comorbidities [7].
Araujo et al. in 2011 [10], prior to the Danish Monitoring Trends and Determinants of Cardiovascular Disease study and EMAS, carried out a systematic review of 21 studies with 12 of these used for meta-analysis with CVD mortality (7 studies, 11,831 individuals) and all-cause mortality (11 studies, 16,184 individuals) as outcomes [10]. Compared to the highest tertile of serum TT distribution (reference), the lowest tertile was significantly associated with all-cause mortality (relative risk: 1.35, 95% CI: 1.13–1.62) [10]. Importantly there appeared significant inter-study heterogeneity; thus, baseline phenotypes such as age could possibly influence the mortality observed in individual studies.
In 2013, Muraleedharan et al. [11] studied mortality rates in 581 men with T2DM who had serum TT checked between 2002–2005 over a mean follow-up of 5.8 years. The men were stratified by a baseline serum TT of 10.4 nmol/L. All-cause mortality was significantly higher (HR: 2.02, 95% CI: 1.2–3.4) in men with baseline serum TT ≤ 10.4 nmol/L compared to those with higher levels, the analysis adjusted for body mass index, haemoglobin A1c (HbA1c), pre-existing CVD, smoking status, statin therapy and angiotensin-converting enzyme inhibitors/angiotensin receptor blocker use [11].
Hackett et al. [12, 13] used the BLAST screened cohort of 857 men with T2DM to study the association between serum TT/FT levels and all-cause mortality. Subjects were stratified into normal (320 men with serum TT > 12 nmol/L, FT > 0.25 nmol/L) and low (537 men with serum TT ≤ 12.0 nmol/L or FT ≤ 0.25 nmol/L) testosterone groups. The latter group of 537 men was further stratified into 362 men not on TTh and 175 men prescribed testosterone undecanoate (TU) [12]. Mortality over a mean follow-up of 3.8 years was significantly lower in the normal testosterone (11.3%) compared with low testosterone group not prescribed TU (16.9%), this was evident from the Cox regression analysis adjusted for baseline age (HR: 0.62, 95% CI: 0.41–0.94).
Mortality rates in the BLAST screened cohort suggested that the increased mortality associated with adult-onset TD not on TTh (682 men) and especially the decrease in mortality following TTh was more pronounced in older men [12, 13]. Accordingly, we stratified the men not on TTh by the median age at study commencement (65.89 years) and carried out a survival analysis in the normal and low testosterone categories in each age category, with the null hypothesis being that no survival difference was evident. The association between serum testosterone and mortality in the subgroup not on TTh appeared significant only in men over the median age. Cox regression model (adjusted for age) showed that men with normal testosterone levels (compared to men with low testosterone) demonstrated significantly lower mortality (HR: 0.61, 95% CI: 0.38–0.96) in men > 65.89 year, but not (HR: 0.74, 95% CI: 0.28–2.00) in men ≤ 65.90 years. Kaplan-Meier plots (based on Cox regression models excluding baseline age) are shown in Figures 2 and 3. The age distribution did not differ (P = 0.12, unpaired t-test) between subgroups stratified by median age. Details of the unadjusted Cox regression models which the Kaplan-Meier plots are based on are found in the footnotes of Figures 2 and 3.
