Abstract
Type 2 diabetes mellitus (DM) and hypertension (HT) are common major cardiovascular disease (CVD) risk factors. They share common pathophysiological mechanisms and are commonly co-existent. Prevalence of HT is increased among diabetic patients but also DM is more common in hypertensive patients. CVD risk increases multiplicatively in coexistence of HT and DM. Lowering blood pressure (BP) has been shown to be associated with improved morbidity related to both macro- and micro-vascular complications. Although there is debate about target BP levels, in many randomized controlled trials and guidelines a goal of < 130/80 mmHg is advocated in patients with DM, if well tolerated. However, an individualized approach should be cared for depending on risk factors, co-morbidities, and frailty of patients. Lifestyle modifications including weight loss, regular exercise, avoiding smoking and excessive alcohol consumption, and a healthy diet including limitation of salt and fat and total energy intake, are important both as a part of preventive therapy and treatment modality for both DM and HT. Among antihypertensive drugs angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) are warranted due to their potential advantages for slowing albuminuria and progression to kidney failure which is more common in DM. Usually, their combination with calcium-channel blockers (CCBs) or thiazide/thiazide-like diuretics, in a step-wise manner, is recommended. Resistant HT is more common in DM and requires the addition of mineralocorticoid receptor antagonists (MRAs). New antidiabetic drugs like glucagon-like peptide 1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors have been found to lower BP. Apart from their antihypertensive effects they also improve CVD and renal outcomes. There’re ongoing new trials for new agents. Development of more potent and longer-term effective BP lowering drugs, single pill multiple drug combinations of antiHT agents and combination of antiHT agents with glucose-lowering and antilipidemic agents will probably improve compliance to treatment and achievement of goals in diabetic patients.
Keywords
Hypertension, blood pressure, diabetesIntroduction
Both diabetes and hypertension (HT) are among the major cardiovascular (CVS) risk factors. HT and type 2 diabetes mellitus (DM) are interrelated conditions and their co-existence is frequent. Prevalence of HT is increased among diabetic patients but also DM is more common in hypertensive patients. Presence of HT increases the risk of developing DM but also the presence of DM increases the risk of future HT significantly. Almost one-third of adult population has HT. Whereas about 75% of adult diabetic patients have HT [1–4].
CVS disease (CVD) risk is doubled among diabetic patients [5]. Diabetes is also a major cause of microvascular events, like retinopathy and nephropathy [6]. In coexistence of HT and DM, CVD risk increases synergistically and multiplicatively [7]. Therefore, diabetic patients should be aware of their blood pressure (BP) levels. Those patients without previously diagnosed HT or renal disease should measure their BP at least annually [8].
Awareness and effective management of HT and DM are of paramount importance for prevention of serious morbidities and mortality. Both conditions share common risk factors and can be modified through lifestyle modifications and medications.
Common risk factors
Sedentary lifestyle, increased body weight, elevated BP, and dyslipidemia are frequently co-encountered and a term metabolic syndrome has been suggested. Therefore, mechanisms, risk factors, and management strategies share common similarities in many diabetic or hypertensive patients.
Insulin resistance, present in most cases of both prediabetes and preHT, is a predictor for progression to DM or HT. About half of HTsive patients have insulin resistance. Apart from the detrimental effects of hyperinsulinemia on carbohydrate metabolism, it leads to stiffness, hypertrophy, fibrosis, endothelial dysfunction, and abnormal remodeling in CVS system. In addition, increased insulin levels stimulate renal sodium and water retention. There’s growing evidence about association of chronic low-grade inflammation with obesity and CVD. Increased secretion of adipokines, cytokines, endothelin-1, and fibroblast growth factors, and reduced nitric oxide production results in stimulation of inflammatory pathways, smooth muscle cell proliferation, angiogenesis, and consequently structural and functional alterations in the endothelium lead to endothelial dysfunction which is an important basis for cardiometabolic disorders including obesity, impaired glucose control, HT and atherosclerosis [9, 10]. In addition, oxidative stress is increased, autonomic nervous system and renin-angiotensin-aldosterone system are activated and aldosterone production is stimulated. Such mechanisms cumulatively impair vascular tone regulation, increase vascular resistance and volume overload, and consequently result in elevated BP. Impaired renal regulatory mechanisms and chronic kidney disease which are more common in diabetics take part in dysregulation of BP and elevated BP has also unfavorable effects on renal functions [11–13].
