Keywords
Endoplasmic reticulum, mitochondria, steroid-producing cell, mitochondrial-associated endoplasmic reticulum membranesWe are only beginning to understand the complexity and vital role of intracellular organelle communication. A striking example of this crosstalk is the coupling of smooth endoplasmic reticulum (SER) with mitochondria (MIT). Steroidogenesis in adrenals and gonads is dependent on an intact exchange between MIT and SER. The substrate of steroid hormones cholesterol is presented to membranes of the characteristic tubule-vesicular MIT of steroid-producing cells. Only the material exchange between SER and MIT involving a series of enzymatic reactions will yield the correct diversity of steroids such as aldosterone, cortisol and sex hormones needed to fulfil its unique biological functions [1]. While this morphological correlation of cellular steroid biochemistry has been known for quite some time, it has recently become evident that endoplasmic reticulum (ER)-MIT interaction fulfils many other functions critical for cell survival and disease. Beyond the concept of MIT as the cellular powerhouse, MIT are living, dynamic signalling organelles [2]. As a “mitochondrial information processing system” with a unique capacity for functional structural remodelling and tuning of other organelles, they transduce metabolic, biochemical, and endocrine adaptation. Particularly, there is a physically intimate ER-mitochondrial interaction-taking place through the mitochondrial-associated ER membranes (MAMs). These MAMs play a crucial role in various cellular functions ranging from lipid metabolism, to calcium signalling and cell death and classical steroidogenesis [3]. Dysregulation of ER-MIT interaction by MAMs has been implicated in insulin resistance, neurodegeneration, cardiac hypertrophy, and cancer [3–7].
Here we present a micrograph of an adrenal steroid-producing cell as a unique endocrine finding underlying these recent observations (Figure 1). The electron micrograph was captured on a section of the inner adrenal cortex of a zona reticularis cell of a male rat 2 h after stimulation with corticotrophin-releasing hormone (CRH). CRH is the central regulator of the hypothalamus-pituitary-adrenal (HPA) axis triggering the production of pituitary adrenocorticotropic hormone (ACTH) and hence adrenal steroid production. Following stress activation, there is a rapid adaptation of the internal mitochondrial membrane from a more tubular to a tubulovesicular pattern providing an optimal stereological configuration for steroid synthesis. Stress-induced hormone production from the adrenals requires constant supplementation of cholesterol from cytoplasm to the inner mitochondrial membrane. This process depends on steroidogenic acute regulatory protein (StAR) and its interaction with voltage-dependent anion channels 1 and 2 (VDAC-1 & 2) and mitochondrial translocase 40 (Tom40), which takes place at the MAM and outer mitochondrial membrane contact sites [8].
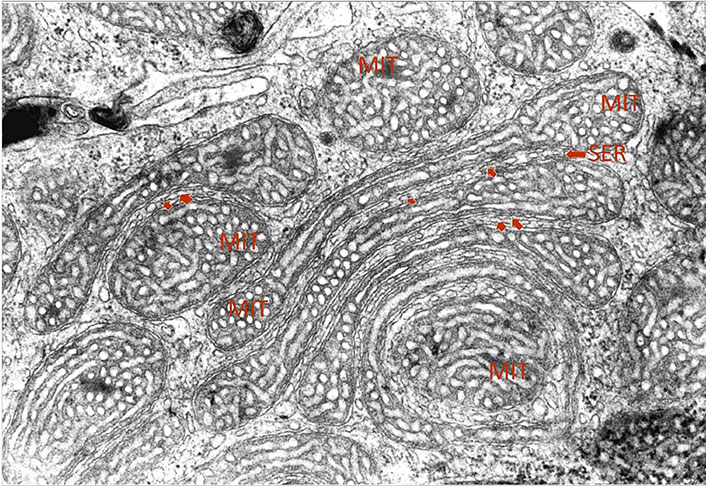
Transmission electron micrograph of MIT of a zona reticularis cell from a section of the inner adrenal cortex of a male rat 2 h after stimulation with CRH. An interorganellar juxtaposition of SER in a close proximity to elongated garland-like shaped tubulovesicular MIT can be seen (arrows). Magnification ×20,000
Furthermore, stress triggers mitochondrial biogenesis. All markers of mitochondrial biogenesis including transcription factors, related kinases, and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) protein, the master regulator of mitochondrial biogenesis are upregulated by stress stimuli [4]. Thus, stress and mitochondrial biogenesis, and morphology are intimately linked allowing a fine-tuning and feedback mechanism of steroid-dependent hormone-regulation. Usually, this adaptation of the steroid-producing organelles of SER and MIT involves an increase in SER droplets and tubules. Furthermore, cells exhibit the production of a densely arranged oval to round-shaped MIT with a transformation to “honeycomb”—like vesicular internal membranes.
The focus of the micrograph presented here is the unique capacity of steroid-producing cells, particularly of the inner adrenal cortex to display unique modelling of joint interorganellar arrangements. In addition to the frequently seen interorganellar juxtaposition of SER with MIT, we are witnessing here the intriguing potential of MIT to form garland-like circular structures in symphony with aligning elongated SER. Like in the modern artwork of impressionism, borders between elongated and stretched-out MIT and intercalated tubes of SER are blurring. The coupling of SER and MIT is forming here a true functional unit providing the optimal terrain for the dynamic maintenance of ER-MIT contact sites. Therefore, this finding allows a unique ultrastructural visualization of crucial biochemical and endocrine processes in the cells.
Abbreviations
| CRH: | corticotrophin-releasing hormone |
| ER: | endoplasmic reticulum |
| MAMs: | mitochondrial-associated endoplasmic reticulum membranes |
| MIT: | mitochondria |
| SER: | smooth endoplasmic reticulum |
Declarations
Author contributions
SRB: Conceptualization, Investigation, Writing—original draft. LSC: Writing—original draft. WK: Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This study has been funded by the
Copyright
© The Author(s) 2024.