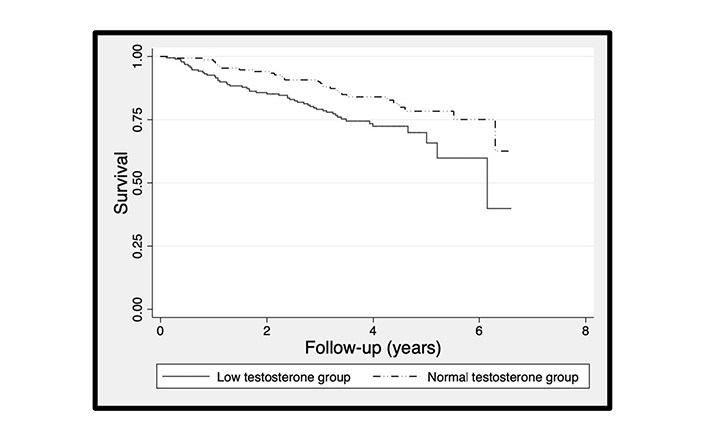
The Kaplan-Meier plots comparing survival of 341 men > 65.89 years of age with T2DM stratified into normal and low testosterone groups. The Kaplan-Meier plot was based on the Cox regression analysis which showed that the normal testosterone group (compared with the low testosterone group) was significantly lower (HR: 0.56, 95% CI: 0.35–0.89)
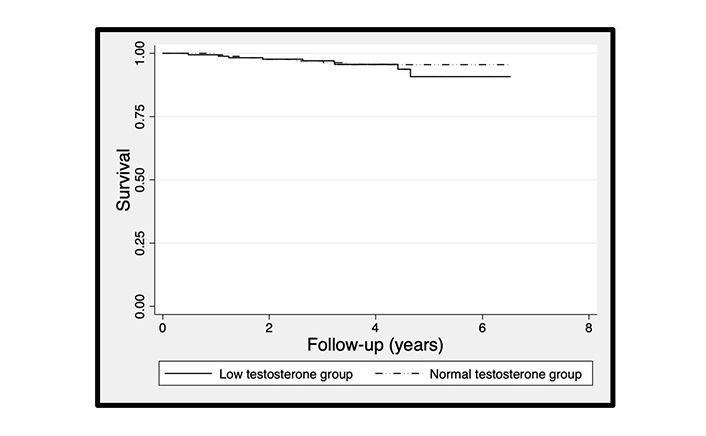
The Kaplan-Meier plots comparing survival of 341 men ≤ 65.90 years of age with T2DM stratified into normal and low testosterone groups. The Kaplan-Meier plot was based on the Cox regression analysis which showed that the normal testosterone group (compared with the low testosterone group) was not significantly lower (HR: 0.74, 95% CI: 0.28–2.00)
Having presented data showing hypogonadism/low testosterone is associated with all-cause mortality, we will consider the impact of restoring testosterone levels on mortality in men with adult-onset TD.
There are a few studies reporting higher all-cause mortality and CVD in men on TTh, though their design and methodology have been widely criticised [14–17]. For example, Vigen et al. [14] in 2013 studied 8,709 patients with serum TT < 10.4 nmol/L enrolled in the Veterans Administration healthcare system who had undergone coronary angiography and subsequently received TTh. They used a composite of all-cause mortality, myocardial infarction, and stroke rates as the outcome [14]. The event rate was lower in those on TTh; 10.1% in testosterone treated and 21.2% in untreated patients over 3 years of follow-up. However, adjustment with over 50 variables led to the rates being reversed. TTh now appeared associated with increased events (25.7% in testosterone treated, 19.9% in untreated men). Importantly, baseline testosterone levels and erectile dysfunction (ED), both factors associated with all-cause mortality, were not included in the analysis. The authors acknowledged that 100 women were included in the original all-male cohort.
Though the principal outcome we focus on is all-cause mortality, it is worth describing other studies that have added to the safety concerns raised by Vigen et al. [14] which could potentially affect TTh compliance. Finkle et al. [15] also in 2014 analysed 55,593 insurance claims and compared myocardial infarction incidence rates during the 12 months and 3 months pre and post TTh initiation respectively [15]. They found an increase in non-fatal myocardial infarctions, mainly in men aged ≥ 65 years whilst, in the younger counterpart, the risk was mainly evident in men with pre-existing heart disease [15]. However, the comparator cohort used men on phosphodiesterase type 5 (PDE5)-inhibitors, which we and others have shown to decrease cardiovascular and all-cause mortality [12, 13, 18, 19]. Hence, any significant differences could have resulted from changes in outcome in controls [15]. Issues in methodology also included unavailability of serum TT pre and post TTh. Additionally, the 3 months post therapy period seems insufficient to study CVD events that could have been a legacy of adult-onset TD. This was appropriately acknowledged by the authors.
