Affiliation:
Department of Science and Mathematics, Mott Community College, Flint MI 48503, USA
Email: jmkokosa@yahoo.com
ORCID: https://orcid.org/0000-0002-5135-6221
Explor Foods Foodomics. 2024;2:275–312 DOI: https://doi.org/10.37349/eff.2024.00038
Received: January 29, 2024 Accepted: March 18, 2024 Published: July 17, 2024
Academic Editor: Cem Erkmen, Ankara University, Türkiye
The article belongs to the special issue New Generation Analytical Technologies in Food Analysis
Food samples require extensive sample preparations for instrumental analyses due to the complex matrices involved. Food safety regulatory agencies also require sample preparation procedures that are accurate, sensitive, robust, and, above all, fast, to handle the requirements for determining the safety of the massive amounts of foods and food products needed for human, pet and livestock consumption. There is also an inseparable interconnection between environmental, agricultural, forensic, cosmetic and industrial analytical chemistry involved in this requirement, and advances in analytical methodology are simultaneously applicable to all of these realms. As a response to these needs, the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method was developed to provide multiclass analysis of agricultural products, and remains the basis for regulatory procedures for large scale analyses of food samples containing a wide variety of possible contaminants. However, since QuEChERS does not enhance analyte concentrations during sample preparation of these complex samples, the methodology also requires very expensive, very sensitive final analytical instrumentation, requiring highly trained personnel and continual maintenance. Smaller regulatory and field laboratories may also need sample preparation procedures for only a limited number of specific pesticides, metals, polycyclic aromatic hydrocarbons (PAHs) or other contaminants, requiring much less expensive and labor-intensive preparations and instrumentation. This is the role of liquid phase microextraction (LPME) in food sample preparation and analysis. LPME, individually or in combination with other sample preparation procedures, such as QuEChERS or traditional techniques such as solid phase extraction (SPE), can meet the requirements for sensitive and accurate analyses of specific analytes found in complex matrices, providing not only cleanup, but concentration of sample extracts, allowing the use of greener, less expensive and low maintenance final determination analytical instrumentation. Crucial review and application publications are tabulated to allow analysts easier access to appropriate publications to use this information for developing new or improved and greener validated methods for plant and animal food analyses.
While reliable, accurate and fast chemical analysis of potential harmful contaminants in agricultural, seafood, livestock or other food sources is necessary for the health and welfare of people, they also represent some of the most challenging requirements for analytical chemists. Food sources also contain a myriad of matrix components which make the extraction, purification and analysis of trace amounts of harmful contaminants a difficult process. Agricultural and livestock products may contain sugars, complex carbohydrates, tannin, chlorophyll, pigments, terpenes, alkaloids phenols, proteins, acids, bases, salts, essential elements, hormones, DNA, proteins, amino acids, and varying amounts of fats and lipids, which may need to be removed from a final sample before instrumental analysis [1]. In addition, food contaminants may be present at trace concentrations, at low parts per billion or less, requiring sample extract concentration and/or use of very expensive and sensitive instrumentation for analytical determination [2].
Food analyses originally derived from the need for determination of the identities and amounts of natural beneficial components, such as flavorings and volatile aroma components, as well as natural food spoilage products, resulting in poor taste or smell. Traditional macro analytical techniques, such as liquid-liquid extraction (LLE), solid-liquid extraction (Soxhlet extraction) and column chromatography, followed by spectrophotometric or chemical analyses were used for this purpose. As concerns have grown over the presence of food adulteration, residual hormonal and antibacterial drugs in livestock products, pesticide residues in agricultural products, and harmful products produced in cooked foods, more sophisticated analytical sample preparation techniques, used for environmental analyses, were applied. However, these analytical methodologies also require large amounts of chemicals, time and personnel, and thus expense [3].