Blood pressure targets
BP reduction is associated with improvements in mortality rate, CVS outcomes, and retinopathy.
In HT optimal treatment (HOT) trial among diabetic hypertensive patients, lowering diastolic BP towards 80 mmHg was associated with 51% reduction in major CVS events compared to lowering towards 90 mmHg [14].
In the UK Prospective Diabetes Study (UKPDS), BP reduction of < 150/85 mmHg resulted in significant risk reduction for mortality (32%), stroke (44%), and retinopathy (34%). There was a linear relation between adverse events and risk reduction down to systolic BP (SBP) of 120 mmHg [6].
In Action in Diabetes and Vascular Disease: Preterax and Diamicron-modified Release controlled Evaluation (ADVANCE) trial [15], microvascular events, CVS deaths, and all-cause mortality were significantly decreased with more aggressive reduction of BP (mean achieved BP of 134/74 mmHg vs. 140/76 mmHg) among diabetic patients.
In Appropriate BP Control in Diabetes (ABCD) trial [16], over a follow-up of 5 years, CVS events and renal functions were not significantly different with intensive treatment (mean BP 128/75 mmHg) and placebo (mean BP 137/81 mmHg) groups. However, the risks of retinopathy, nephropathy, or stroke were significantly lower with intensive treatment.
In Action to Control CVS Risk in Diabetes (ACCORD) BP trial [17], 4,500 diabetic patients were followed for 4.7 years. No significant difference was found in risks of CVS death, myocardial infarction, or stroke between intensive BP lowering (target SBP < 120 mmHg) and standard BP lowering (target SBP < 140 mmHg) groups. The study was claimed to be underpowered due to possible potential interactions between the intensive glycemia and SBP interventions that might have masked beneficial effects of the SBP intervention [18]. Nevertheless, these results created a question mark about targeting SBP below 130 mmHg as suggested in view of previous studies [6, 15, 16].
In the SBP Intervention Trial (SPRINT) [19], BP measurements were made by patients themselves (unattended). Mortality rate and CVS events were significantly lower with intensive BP lowering with target SBP of < 120 mmHg than targeting SBP of < 140 mmHg. However, since diabetes was among exclusion criteria, the results of this trial, may not support lower BP goal in diabetic patients. Also, it should be noted that unattended SBP may be as much as 10 mmHg lower than attended office SBP measured with medical staff. The results of SPRINT have been heavily criticized: the trial was stopped early; although CVS mortality was reduced significantly the absolute numbers were low and an important number of deaths were grouped as unclassifiable; heart failure rate was reduced but the use of diuretics in the intensive BP control group was significantly higher; and there was a high rate of loss of follow-up. Another issue heavily discussed after this trial was the reliability of BP measurements. It should also be kept in mind that erroneous measurements related to factors including training for proper BP measurement or validation of devices may be misleading [20, 21]. Nevertheless, BP measurements from either 24-hour ambulatory or home BP measurements (HBPMs) can predict CVS risk. HBPM may improve awareness of HT and adherence to lifestyle measurements and antiHT treatment.
A further analysis of ACCORD and SPRINT suggested that SPRINT-eligible patients from the ACCORD-BP trial not receiving intensive glycaemic control benefited from intensive BP control and therefore the results of two studies may be consistent [22]. Another secondary analysis of these two trials revealed that the chronic kidney disease risk was increased with lowering SBP to less than 120 mmHg in patients both with DM and without DM. However, the absolute risk was higher in people with DM [23]. Furthermore, in a post hoc analysis of ACCORD and the Veterans Affairs Diabetes Trial (VADT), heart failure risk was increased in diabetic patients having variable BP, especially dips of BP were more dangerous in this sense. This finding may be explained by ischemia of vital organs including heart, secondary to low BP values as previously discussed for J-curve BP phenomenon [24]. However, whether increased mortality and morbidity with excessive BP reduction suggested with J-curve phenomenon is secondary to low BP itself or other co-morbidities like frailty or ischemic heart disease is controversial [25].
Diabetic patients having higher baseline SBP levels above 140 mmHg seem to benefit more from BP lowering [26]. According to another meta-analysis, for achieved SBP of < 140 mmHg, most CVS outcomes were significantly more reduced in diabetic patients compared to nondiabetics. However, for achieved SBP of < 130 mmHg, the difference was not current, or even CVS outcomes were higher in diabetics compared to nondiabetics. Lowering BP reduced end-stage renal disease significantly only in diabetics, but this reduction was greatest when achieved SBP was ≥ 140 mmHg and no further reduction was found with achieved SBP below 140 mmHg [27].