A few years previously Basaria et al. [16] reported outcomes from the ‘Testosterone in Older men with Mobility Limitations (TOM) Trial’ of 209 men (mean age: 74 years) with restricted mobility randomised to either testosterone or placebo gel. The primary outcome, change in maximal voluntary muscle strength during leg press exercise, was significantly different between groups [16]. The trial was discontinued prematurely as 23 men given TTh and 5 men given placebo appeared to develop CVD related adverse events. These events in a relatively small group of men with no baseline CVD assessment were wide ranging (even including syncope and peripheral oedema) and classification was based on self-reports not subsequently verified [16].
In contrast there is an increasingly large body of data from longitudinal studies, that TTh in addition to improving sexual health, reduces all-cause mortality. Shores et al. [20] showed in 1,031 men aged over 40 years with serum TT ≤ 8.7 nmol/L that over a 4 years follow-up, all-cause mortality appeared lower in 398 men prescribed TTh (10.3%) compared to 633 untreated counterparts (20.7%) after taking into account confounding variables [20]. Significance in subgroup analysis was only evident in men with T2DM and those without coronary heart disease (CHD), suggesting cohort heterogeneity was a factor mediating the association between TTh and all-cause mortality. This finding in men with T2DM has been supported by other longitudinal studies [11, 12, 21].
Muraleedharan et al. [11], in addition to studying mortality associated with low serum TT, also investigated the effect of TTh on all-cause mortality. Of the 238 men with baseline serum TT ≤ 10.4 nmol/L, the 174 men not on TTh were at significantly higher risk of mortality (HR: 2.3, 95% CI: 1.3–3.9) compared to 64 men given TTh [11]. The BLAST screened cohort contained 175 men with T2DM and low serum TT who were treated with TU [12]. Mortality in these men was significantly lower (HR: 0.38, CI: 0.16–0.90) than in the 362 men with low serum TT who remained untreated, the Cox regression model was adjusted for baseline age [12]. As baseline age significantly differed (P < 0.0001, unpaired t-test) between the treated and untreated men with low serum TT, we refrained from plotting a Kaplan-Meier graph based on a Cox regression model, unadjusted for baseline age. Haider et al. [21] studied 356 men with T2DM, symptoms of TD and serum TT ≤ 12.1 nmol/L with 178 men receiving TU (median follow-up of 8 years) whilst the remaining 178 men were not on TTh (median follow-up of 10 years) [21]. Mortality was significantly lower (P < 0.0001) in the men on TU (7.3%) compared to the untreated men (27.0%). Hudson et al. in 2022 [22] published a systematic review/meta-analysis of 35 RCTs (with 17 providing individual participant datasets) using testosterone preparations with a mean follow-up of 9.5 months (inclusion criteria for follow-up over 3 months) in men >18 years of age. Mortality data were available in 14 RCTs and though lower in the 1,621 men (0.4%) on TTh compared to those on placebo (0.8%), statistical significance was not reached [odds ratio (OR): 0.46, 95% CI: 0.17–1.24, P = 0.13) [22].
The data above indicate that at least in some men with adult-onset TD, low serum testosterone is associated with increased mortality and that mortality risk can be reduced by TTh. The mechanism(s) for increased mortality associated with adult-onset TD and reversal following TTh has not been elucidated. Clinical presentation phenotypes due to low testosterone levels will depend not just on serum hormone concentration, but also other factors such as the SHBG affecting FT levels, androgen receptor sensitivity, etc. [4]. The definition used to classify adult-onset TD is pragmatic as the addition of symptoms to low serum TT may be considered a summation of factors both unknown such as validity of the free hormone hypothesis and those not measured in routine clinical practice such as CAG repeats in the androgen receptor. However, this flexible classification can lead to presentation heterogeneity which can lead to confusion as many of the clinical outcomes can be restricted to subgroups. Hence, when evaluating clinical outcomes in adult-onset TD and following TTh it is useful to also study these outcomes in subgroups stratified by the symptoms that resulted in the diagnosis.