During the last three decades, efforts have been made to make environmental and food analyses more environmentally friendly, with methodology requiring reduced use of chemicals, energy, time and personnel, as well as reduction of hazardous waste. These approaches have been incorporated into the concepts of green analytical chemistry (GAC), which have been widely accepted by analytical chemists in government and industry, and have resulted in the development of microextraction sample preparation techniques, including solid phase extraction (SPE), solid phase microextraction (SPME), and liquid phase microextraction (LPME) [4, 5]. Analysis of food products has been especially challenging for those responsible for ensuring their safety and quality, given the need for fast, reliable, inexpensive and sensitive methods for samples potentially contaminated with more than 1,000 possible pesticides, toxic metals, natural toxins and metabolites. The development of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) by Anastassiades et al. [6] in 2003 (USDA) provided an extraction-purification methodology, requiring reduced amounts of sample (5–20 g) and solvents (5–20 mL), for analyte extraction from the food matrix. This technique involved two steps: (1) a salting out step to extract analytes into acetonitrile (ACN) and (2) dispersive SPE (d-SPE) to remove addition co-extracted lipids, sugars, chlorophyll, pigments, fatty acids and water. Originally developed for the sample preparation of fruits and vegetables, QuEChERS has been modified and developed for a wide variety of agricultural, aquatic and livestock foods, and further refined and validated to provide analytical laboratories with standardized multiclass contaminant methodology for the analysis of environmental and forensics samples as well [7, 8]. However, no one method can possibly cover all possible sample types and over the last two decades well over 5,000 research and application papers using QuEChERS or modified QuEChERS approaches have been published, increasing the scope of samples for analysis, making it difficult to determine an appropriate method to adopt for a new sample preparation method [9]. In addition, QuEChERS, alone, is not a microextraction-sample preparation technique, and suffers from two major flaws. First, QuEChERS extracts do not concentrate the analytes of concern: the enrichment factors (EFs) are 1, or even less [1, 8]. Second, a sample preparation method should prepare the extract in a format (solvent) appropriate for instrumental analysis. To do so, QuEChERS extracts may need to undergo additional preparation steps. Many published methods require additional solvent evaporation and solvent changeover steps, as well as cartridge-based SPE for further purification and to provide extracts concentrated enough and purified enough for instrumental analysis. Most QuEChERS sample preparation requires expensive, very sensitive analytical instrumentation, such as gas chromatography-tandem mass spectrometry (GC-MS/MS), high-resolution GC-MS, quadrupole time-of-flight (QTOF)-MS or ultra-high performance liquid chromatography (UHPLC)-MS/MS to provide the sensitivity needed for trace analysis of the extracts due to the lack of analyte enrichment. However, residual matrix components in the extracts can be damaging to these sensitive and expensive instruments, without further purification steps. In addition, highly skilled personnel are also required for the operation and maintenance of these instruments [8, 10].
QuEChERS, in its many modified formats, has become an indispensable tool, with its “brute force” sample preparation approach, for regulatory laboratories to rapidly deal with vary large numbers of food product samples containing large number of possible multiclass contaminants. However, with the requirements of highly trained personnel, and very expensive instrumentation, this approach may not be suitable for smaller laboratories and samples containing fewer contaminants or beneficial natural components of interest.
As a result, alternative sample preparation methods involving microextraction techniques, including LPME, as replacements or for accompanying QuEChERS have been developed for food analyses. While QuEChERS uses much less solvent and sample than traditional macro sample preparation techniques, it is generally not considered to be a microextraction technique, LPME typically requires sample sizes of 1 g or less of solid, 5 mL or less of liquid, and 400 µL or less of extraction solvent. Reduction in size for QuEChERS is possible, however, the micro-QuEChERS (µ-QuEChERS) modification reduces solid sample sizes to 1 g and liquid samples to less than 5 mL, with reduction of extraction solvent volumes to 1–2 mL, placing the technique in the range of microextraction [11].
This review discusses the role that LPME has in developing green analytical methods for food analyses. LPME consists of three distinct sample preparation techniques: (1) single drop microextractions (SDMEs); (2) membrane protected microextractions; and (3) dispersive microextractions. These techniques will be briefly described, along with their importance for GAC. Selected relevant applications for food methods using LPME alone and LPME with QuEChERS and SPE, as well as future directions involving LPME in food analysis will be discussed. Rather than providing detailed theory and diagramed instructions for LPME modes, crucial review and application publications are tabulated to allow analysts easier access to appropriate publications needed for understanding the role of LPME in sample preparations and, in turn, use this information for developing new or improved and greener validated methods for plant and animal food analyses.
The 12 principles of GAC have been widely accepted by the industrial, governmental and academic communities for developing green analytical methods for environmental, industrial and food analyses [12]. More recently, GAC has been refined to include the 10 principles of green sample preparation (GSP), with an emphasis on sample preparation techniques for overall analytical methods [13]. The 12 GAC and 10 GSP principles can be summarized (Figure 1) into four main categories that need to be adhered to for a green analytical method [14].
These four minimization categories reduce the time, cost and hazards for personnel, while also minimizing the environmental impact of an analytical method. Converting from a traditional macro-LLE (separatory funnel) or semi-micro solid-liquid method (SPE) sample preparation method to a micro extraction technique such as LPME, can reduce solvent, time, and waste requirements, as well as potential personnel hazards. Micro extraction techniques also provide sample cleanup and reduced final analyte volumes, thus higher extract concentrations, decreasing demands on analytical instrumentation requirements [5, 14]. The application of these green microextraction techniques for analytical sample preparations, constitutes the role of LPME sample preparations in monitoring the nutritional value and safety of food products, as illustrated in Figure 2.