The Strategy of BP Intervention in the Elderly Hypertensive Patients (STEP) study included 60–80 (mean 65) years old elderly HTsive patients of whom about 19% had DM. Compared to the target SBP of 130–150 mmHg, the results have shown decrease in CVS outcomes with the target SBP of 110–130 mmHg [28].
Recently, the results of a large randomized Effects of Intensive BP Lowering Treatment in Reducing Risk of CVS Events Trial (ESPRIT) that compared intensive (office SBP target < 120 mmHg) and standard (office SBP target < 140 mmHg) BP reduction, have been published. The study included 11,255 patients of whom 4,359 had diabetes. Over a median follow-up of 3.4 years, CVS death and events were significantly lower in the intensive treatment group (9.7% vs. 11.1%, p = 0.028) whereas acute renal event rates were similar. Although frequencies of hypotension were similar between groups syncope was more common in the intensive treatment group (0.4% vs. 0.1%). Presence and duration of DM had no differential effect on results [29].
In the recent large Intensive Blood-Pressure Control in Patients with Type 2 Diabetes (BPROAD) trial > 50 years old hypertensive diabetic patients were followed for a median of 4.2 years. Similar to ESPRIT and ACCORD trials attended office BP measurements were used (HBPM during COVID-19). Major outcomes occurred in 393 patients among < 120 mmHg SBP target group (n = 6,414) and in 492 patients among < 140 mmHg SBP target group (n = 6,407) [hazard ratio (HR), 0.79; 95% confidence interval (CI), 0.69–0.90; p < 0.001] primarily driven by reduced stroke. Although the rate of serious adverse events was similar between groups, frequencies of symptomatic hypotension and hyperkalemia were higher in intensive BP lowering group [30].
According to several randomized controlled trials and meta-analyses, we can conclude that lowering BP levels may decrease mortality rate and may prevent or slow down progression of morbidities in people with and without DM. However, diabetic patients seem to be more susceptible to HT-related CVS and renal morbidities. Nevertheless, controversial findings and lack of a clear benefit of intensive BP reduction on cardiac outcomes in several studies and metanalysis outlined above resulted in cautious recommendations about deep cut-off value for target BP in diabetic patients.
Individual risk factors and co-morbidities should be considered in the treatment of HT in diabetes. Young patients will probably tolerate BP lowering without any adverse effects and since they will probably survive longer they will benefit from long-term BP lowering effects with reduced risk of CVS events, renal disease, retinopathy, and other complications. In contrast, elderly patients having relatively short survival time may be less likely to get long-term benefits and may be more prone to adverse side effects like hypotension and hyperkalemia due to co-morbidities and frailty.
In many randomized controlled trials and guidelines, a goal of < 130/80 mmHg is advocated in patients with diabetes if well tolerated (Table 1) [31–36]. European Society of HT (ESH) guidelines recommend < 130/80 mmHg in most patients if well tolerated and suggest avoiding SBP of < 120 mmHg and DBP of < 70 mmHg. However, If a target of < 130/80 mmHg is not tolerated a BP range of 130–139/80–89 mmHg is acceptable [35]. American Association of Clinical Endocrinology (AACE) has also offered SBP goal of < 130/80 mmHg for most diabetics. A lower level can be targeted for patients with established CVD, albuminuria, moderate/high risk for CVD, or retinopathy. Lower targets may not be tolerated in elderly, frailty, and patients with autonomic dysfunction and orthostatism, medication intolerance, or acute coronary syndromes [37].