Adult-onset TD has been associated with the metabolic syndrome (MetS) and we have previously considered whether TD should be included as one of its classifying features [23]. The MetS, as classified in 2009, describes a cluster of presentation phenotypes; central obesity (defined by ethnicity dependent waist circumference cut-off values), which appears a principal driver of the condition, and at least two of the following 4 risk factors: triglyceride ≥ 1.7 mmol/L, high density lipoprotein cholesterol < 1.03 mmol/L (males) or < 1.29 mmol/L (females), blood pressure ≥ 135/85 mmHg and fasting plasma glucose ≥ 5.6 mmol/L [24]. The MetS has been associated with CVD and TTh has been shown to improve the classifying presentation phenotypes, which are predictors of CVD [25]. Thus, an attractive hypothesis would be that TTh reduces all-cause mortality via a positive impact on these classifying cardiometabolic risk factors. TTh also appears to increase insulin sensitivity [26, 27]. However, as with adult-onset TD, we expect MetS to include significant heterogeneity based on the classification. Furthermore, many of the classifying factors are dichotomized and could affect the syndrome as a factor predicting clinical outcomes. The effect of TTh in men with the MetS could be affected by heterogeneity observed in both adult-onset TD and the MetS.
However, in the BLAST screened cohort we showed that the decreased mortality following TU did not appear to be mediated by CVD risk factors [28]. Whilst acknowledging the lack of evidence of the association between TTh and all-cause mortality in RCTs, we speculate that the scale of mortality reduction seen in these studies appears to be in excess of the prevalence of CVD deaths (clearly caution must be exercised when considering HR values in such studies) [29, 30]. Further, there are no clear data that TTh is associated with a reduction in CVD, although as mentioned previously cohort heterogeneity affecting outcomes may be present.
Corona et al. [31] demonstrated higher CVD in frail men and those provided higher than recommended TTh, however lower CVD was evident post TTh in men with body mass index in excess of 30 kg/m2 [31]. Hudson et al. [22] in their review/meta-analysis of RCTs in 2022 found no evidence that TTh increased short to medium-term CVD (OR: 1.07, 95% CI: 0.81–1.42, P = 0.62) in hypogonadal men [22]. CVD safety following testosterone gel therapy in hypogonadal men was evaluated in the Testosterone Replacement Therapy for Assessment of Long-term Vascular Events and Efficacy Response in Hypogonadal Men (TRAVERSE study); a non-inferiority RCT with a mean follow-up of 21.7 months [32]. This RCT was carried out in response to the Food and Drug administration guidance (https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-cautions-about-using-testosterone-products-low-testosterone-due, accessed on 03.12.2023) in 2015 [33] following the conflicting outcomes regarding CVD and TTh that we have described. The above-described evidence suggests that factors other than CVD risk predictors must be considered as mediators driving the association between adult-onset TD, TTh and all-cause mortality.
Higher values of SHBG have been associated with increased mortality in men with T2DM [34, 35]. Rastrelli et al. [36] demonstrated a positive correlation (independent of serum TT) between SHBG levels and the classifying symptoms of TD. An elevated SHBG could lead to lower serum FT concentrations and this could perhaps result in reduced biological activity exerted by testosterone. Tint et al. [34] showed that SHBG levels (which increased with age) were positively associated with all-cause mortality, the analysis being adjusted for age. We used the BLAST screened cohort, and studied the association between SHBG, testosterone, variables related with SHBG (e.g., age, serum TT, etc) and all-cause mortality in men with T2DM not on TTh [35]. Age, SHBG (independent of calculated FT levels) were positively associated and serum TT was inversely associated with mortality, all of which were significant. Our view is that the association between SHBG and mortality is not causative given the wide distribution range. The role of SHBG could be via changing FT levels, despite our analysis showing independence, as the algorithm used to calculate FT may not reflect physiological activity [37].