In the past few years, metrics have been developed to aid in assessing the relative greenness of analytical methods. Reviews and pertinent references for GAC, GSP and green assessment metrics are listed in Table 1 [4, 12, 13, 15–39]. Recently developed metrics [19–24], have become increasingly popular for assessing the development of greener analytical methods. Of particular relevance for this review are three recent papers by Nowak et al. [25–27], which assess the relative greenness of analytical solvents (ChlorTox Scale) [25, 26] and the energy requirements and environmental impact of analytical instrumentation. The instrumentation analyses clearly show the environmental impact of instrumentation like UHPLC-MS/MS to be up to 10 times higher than less sophisticated instrumentation, such as HPLC/ultraviolet (UV), based on energy requirements alone, without counting the expenses involved in their manufacture, purchase, operation and maintenance. In the following sections, examples will show that LPME methodology can provide sample preparations of sufficient purity and concentrations to allow less the use of greener, less expensive, and lower maintenance requirement instrumentation for Food analyses, especially for analyses for samples containing only a limited number of potential contaminants of concern. LPME can also provide increased clean-up and extract concentration when used in tandem with more comprehensive techniques, including QuEChEERS and SPE.
Green analytical metrics for LPME
| Green analytical metrics | Subject | Author(s) | Year | References |
|---|---|---|---|---|
| Green metrics for selecting and evaluating analytical methods | National environmental methods index (NEMI) | NEMI | 2002 | [15] |
| Green analytical methodologies, NEMI applications | Keith et al. | 2007 | [16] | |
| Analytical eco-scale (ECO) | Gałuszka et al. | 2012 | [17] | |
| Green analytical procedure index (GAPI) | Płotka-Wasylka | 2018 | [18] | |
| Summarizing the information in a hexagon (HEXAGON) | Ballester-Caudet et al. | 2019 | [19] | |
| Red-green-blue (RGB) additive color model | Nowak, Kościelniak | 2019 | [20] | |
| Analytical GREEnness (AGREE) | Pena-Periera et al. | 2020 | [21] | |
| White analytical chemistry (WAC) | Nowak | 2021 | [22] | |
| Complementary green analytical procedure index (ComplexGAPI) | Plotka-Wasylka, Wojnowski | 2021 | [23] | |
| Analytical greenness metric for sample preparation (AGREEprep) | Wojnowski et al. | 2022 | [24] | |
| Green analytical chemistry (GAC)—theory and practice | Tobiszewski et al. | 2010 | [4] | |
| 12 principles of GAC | Gałuszka et al. | 2013 | [12] | |
| 10 principles of green sample preparation (GSP) | López-Lorente et al. | 2022 | [13] | |
| Total chemical risk-ChlorTox scale | Nowak et al. | 2023 | [25] | |
| Clortox base-chemical hazards, greenness assessment | Nowak et al. | 2023 | [26] | |
| Carbon foot of the analytical laboratory | Nowak et al. | 2023 | [27] | |
| Reviews of green metric assessment tools | Overview of hexagon and RGB algorithms | Nowak et al. | 2020 | [28] |
| Impact of green assessment tools in HPLC (NEMI, EOC, GAPI, AGREE) | Kannaiah et al. | 2021 | [29] | |
| Green chemistry metrics (ECO, GAPI, AGREE) | Sajid, Plotka-Wasylka | 2022 | [30] | |
| Green chemistry metrics (GAPI, ComplexGAPI, AGREE, AGREEprep) | Martinez et al. | 2022 | [31] | |
| Software tools for green and sustainable chemistry (ECO, GAPI, AGREE) | Derbenev et al. | 2022 | [32] | |
| Research and application papers using green assessment tools | Natural deep eutectic solvent (NADES) for vegetable samples (NEMI, GAPI, ECO, AGREE, WAC) | Ferreira et al. | 2022 | [33] |
| HPLC methods for dyes (HEXAGON) | Ballester-Caudet et al. | 2022 | [34] | |
| Fluorescence detection of ergosterol (AGREE, AGREEprep, WAC) | Dazat et al. | 2022 | [35] | |
| Determination of fatty acids in milk (ECO, GAPI, AGREEprep) | Narloch, Wejnerowska | 2022 | [36] | |
| Sample preparations for anthocyanin samples (AGREE) | Mandrioli et al. | 2022 | [37] | |
| Safety assessment of citrus and olive by-products (AGREE) | Socas-Rodríguez et al. | 2022 | [38] | |
| Spectroscopic methods for analysis of betrixaban (ECO, GAPI, AGREE) | El-Masry et al. | 2022 | [39] |
Note. Adapted from “Green microextraction methodologies for sample preparations,” by Kokosa JM, Przyjazny A. Green Anal Chem. 2022;3:100023 (https://www.sciencedirect.com/science/article/pii/S2772577422000222?via%3Dihub). CC BY 4.0 Deed; adapted with permission from “Principles for developing greener liquid-phase microextraction methods” by Kokosa JM. TrAC Trends Anal Chem. 2023;167:117256 (https://www.sciencedirect.com/science/article/abs/pii/S0165993623003436?via%3Dihub). © 2023 Elsevier B.V.