Target BP levels and preference of drugs in diabetic patients in several guidelines
| Guidelines | Hypertension | Target BP | Preference of drugs |
|---|---|---|---|
| ACC/AHA 2017 | 140/90 mmHg | < 130/80 mmHg | No preference |
| HCGC 2020 | 130/80 mmHg | < 130/80 mmHg | A (if not satisfactory A + D) |
| ISH 2020 | 140/90 mmHg | < 130/80 mmHg (< 140/80 mmHg in elderly) | A + C (C + D for blacks) |
| ESH 2023 | 140/90 mmHg | < 130/80 mmHg | A + C or D |
| ESC 2024 | 140/90 mmHg | < 130/80 mmHg | A + C or D |
| ADA 2025 | 130/80 mmHg | < 130/80 mmHg | < 150/90 mmHg: A or C or D; A (if albuminuria or CAD)> 150/90 mmHg: two of A or C or D; A + C or D (if albuminuria or CAD) |
β-blockers are recommended in the presence of compelling indications like left ventricular dysfunction, coronary artery disease (CAD), or tachycardia. ACC: American College of Cardiology; ADA: American Diabetes Association; AHA: American Heart Association; BP: blood pressure; ESC: European Society of Cardiology; ESH: European Society of Hypertension; HCGC: Hypertension Canada Guidelines Committee; ISH: International Society of Hypertension. A: angiotensin-converting enzyme inhibitor/angiotensin receptor blockers; C: dihydropyridine calcium-channel blockers; D: diuretics
Treatment of HT in type 2 diabetes mellitus
It’s reasonable to give a chance to lifestyle modification, up to 3 months, for patients not having comorbidities and high BP levels. If this fails to get office BP levels below 130/80 mmHg, to reduce CVD risk in DM, drug treatment, targeting 120–129/70–79 mmHg, is recommended [36]. For obese and/or prediabetic patients antiHT drug treatment is recommended when office BP follow-up is ≥ 140/90 mmHg or when 130–139/80–89 mmHg if 10-year CVD risk exceeds 10% [36].
It should be emphasized that, since DM and HT are interrelated diseases and share common risk factors and pathophysiological mechanisms lifestyle measures are important in prevention and progression of these two major CVS risk factors and their CVS outcomes. American Diabetes Association (ADA) and other clinical practice guidelines suggest prompt initiation of lifestyle measures, such as regular moderate-intensity dynamic exercise of 30 minutes per day for at least five days per week, avoiding smoking and alcohol, and adapting a healthy diet including limitation of salt to < 5 g/day, increasing amount of vegetables and fruits and potassium-rich foods like nuts, seeds and legumes, and avocado, reduction of fat and high caloric foods and weight loss, as a part of preventive therapy and as well as treatment modality for both diabetes and HT [31–37]. Dietary Approaches to Stop HT (DASH) diet, including more fruits, vegetables, whole grains, and low-fat dairy, and reducing saturated fats, sugar, and salt not only reduces BP but also improves metabolic parameters [38]. DASH diet may reduce mean SBP by 3.2 mmHg and mean diastolic BP by 2.1 mmHg with greater reductions in patients having baseline BP > 140/90 mmHg [39]. In a meta-analysis of ten trials, restriction of salt in diet significantly reduced both systolic and diastolic BP with weighted mean differences of 5.5 mmHg and 1.7 mmHg among diabetic patients [40]. Yoga which includes special physical movements and breathing techniques seems a new player in preventive CVD lifestyle changes. Yoga-based lifestyle modification regulates stress, has positive effects on sympathetic and neuroendocrine systems, and improves endothelial dysfunction. Although there’s some negative data there’s substantial evidence that it improves BP regulation [41, 42].
Although there may be individual variation, lifestyle modification usually has modest anti-HTsive effects, with 5–10 mmHg BP reduction. Lifestyle modification alone may be a solution in patients with office BP of < 140/90 mmHg, but patients with higher BPs ideally should be given pharmacotherapy along with lifestyle modification.
Among antihypertensive drugs, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium-channel blockers (CCBs), and thiazide/thiazide-like diuretics were associated with reduced CVS complications in patients with concomitant DM and HT. However, ACEIs/ARBs are warranted since they have potential to prevent or retard albuminuria and/or kidney failure which are frequent in DM [43, 44].
β-blockers are recommended to be prescribed whenever there’s an additional indication for their usage, like presence of tachycardia, angina, myocardial infarction, or left ventricular dysfunction [31–36].
In most of the patients a combination therapy of ACEI/ARB with a dihydropyridine CCB or thiazide/thiazide-like diuretic is suggested during initial drug therapy. Among elderly and frail patients, those having orthostatic hypotension and mildly elevated BP (120–139/70–89 mmHg) initial monotherapy may be preferred [36].