A factor worthy of consideration as a mediator of the association between TTh and all-cause mortality is haematocrit (HCT). The National Institute for Health and Care Excellence in the United Kingdom clinical knowledge summary in 2020 (https://cks.nice.org.uk/topics/polycythaemia-erythrocytosis/), suggested that increased HCT levels (polycythaemia) could be associated with increased CVD and mortality [38]. Polycythaemia is classed as primary (polycythaemia vera) or secondary, often associated with smoking, dehydration and TTh [39]. Gagnon et al. in 1994 [40] examined the association between CVD morbidity, mortality and HCT in the Framingham cohort comprising 5,209 individuals during 34 years of follow-up [40]. The highest HCT subgroup was associated with greater all-cause mortality as well as CVD. There was an increased risk of all-cause death as well as morbidity and mortality due to CVD in subjects with HCT values in the highest quintile. The correlation between HCT and CVD appeared non-linear with hint of a J or U shape with heterogeneity based on age and sex also apparent [40].
Further evidence for a non-linear association between HCT and all-cause mortality was evident in a study by Boffetta et al. [41] in 2013 of 49,983 adult Iranians. A U-shaped relationship between categories of HCT and mortality was seen in both males and females with both low and high HCT appeared associated with greater mortality, although the optimal HCT level offering lowest risk differed for the sexes; all-cause mortality and CVD mortality increased when the HCT was < 39% or > 45% and < 30% or > 40%, in males and females respectively [41]. Locatelli et al. in 1998 [42] analysed the Lombardy registry for the effects of erythropoietin in 5,302 patients with a low mean HCT (mean HCT: 30.1%) with end stage renal disease. All-cause mortality risk appeared inversely associated with change in HCT following erythropoietin therapy. This finding is in keeping with a negative slope in a U-shaped relationship between mortality and HCT at lower values of HCT.
Regarding the U-shaped association between HCT and mortality/CVD, we speculate that the negative slope is possibly driven by improved oxygenation of tissues due to increased haemoglobin, whilst the positive gradient beyond an optimal HCT value is due to increased viscosity compromising blood flow. Some studies suggest that in the case of ageing the fluidity of blood is impaired; a rise in blood and plasma viscosity, impaired erythrocyte deformability and enhanced aggregation have been reported [43]. In a case control study, we compared differences in blood flow characteristics and computational flow dynamics in 27 subjects diagnosed with CHD and 30 individuals not demonstrating any symptoms of CHD [44]. Our analyses suggested that peak systolic velocity predicted CHD; despite the modest number of patients a significant difference was observed (patients without CHD, mean peak systolic velocity: 62.8 cm/s, patients diagnosed with CHD, mean peak systolic velocity: 53.6 cm/s, P = 0.042). We also found that wall shear stress was associated with peak systolic velocity, leading to the speculation that peak systolic velocity could be a composite surrogate factor as it may be associated with many of the blood flow related risk factors associated with atherogenicity [44].
We analysed a registry database comprising 737 men from Bremerhaven, Germany with adult-onset TD who were all offered TU in accordance with the guidelines at the time [45]. TU was prescribed to 353 men (median follow-up: 105 months) with the remaining 384 men (median follow-up: 114 months) opting against it in view of safety concerns and financial considerations [45]. Secondary polycythaemia is the commonest adverse effect of TTh and we showed a median increase in HCT of 5% following TU [45]. All the men on TU had HCT levels at final assessment ≤ 52%. All-cause mortality was lower in men on TU (5.7%) compared to those who opted against treatment (19.3%) [45]. Logistic regression analysis showed that in the men on TU, baseline HCT (OR: 0.49, 95% CI: 0.31–0.79) and increase in HCT (OR: 0.54, 95% CI: 0.34–0.86) were inversely associated with all-cause mortality, the statistical model adjusted for age at final assessment/death. When the cohort was stratified into tertiles based on final HCT values the mortality rates during follow-up were 7.4% (HCT of 46–48%), 8.9% (HCT of 49%) and 0.8% (HCT of 50–52%). Our data did not permit analysis of men with a final HCT > 52% to determine whether the association between HCT and all-cause mortality remained negative or switched to a positive coefficient. HCT > 52% was studied by Ory et al. in 2022 [46] demonstrating that a HCT > 52% following TTh over 12 months was associated with increased CVD and venous thromboembolism, but no change in all-cause mortality was evident [46].