LPME extractions which involved extraction of non-polar analytes from aqueous samples have been accomplished with several traditional LLE solvents, including 1-octanol, hexane, chloroform, carbon tetrachloride and toluene. Other traditional extraction solvents, such as ethyl acetate and diethyl ether have been less frequently used because they are too water soluble [40]. A few chlorinated solvents are still used, but their use is discouraged, due to their toxicity and environmental impact. Volatile hydrocarbons are also discouraged due to their flammability hazards [41]. More recently, new classes of solvents, considered to be greener, have been more commonly used. These include bio-derived solvents, ionic liquids (ILs), deep eutectic solvents (DESs), natural DESs (NADESs) and magnetic ILs (MILs) and magnetic DESs (MDESs). Review articles covering LPME solvents are included in Table 2 [40–72].
LPME solvents and solvent selection reviews
| Review subject | Author(s) | Year | References |
|---|---|---|---|
| Green solvent selection guides | Byrne et al. | 2016 | [40] |
| Selection a greener liquid phase microextraction (LPME) mode | Kokosa | 2019 | [41] |
| Green solvents for LPME using gas chromatography-mass spectrometry (GC-MS) | Zhang et al. | 2024 | [42] |
| Ionic liquid (IL) toxicity | Flieger J, Flieger M | 2020 | [43] |
| Deep eutectic solvent (DES) toxicity | Martínez et al. | 2022 | [44] |
| DESs in food analysis | Chen et al. | 2019 | [45] |
| Ionic DESs for extraction of natural products | Huang et al. | 2019 | [46] |
| DESs, dispersive liquid-liquid microextraction (DLLME), foods | Lu et al. | 2022 | [47] |
| DESs preparations and applications | Farooq et al. | 2020 | [48] |
| DESs in food analysis | Ortega-Zamora et al. | 2021 | [49] |
| Hydrophilic to hydrophobic DESs | Tang et al. | 2021 | [50] |
| Hydrophobic DESs in food analysis | Boateng | 2022 | [51] |
| Properties of DESs | Omar, Sadeghi | 2022 | [52] |
| DESs in LPME | Santos et al. | 2022 | [53] |
| DESs in microextractions of biologicals | Coelho de Andrade et al. | 2022 | [54] |
| DESs in microextraction, chromatography | Kamal El-Deen et al. | 2023 | [55] |
| DESs in LPME, 2017−2022 | Andruch et al. | 2023 | [56] |
| Natural deep eutectic solvent (NADES) in green extraction techniques for foods | Cannavacciuolo et al. | 2022 | [57] |
| Surfactants in sample preparation techniques | Vakh, Koronkiewicz | 2023 | [58] |
| DES solvation | Trivedi et al. | 2023 | [59] |
| CO2 DES-switchable solvent DLLME (DES-SS-DLLME) | Nazraz et al. | 2021 | [60] |
| Switchable DESs in DLLME | Zhang et al. | 2023 | [61] |
| ILs and DESs for LPME of metals | Herce-Sesa | 2021 | [62] |
| Task-specific ILs in sample preparation | Llaver et al. | 2022 | [63] |
| ILs in extraction of pesticides in foods | Delińska et al. | 2021 | [64] |
| ILs in food analysis | Fiorentini et al. | 2022 | [65] |
| ILs and magnetic ILs (MILs) in microextractions | Llaver et al. | 2021 | [66] |
| MILs in sample preparations | Chatzimitakos et al. | 2021 | [67] |
| MILs synthesis, microextractions | Alves et al. | 2022 | [68] |
| MILs in analytical microextractions | González-Martín et al. | 2022 | [69] |
| Magnetic DES (MDES) formation and properties | Shi et al. | 2022 | [70] |
| MDES fundamentals and applications | Makoś-Chełstowska et al. | 2022 | [71] |
| MDESs in microextraction techniques | Aguirre, Canals | 2022 | [72] |
The term LPME is sometimes misused to refer to only one of the LPME modes. As the various modes of LPME have developed, LPME nomenclature has been refined, so that LPME is now a general term which refers to all of the modes using solvent microextraction. Each mode is given a unique name, with prefixes or a suffix to indicate the specific modification of the principle mode. For examples, dispersive liquid-liquid microextraction (DLLME) utilizing vortex agitation as an aid for dispersion is abbreviated as vortex assisted (VA)-DLLME. This DLLME modification is sometimes referred to in the literature as VALLME. This will not be used here so that the nomenclature will be consistent and discriptive. A more complex, but understandable mnemonic for DLLME, using a DES, which is solidified in ice-water [solidified floating organic droplet (SFO)], after ultrasonic aided dispersion extraction would then be listed as DES-UA-DLLME-SFO. The principle LPME modes, prefixes and suffix are listed in the abbreviations section. A number of excellent LPME reviews are available, covering the various LPME modes, their theory, and thousands of applications. A section of some useful reviews is included in Table 3 [5, 14, 73–85].