CCB has no metabolic derangements and can be prescribed to almost all patients with DM without restriction. CVS events were lower with CCB/ACEI combination than ACEI/diuretic combination in Avoiding CVS Events through Combination Therapy in Patients Living with Systolic HT (ACCOMPLISH) trial [45]. However, in Swedish Trial in Old Patients with HT (STOP-HT), diuretics, ACEIs, and CCBs were found to be similar in prevention of CVS events in diabetic patients [46]. ACEI/CCB combination was associated with reduced CVS events and induced less diabetes than β-blocker/diuretic regime in Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) [47]. Some guidelines prefer ACEI/ARB + CCB combination instead of combination with diuretics [33]. If a CCB is not tolerated, for example, because of edema, a thiazide/thiazide-like diuretic can be offered.
Use of diuretics has been debated because of possible metabolic side effects like hyperglycemia, hyperuricemia, and hypokalemia. However, these potential effects are so rare with current doses used in HT management. Also, since they’re usually used in combination with ACEI/ARB, potassium-sparing diuretic, or mineralocorticoid receptor antagonists (MRAs) the risk of hypokalemia is usually avoided [43]. Hydrochlorothiazide and chlorthalidone are effective in lowering BP moderately among patients with preserved or moderately impaired renal functions [glomerular filtration rate (GFR) > 50 mL/min]. However, in patients with significant renal dysfunction (GFR < 30 mL/min) loop diuretics or a combined usage of loop diuretics and thiazides are preferred [35, 36, 43].
2024 European Society of Cardiology (ESC) guideline recommends low dose double combination at initial therapy. If this is not sufficient during 1–3 months of follow-up, low dose triple therapy then full dose or maximally tolerated triple therapy is suggested in a step-wise fashion [36]. Some guidelines prefer full dose double therapy in the second step and if not sufficient triple therapy thereafter [33, 35]. A blended flow chart for a general approach to HT in DM is illustrated in Figure 1.
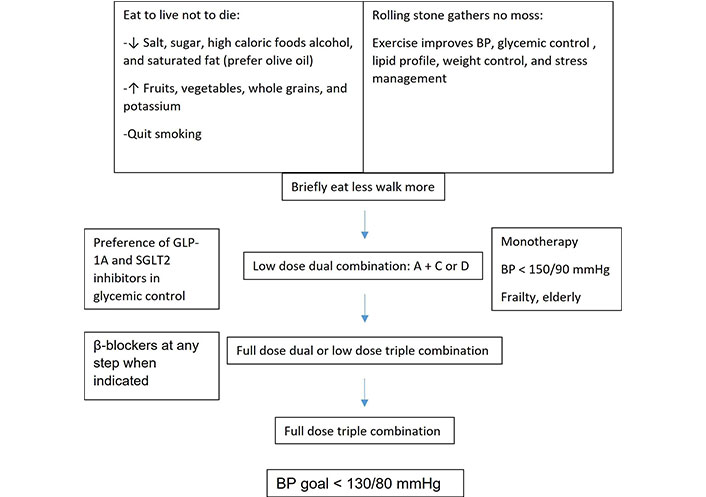
A simple flow chart for management of hypertension in diabetic patients. BP: blood pressure; GLP-1A: glucagon-like peptide 1 agonist; SGLT2: sodium-glucose cotransporter 2. A: angiotensin-converting enzyme inhibitor/angiotensin receptor blockers; C: calcium-channel blockers; D: diuretics. ↓: decrease, ↑: increase
Timing of drug administration
Nocturnal BP drop is diminished in diabetes (nondipping HT). Therefore, it was speculated that giving antiHT drugs before bedtime instead of morning may have better control of BP in diabetics. However, recent studies suggested that giving antiHT medication in the morning or evening had similar effects [48]. Nevertheless, individualized timing of drug administration may be required. Repeated 24-hour ambulatory blood measurements may be helpful in this sense.
Unachieved BP target levels
Many HT patients with DM do not achieve BP target goals. This may be associated with several factors including socioeconomic status, healthcare system-related problems, poor medication adherence, therapeutic inertia, co-administered drugs or products increasing BP, and other patient-related factors but also the pathophysiology of DM itself [49].
Resistant HT is more common in diabetes and requires the addition of MRA (spironolactone, eplerenon), α-blockers, or β-blockers if not given yet for compelling indications [50]. In a meta-analysis, as a fourth-line therapy, compared to doxazosin, bisoprolol, or furosemide, addition of MRA reduced BP more effectively [51]. Nonsteroidal MRA Fineronone has recently been shown to reduce CVD events and slow deterioration of renal functions in patients with chronic kidney disease and DM [52].