Marchioli et al. in 2013 [47] demonstrated that more aggressive venesection in patients with primary polycythaemia and decreasing HCT levels to < 45% was associated with significantly lower CVD when compared with less aggressive venesection leading to HCT levels between 45–50% [47]. This suggests that a possible base of a U-shaped association between CVD and HCT in primary polycythaemia was around 45%, unlike in secondary polycythaemia based on the report by Ory et al. which hinted a corresponding HCT to be around 52% [46].
The results of the T4DM study and our own work (using HCT/haemoglobin ratios following TU) have led to speculation that the increase in HCT following TTh is associated with increased erythrocyte lifespan, perhaps related to TTh changing erythrocyte membrane characteristics in men with adult-onset TD [48, 49]. Angelova et al. [50] investigated the effects of TTh on erythrocyte membrane lipid composition and physico-chemical properties in 3 hypogonadal men showing structural changes of the erythrocyte membrane that may have led to more flexibility (and deformability) [50]. Thus, it would be interesting to study changes in rheology parameters before and after TTh or androgen deprivation therapy.
ED, a common symptom of adult-onset TD has been associated with CVD, cerebrovascular disease, and all-cause mortality [51, 52]. Many studies show that PDE5 inhibitor use in men with ED appears associated with reduction in all-cause mortality and morbidity. The Swedish nationwide cohort study included 43,145 men following their first myocardial infarction for a mean follow-up of 3.3 years showed that the 3,068 on PDE5 inhibitors showed significant reduction (HR: 0.67, 95% CI: 0.55–0.81) in mortality with the analytical model adjusted for comorbidities and other treatments [18]. A study of 5,956 men (40–89 years of age) with T2DM in the United Kingdom with a median follow-up of 7.5 years showed that PDE5 inhibitors were associated with a lower all-cause mortality (unadjusted HR: 0.69, 95% CI: 0.60–0.79), this remaining significant after the model was adjusted for factors such as age, myocardial infarction, stroke, peripheral vascular disease, age, renal function, hypertension and use of drugs such as beta-blockers and statins [HR (adjusted): 0.54, 95% CI: 0.36–0.80] [19]. Further, PDE5 inhibitor use was also associated with myocardial infarction [HR (adjusted): 0.60, 95% CI: 0.54–0.69]. Kloner et al. [53] studied 48,692 men, reporting that PDE5 inhibitors were associated with a 25% reduction in all-cause mortality over 3 years. Subgroup analysis showed 41% and 21% decrease in all-cause mortality in men at high cardiovascular risk and men with T2DM respectively [53].
Our group investigated the effects of PDE5 inhibitors in the BLAST screened cohort of 857 men (175 of the men were on PDE5 inhibitors for ED) with hypogonadism and T2DM. PDE5 inhibitors were prescribed to 175 of the 857 men for ED. PDE5 inhibitor use was significantly associated with decreased mortality [HR (adjusted): 0.21, 95% CI: 0.066–0.68], the model adjusted for age, testosterone status, TTh and statin treatment [12]. A subsequent analysis of the effect of PDE5 inhibitors on the age-related mortality probability showed increased benefit in older men [13].