Key reviews for LPME modes and theory
| Review subject | Author(s) | Year | References |
|---|---|---|---|
| Liquid-liquid liquid microextraction (LLLME), theory | Ma, Cantwell | 1999 | [73] |
| Solvent microextraction (SME) text, modes, applications, theory | Ramos | 2010 | [74] |
| Single drop microextraction (SDME), theory, applications, trends | Jeannot et al. | 2010 | [75] |
| SME [liquid phase microextraction (LPME)], theory, applications | Kokosa | 2015 | [76] |
| LPME principles and configurations | Yamini et al. | 2018 | [77] |
| LPME review of reviews | Rutkowska et al. | 2019 | [78] |
| Green miniaturized technologies in analytical chemistry | Agrawal et al. | 2021 | [79] |
| Overview of LPME modes and applications | Câmara et al. | 2022 | [80] |
| Green microextraction methods and applications | Kokosa, Przyjazny | 2022 | [5] |
| LPME for polycyclic aromatic hydrocarbons (PAHs) analyses | Jalili et al. | 2020 | [81] |
| Microextractions of heavy metals | Song, Huang | 2022 | [82] |
| Green principles for green solvents | Nithya, Sathish | 2023 | [83] |
| Principles for green LPME methods | Kokosa | 2023 | [14] |
| LPME for improving greenness of standard analytical methods | Tintrop et al. | 2023 | [84] |
| LPME method greenness comparisons, 2019−2023 | Esteve-Turrillas et al. | 2024 | [85] |
In the following sections, the more commonly used LPME modes used for food product analyses will be briefly described. Reviews covering the use of LPME for sample preparations for food analyses are listed in Table 4 [2, 86–104]. These reviews cover a wide range of food analyses, including multiple classes of pesticides, polycyclic aromatic hydrocarbons (PAHs), mycotoxins, dairy products and toxic metals analyses, as well as analyses for beneficial materials, such as essential oils.
LPME sample preparations for analysis of food-based products: reviews
| Review subject | Author(s) | Year | References |
|---|---|---|---|
| LPME, mycotoxins in food, SDME, HF-LPME, DLLME | Alsharif et al. | 2015 | [86] |
| HF-LPME, DLLME in analytical toxicology | Sharifi et al. | 2016 | [87] |
| DI-, HS-SDME, DLLME, HF-LPME, sample preparations, food | Demirhan et al. | 2017 | [88] |
| DI-, HS-SDME, DLLME, analysis of plant and herbal samples | Diuzheva et al. | 2020 | [89] |
| DI-, HS-SDME, DLLME, HF-LPME, pesticide residue, food | Eticha | 2020 | [90] |
| SDME, HF-LPME, DLLME, endocrine disruptives, food | Chormey et al. | 2020 | [91] |
| SDME, HF-LPME, DLLME, antibiotics in foodstuff | Khatibi et al. | 2022 | [92] |
| SDME, HF-LPME-DLLME, pesticides in food | Jagirani et al. | 2022 | [93] |
| DI-, HS-SDME, HF-LPME, EME, DLLME, bio-food analyses | Kokosa | 2021 | [2] |
| SDME, HF-LPME, DLLME pesticides, environmental, food | Jagirani et al. | 2022 | [94] |
| SDME, HF-LPME, SBME, DLLME, µ-QuEChERSg, histamine/food | Jayasinghe | 2022 | [95] |
| SDME, HF-LPME, DLLME, µ-QuEChERS, green preparations | Câmara et al. | 2022 | [96] |
| SDME, HF-LPME, DLLME, microextractions of cosmetic products | Schettino et al. | 2022 | [97] |
| SDME, HF-LPME, EME, DLLME, bio-actives in foods | Pereira et al. | 2022 | [98] |
| QuEChERS/DLLME, DLLME, fruit, vegetables safety | Berenguer et al. | 2023 | [99] |
| DLLME, green assessments of methods for antibiotic residues in food | Vokh, Tobiszewski | 2023 | [100] |
| SDME, HF-LPME, DLLME, dairy products | Pourali et al. | 2023 | [101] |
| EME, DLLME contaminants in plastic, paper food contact materials | Chen et al. | 2023 | [102] |
| DES, IL based DLLME for analysis of essential oils | Zhao et al. | 2023 | [103] |
| DLLME, acrylamide in foods | Sabastià et al. | 2023 | [104] |
LPME: liquid phase microextraction; SDME: single drop microextraction; HF-LPME: hollow fiber liquid phase microextraction; DLLME: dispersive liquid-liquid microextraction; DI: direct immersion; HS-SDME: headspace SDME; EME: electromembrane extraction; SBME: solvent bar microextraction; µ-QuEChERS: micro-quick, easy, cheap, effective, rugged, and safe; DES: deep eutectic solvent; IL: ionic liquid
SDME was the first LPME technique developed and has two principle modes: (1) direct immersion (DI)-SDME (often just referred to as SDME) and (2) headspace (HS)-SDME. Less widely used techniques, including directly-suspended microextraction (DSME), liquid-liquid liquid microextraction (LLLME) and continuous flow (CF)-SDME, have also been used successfully in food preparations. Representative examples of these techniques are also included in Table 5 [105–123].