Single pill combinations increase patient adherence and BP control rates. Quarter dose single pill combination of telmisartan, amlodipine, and chlorthalidone was successful in achieving the BP target in patients with mild to moderate HT, both in patients with and without DM. However, BP reductions were less in diabetic patients. Therefore, presence of DM should prompt for more aggressive antiHT therapy since DM might reduce the efficacy of drugs [53, 54]. Recently, compared to initial monotherapy, a fixed-dose quadruple quarter-dose combination (37.5 mg irbesartan, 1.25 mg amlodipine, 2.5 mg bisoprolol, and 0.625 mg indapamide) was reported to achieve greater and sustained BP reduction with less side effects [55].
Direct renin inhibitor aliskiren has been tested in diabetic patients in combination with ACEIs/ARBs. Because of increased CVS events and side effects including hyperkalemia and hypotension, the study was terminated prematurely [56].
In recent years, apart from their antihyperglycemic effects some new antidiabetic drugs like glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 (SGLT2) inhibitors, have been found to reduce BP. Metabolic syndrome (obesity, type 2 DM, HT, and dyslipidemia) and metabolic dysfunction-associated steatotic liver disease (MASLD) share a common pathogenesis with insulin resistance playing a major role. GLP-1RAs have been associated with decreased CVS outcomes and improvement in metabolic dysfunction including MASLD [57–59]. SGLT2 inhibitors decrease BP through glycosuria, osmotic diuresis, and natriuresis. Apart from its effects on BP, they also provide reduction in mortality and CVS events and slow down renal dysfunction. They’re specifically valuable in improvement of life quality and decreased rate of hospitalization in heart failure. Furthermore, the beneficial effects of SGLT2 inhibitors appear to include both diabetic and nondiabetic patients [43, 60].
Development of single pill drug combinations of antiHT agents and combination of antiHT agents with glucose-lowering and antilipidemic agents will probably improve compliance to treatment in diabetic patients. Also, long-term effects of new pharmacological (i.e., zilebesiran: subcutaneously administered RNAi therapeutic targeting angiotensinogen applied quarterly or bi-annually; baxdrostat: aldosterone synthase inhibitors; aprocitentan: dual endothelin-A and -B receptor antagonist) [61–63] and nonpharmacological (i.e., renal denervation) [64] modalities may aid in the treatment of HT in diabetic and nondiabetic HT patients in near future.
Conclusions
Lifestyle modification including weight loss, regular exercise, and appropriate diet prevents and aids in treatment of both HT and type 2 DM which are commonly encountered CVS risk factors. Although, there’re debates about dips in target BP levels in diabetic patients even a small decrease in BP significantly improves macrovascular and microvascular outcomes. A BP of < 130/80 mmHg should be targeted in most patients if tolerated. Single combination pills aid in patient compliance and BP management. Newer, antidiabetic drugs having BP lowering effects and decreasing CVS and renal outcomes should also be preferred for glycaemic control. More potent and long-acting agents and modalities will probably change current approaches in near future.
Abbreviations
| ACCORD: | Action to Control Cardiovascular Risk in Diabetes |
| ACEIs: | angiotensin-converting enzyme inhibitors |
| ARBs: | angiotensin receptor blockers |
| BP: | blood pressure |
| CCBs: | calcium-channel blockers |
| CVD: | cardiovascular disease |
| CVS: | cardiovascular |
| DASH: | Dietary Approaches to Stop Hypertension |
| DM: | diabetes mellitus |
| ESPRIT: | Effects of Intensive Blood Pressure Lowering Treatment in Reducing Risk of Cardiovascular Events Trial |
| GFR: | glomerular filtration rate |
| GLP-1RAs: | glucagon-like peptide 1 receptor agonists |
| HBPMs: | home blood pressure measurements |
| HT: | hypertension |
| MASLD: | metabolic dysfunction-associated steatotic liver disease |
| MRAs: | mineralocorticoid receptor antagonists |
| SBP: | systolic blood pressure |
| SGLT2: | sodium-glucose cotransporter 2 |
| SPRINT: | Systolic Blood Pressure Intervention Trial |
Declarations
Author contributions
YG: Writing—review & editing.
Conflicts of interest
The author declares that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.