Patient numbers in the BLAST screened cohort did not permit us to examine the interaction between TTh and PDE5 inhibitors, our data suggesting independent effects. However, there is a body of evidence suggesting that TTh could possibly mediate the effects of PDE5 inhibitors. Studies have shown PDE5 expression to be modified in hypogonadism and TTh appears to reduce circulating endothelial microparticles (fragments of plasma membrane released from damaged and endothelial progenitor cells and considered a marker of endothelial dysfunction) and endothelial progenitor cells (cells associated with vasculogenic reparative process and re-endothelisation post-vascular injuries) [54]. Thus, it is reasonable to speculate the possibility of synergistic action between TTh and PDE5 inhibitors [55].
The above sections have provided details of the studies that have identified possible associations between adult-onset TD and mortality, and reduction in mortality following TTh (study details are shown in Table 1). It is important that age is considered a confounder as it is associated with both adult-onset TD and mortality [56]. We have also speculated on possible contributory mechanism(s) and it is clear that much work is necessary to gain further understanding. Current use of TTh in men with adult-onset TD is based on improving the classifying symptoms of the condition [4, 57, 58]. There are potential issues in diagnosing adult-onset TD that are currently leading to variation in clinical practice.
There is variation in testosterone assay methodology with most laboratories in the United Kingdom and the United States of America opting for immunoassays as opposed to liquid chromatography-tandem mass spectrometry [59, 60]. The reference ranges quoted by the laboratories varied between and within assays [59]. Considerable inter and intra-assay variability in performance appears to exist [59, 61]. Thus, harmonisation and assay standardisation is essential for safe clinical practice. Sample collection times should also be standardised as testosterone levels are possibly affected by diurnal rhythm [62, 63]. Studies by Lehtihet et al. [64], Kanakis et al. [65], Van de Velde et al. [66] suggested a lowering of serum TT in the post prandial state. However, we did not see any significant change between prandial status and serum TT in an analysis of 213 men with serum sampling taking place within a 2 hour window to negate the impact of diurnal rhythm [67]. It is also important that further work be carried out to understand the effect of seasonality on the measured serum testosterone in view of some conflicting reports [68–72]. Further, it is essential that clinicians are made aware that serum TT values are often lower during an acute illness (negative acute phase response) [73, 74].
The role of FT in clinical practice is also not clear. Lipophilic hormones such as testosterone are principally bound to specific transport proteins for transport in plasma. The unbound free fraction of testosterone is determined by concentrations of the carrier proteins, particularly SHBG. The free hormone hypothesis is based on the premise that only the unbound fraction is physiologically functional after passively crossing into the target cell [75]. However, it is possible that the free hormone hypothesis is overly simplistic when assuming that the role of SHBG is only passive [76–78]. However, there is evidence from EMAS for including FT in clinical management [8]. It appeared that men with normal serum TT but low FT (using the algorithm of Vermeulen et al. [79]) had higher LH levels, and more sexual and physical symptoms of adult-onset TD than men with both normal serum TT and FT, or men with low serum TT together with normal FT concentrations [8]. In routine practice FT is usually calculated via algorithms although many issues remain unresolved and a detailed description of this is beyond the scope of this review [79–83]. However, it must be stated that direct measurement of FT is also problematic with the reference method (equilibrium dialysis) lacking standardisation [80, 84]. Thus, routine clinical laboratories will likely continue to report calculated FT in the immediate future [59].
There are numerous testosterone preparations and formulations available with varying routes of delivery and pharmacokinetics. The modes of delivery include buccal, nasal, subdermal, transdermal, and intramuscular routes [85]. Factors influencing preparation choice are usually based on patient preference with intramuscular preparations and topical gels being the front-line agents in our practice [85]. Some intramuscular preparations, have been seen to lead to significant supraphysiological or fluctuating hormone levels, with this phenomenon more common with the short-acting formulations [84, 86]. In contrast longer-acting intramuscular preparations such as TU resulted in testosterone levels within the normal range throughout treatment. Topical testosterone gels enjoy some advantages such as easy application, ready bioavailability, tolerability and lower likelihood of supraphysiological hormone levels [84, 87].