SDME reviews and application papers
| SDME | Subject | Author(s) | Year | References |
|---|---|---|---|---|
| SDME modes, reviews | DI-, HS-SDME trends | Kokosa | 2015 | [105] |
| DI-, HS-SDME | Tang et al. | 2018 | [106] | |
| HS-SDME | Mogaddam et al. | 2019 | [107] | |
| DI-SDME for environmental samples | Delove Tegladza et al. | 2020 | [108] | |
| DI-, HS-SDME | Jain, Verma | 2020 | [109] | |
| SDME overview of reviews | Dmitrienko et al. | 2021 | [110] | |
| DI-SDME mode food applications | DI-SDME, GC-MS multiclass pesticides in fruit | Pano-Farias et al. | 2017 | [111] |
| MIL-DSDME, voltametric determination of ascorbic acid | Jahromi et al. | 2017 | [112] | |
| LLLME, UHPLC-MS/MS, patulin in apple joice | Li et al. | 2018 | [113] | |
| DI-SDME, GC/ECD acrylamide in food | Saraji, Javadan | 2019 | [114] | |
| MIL-DI-SDME HPLC/UV, 96-well device, pesticides | Mafra et al. | 2019 | [115] | |
| CF-DI-SDME, EDX, Cr, Mn, Ni in vegetable oils | Ferreiro et al. | 2023 | [116] | |
| HS-SDME mode food applications | Microwave distillation, HS-SDME, GC-MS, spices | Gholivand et al. | 2013 | [117] |
| HS-SDME, GC-MS, 2-phenoxyethanol anesthetic in fish | Abreu et al. | 2019 | [118] | |
| DES-HS-SDME, GC-MS, terpenes in spices | Triaux et al. | 2020 | [119] | |
| DES solvents in HS-SDME GC-FID, pesticides | Abolghasemi et al. | 2020 | [120] | |
| DES solvent HS-SDME GC-MS, PAHs in water | Mehravar et al. | 2020 | [121] | |
| HS-SDME, LLME, Vis, ammonia in foods | Jain et al. | 2021 | [122] | |
| CF-HS-SDME, GC-MS, 4-methylimidazole in foods | Rafiei Jam et al. | 2022 | [123] |
SDME: single drop microextraction; DI: direct immersion; HS: headspace; MIL: magnetic ionic liquid; GC-MS: gas chromatography-mass spectrometry; LLLME: liquid-liquid liquid microextraction; UHPLC-MS/MS: ultra-high performance liquid chromatography-tandem mass spectrometry; ECD: electron capture detector; UV: ultraviolet; CF: continuous flow; FID: flame ionization detector; DES: deep eutectic solvent; EDX: energy dispersive X-ray spectroscopy; PAHs: polycyclic aromatic hydrocarbons; Vis: visible
Note. Adapted from “Green microextraction methodologies for sample preparations” by Kokosa JM, Przyjazny A. Green Anal Chem. 2022;3:100023 (https://www.sciencedirect.com/science/article/pii/S2772577422000222?via%3Dihub). CC BY 4.0 Deed.
DI-SDME, in its simplest format, involves the immersion of a drop of solvent at the tip of a syringe, into a water sample, withdrawal into the syringe and analysis by GC, GC-MS atomic absorption spectroscopy (AAS), or HPLC. The theoretical concentrating, or EF can range from 10 to 1,000 SDME, like all LLE extractions, is an equilibrium process, with actual analyte extraction amounts ranging from 10% to 100%, though it is consistent when all extraction parameters are consistent. It is also possible, however, in some cases to achieve nearly 100% recovery, when a complexing, or derivatizing agent or acid/base conversion reaction is used to force the equilibrium towards the extraction solvent. Typical sample sizes range from 0.5–10 mL. Typical solvent volumes range from 0.5–10 µL. Extraction times vary from 5 min to 90 min, though typical extraction times are about 15 min. Reverse DI-SDME, where the sample is an oil and the extraction solvent is aqueous or very polar solvent (ionic DES, IL) is also possible. DI-SDME suffers from drop instability, as well solvent water solubility or evaporation, and the limited solvent volume possible. These problems have been addressed, in part, by using magnetic solvents and the simple directly suspended droplet microextraction (DSDME) and LLLME methods. Nevertheless, this is an important sample preparation technique and has been found useful for food analyses [105–110].
DSDME is a technique that eliminates the syringe during the extraction process. In this process, a few microtiters low density solvent is added to the vortex of a stirring aqueous sample. After extraction, the solvent is removed with a microsyringe and analyzed by GC. Particulates can be a problem with this technique, so filtration of the sample is usually necessary. Since larger volumes of solvent can be used in this extraction, compared to syringe DI-SDME, recoveries can also be enhanced, without worry about drop loss [105, 106, 109].
This technique extends DSDME by using a syringe to place a drop of final aqueous extractant into the organic drop in the vortex, to back extract polor or ionized analytes from the sample. The aqueous drop is then usually subjected to HPLC, ion chromatography (IC), or AAS determination, though UV-visible (Vis) can be used when a derivatizing or complexing agent is present in the extraction drop [105, 106, 113].
The use of more environmentally favorable solvents, such as DESs and ILs, NADESs, bio-derived solvents, and supramolecular solvents (SUPRASs) have rapidly displaced traditional solvents, such as CHCl3, hexane, and toluene. MDESs and MILs can also be successfully used with 96 well autosamplers, replacing the syringe with a neodymium magnet [115]. DI- and HS-SDME can also be completely automated using commercial XYZ-autosamplers, as well as 96 well devices [105, 106, 118]. SDME extracts are also compatible with a wide range of analytical instruments and detectors, depending on the solvent used. Importantly, due to the EFs and cleanups obtained in LPME, these techniques include widely available and relatively inexpensive, and low maintenance instrumentation, such as UV-Vis spectroscopy, GC with flame ionization detector (FID), HPLC, with diode array detector (DAD), and flame AAS (FAAS).
While most DI-SDME applications involve the analysis of single, or related families of food contaminants, DI-SDME can also be useful for multiclass residue analyses as well, while obtaining EFs and matrix reduction levels that allow final analytical determinations with standard benchtop GC-MS and HPLC/UV instruments. An example by Pano-Farias et al. [111], involved the extraction of 17 pesticides from a 3 g samples of mango fruit. The sample was first diluted with 9 mL of 10% ACN, the addition of 10% salt, and extracted with 2 µL of toluene for 30 min. The procedure was optimized and in-house validated with limits of quantification (LOQs) ranging from 6–124 µg kg-1, recoveries ranging from 69–119%, and linear ranges from 5–1,000 µg kg-1. A fourth example by Mafra et al. [115] involved the use of a MIL [(trihexyl(tetradecyl)phosphonium chloride: MnCl2 tetrahydrate, 5.5 mg] on the base of a neodymium magnet attached to the pin of a 96 well sampler, for extraction of 1.5 mL of pesticides, bisphenol A and benzophenone from environmental waters. After a 90 min extraction, the MIL was back extracted into ACN and the extract analyzed by HPLC/UV. Limits of detection (LODs) ranged from 1.5–3 µg L-1.
An illustration of the utility of DSDME is a unique and simple extraction and analysis of ascorbic acid in aqueous solutions developed by Jahromi et al. [112]. A solution of MIL (Aliquat iodide:MnCl42-, 2:1, 8 mg) and ethanol (1 µL) was placed in the vortex of a stirring sample (diluted, filtered orange juice) for extraction of ascorbic acid for 15 min. A neodymium magnet was used to hold the MIL to the wall of the sample tube and the aqueous sample decanted. The MIL was dissolved in 3 µL of ethanol and transferred to the surface of a TiO2 coated electrode. Ascorbic acid was then determined by differential pulse voltammetry. The LOD for the procedure was 0.43 nmol L-1 (0.076 µg L-1), and the EF was 111. A second example by Li et al. [113] involved LLLME for determination of patulin (a toxic fungus metabolite) in apple juice. This involved the addition of 1.5 mL of ethyl acetate to the vortex of 5 mL of the stirring sample, and then back extraction into 5 µL of water for 20 min. The procedure removed all sugar matrix interference from the extract, which was analyzed by UHPLC-MS/MS, with a LOQ of 2 µg L-1, and a linear range from 2−2,000 µg kg-1.