Another measure that would be useful in routine practice is estimation of the CAG repeat numbers in exon 1 of the androgen receptor gene, as the number of CAG repeats has been shown to mediate response to TTh [88]. Zitzmann et al. [88] in a single arm observational study demonstrated injectable TU to be associated with reduction in lipids, systolic and diastolic BP and increase in HCT, with the number of CAG repeats perhaps by altering androgen receptor sensitivity modifying treatment outcomes [88]. A recent report by Heald et al. [89] evaluated the association between the number of CAG repeats and all-cause mortality in 1,977 men (mean age ± SD: 60 ± 11.1 years) followed up over a mean 12.2 years (mortality rate = 25.1%) [89]. No association was observed between CAG repeats and mortality in the total cohort. However, in men with serum TT < 14.2 nmol/L a ‘U’ shaped association between CAG repeats and all-cause mortality was observed; compared to the reference group of 22–23 CAG repeats, < 22 (HR: 1.53, 95% CI: 1.01–2.33) and > 23 (HR: 1.70, 95% CI: 1.07–2.69) CAG repeats demonstrated higher mortality respectively, the statistical models adjusted for age, serum TT and serum oestradiol, but not SHBG [89].
As far back as 1825, Benjamin Gompertz showed that mortality increased exponentially with age and data from the United Kingdom Government web archive (https://webarchive.nationalarchives.gov.uk/ukgwa/20160105202750/http:/www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2013/sty-mortality-rates-by-age.html, accessed on 20.08.2023) confirmed that this association still applied in men and women [56]. In accordance with Gompertz’s model, analysis of the BLAST screened cohort showed that mortality in men with adult-onset TD and T2DM was exponentially associated with age [13]. In this review using the BLAST screened cohort data we showed that low serum TT levels, highly prevalent especially in men with T2DM, was associated with significantly greater all-cause mortality (compared to their counterparts with normal serum TT) in men ≥ 65.90 years of age. Any measure reducing this mortality would lead to significant benefit in view of the high prevalence. TTh appears to be associated with decreased all-cause mortality in men with T2DM [11–13]. Although the mechanisms of this benefit remain unproven it is essential that the condition is diagnosed. We speculate that improvements in the classifying clinical phenotypes of the MetS, synergistic action with PDE5 inhibitors and, changing levels of SHBG and HCT may mediate the association between TTh and reduced all-cause mortality; further research taking into account heterogeneity is essential. In view of the association with the MetS, we propose that all men with this cluster phenotype be screened for serum TT and FT with TTh subsequently offered to those with adult-onset TD unless contraindicated. TTh has been known to lead to improvement in quality-of-life parameters and morbidity [57, 58] in men with adult-onset TD and the gathering evidence highlighted in this review suggests that we perhaps should perhaps add longevity to this.
BLAST: Burntwood Lichfield Atherstone Sutton Coldfield Tamworth
CHD: coronary heart disease
CI: confidence interval
CVD: cardiovascular disease
ED: erectile dysfunction
EMAS: European Male Ageing Study
FT: free testosterone
HCT: haematocrit
HR: hazard ratio
LH: luteinising hormone
MetS: metabolic syndrome
OR: odds ratio
PDE5: phosphodiesterase type 5
RCT: randomised controlled trials
SHBG: sex hormone binding globulin
T2DM: type 2 diabetes
TD: testosterone deficiency
TT: total testosterone
TTh: testosterone therapy
TU: testosterone undecanoate
AM and SR: Conceptualization, Writing—original draft, Writing—review & editing, Visualization. RCS, GH, and CK: Writing—original draft. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics were obtained for BLAST study: Multi centre UK Research Ethics Committee approval: 08/H1208/30; European Union Clinical Trials Register: EudraCT 2008-000931-16.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Datasets are available on request.
Grant from North Staffordshire Medical Institute (Grant Number: [PID-200078]). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Pravinath Ramachandran ... Geoffrey Hackett
Eugenie Macfarlane ... Markus Joachim Seibel
Carter Coggins ... Vikrant Rai