HS-SDME involves exposing the solvent drop in the HS of a liquid (aqueous or oil) or solid. This technique is much less susceptible to drop instability and has been used successfully for analysis of volatiles in spices, wine and other food products [118–123]. Typically, sample temperatures range from 25–40℃, to avoid evaporation of the solvents, such as hexane. Less volatile solvents, such as DESs, NADESs, and ILs can be used at higher temperatures. Sample sizes range from 10 mg–10 g for solid samples and and 10 µL–10 mL for liquid samples. This technique can compete quite effectively with purge and trap for volatiles analysis, and the extraction can be fully automated as well and is comparatively economical [108].
In an example by Abreu et al. [118], HS-SDME analysis for 2-phenoxyethanol (an anesthetic) was performed on 2 g tilapia homogenate using 1.8 µL of octane for 30 min at 35℃. The octane extract (0.5 µL) was analyzed by GC-MS. The LOQ was 0.56 mg L-1 of sample. The results were comparable to those obtained by SPME, but at requiring less extraction time and cost, with no carryover. In a second example, Triaux et al. [119], analyzed 6 spices for 29 terpenes. The powdered spices (50 mg were extracted for 90 min with 1.5 µL of DES solvent (tetrabutyl bromide:dodecanol, 1:2). The DES extract was analyzed by GC-MS, using a DB-WAX column. LOQs ranged from 0.47 µg/g (borneol) to 86 µg/g (α-farnecene), with most terpenes at less than 2 µg/g. In a third example by Abolghasemi et al. [120], HS-SDME was used to analyze the amounts of 7 triazole fungicides in fruit juices and vegetables. Prepared aqueous samles (10 mL containing 10% salt were extracted for 30 min at 85℃ with 2 µL of DES (choline chloride:4-chlorophenol, 1:2) and the extract analyzed by GC-FID. The LODs for the fungisides ranged from 0.82−1.0 µg L-1.
Reviews and applications for hollow fiber liquid phase microextraction (HF-LPME), EME and parallel artificial liquid membrane extraction (PALME) are listed in Table 6 [124–152]. Membrane based LPME techniques, including HF-LPME were developed , in part, to overcome the drop instability of DI-SDME, as well as for applicability to biological fluids and environmental samples, which contain proteinaceous and particulate matrices [131, 132]. Membrane LPME uses either a so-called hollow fiber-actually a tube constructed of a porous polymer, such as polypropylene (HF-LPME), or a flat sheet of porous polymer membrane. The membrane usually contains a water immiscible solvent (such as 1-octanol or dihexyl ether) separating the aqueous sample, from the extraction solvent in the lumen of the fiber. When the extraction solvent and solvent in the membrane [a supported liquid membrane (SLM)] are the same. This 2-phase system mode is used to extract relatively non-polar analytes from the sample. When the solvent in the lumen is aqueous (acidic or basic), the resulting 3-phase system is used to extract ionized analytes from the sample. The membrane also protects the extraction solvent from particulates, salt or proteinaceous matrix components in the sample. A flat sheet or envelope can also be used for the extraction to separate sample and extraction solvent. This has been used with 96 well and microfluidic systems. When used with a 96 well system, the technique is referred to as PALME, which allows multiple extractions to be carried out simultaneously [129]. Migration through the membrane can be slow (45−90 min). The extraction of three-phase HF-LPME of ionized analytes can be increasesd, with decreased extraction times, using electrokinetic migration, referred to as electromembrane extraction (EME) [132]. This technique can also be used in the PALME mode [parallel EME (pEME)]. As with SDME, EFs for membrane techniques can range from 10 to 1,000. Given the slow migration through the membrane, and long equilibrium times, typical EFs for most membrane-based extractions are arount 25–50. However, these EFs generally allow the use of less sensitive instrumentation for analysis than QuEChERS or macro extraction techniques [124].
Membrane-based LPME: HF-LPME, EME, PALME review and application papers
| Membrane LPME | Subject | Author(s) | Year | References |
|---|---|---|---|---|
| Membrane-based LPME reviews | Efficiencies of HF-LPME | Alsharif et al. | 2017 | [124] |
| HF-LPME, SBME and EME for pesticide analyses | Prosen | 2019 | [125] | |
| Microextraction with supported membranes | Pedersen-Bjergaard | 2020 | [126] | |
| HF-LPME for analysis of metal ions and pharmaceuticals | Khan et al. | 2020 | [127] | |
| HF-LPME with polymer inclusion membrane (PIM) | Olasupo, Suah | 2022 | [128] | |
| 3-phase HF-LPME and PALME | Gjelstad | 2019 | [129] | |
| Mass transfer in EME | Huang et al. | 2016 | [130] | |
| EME | Huang et al. | 2017 | [131] | |
| EME overview | Druoin et al. | 2019 | [132] | |
| Conductive vial EME/PALME, drugs | Skaalvik et al. | 2021 | [133] | |
| Environmental applications of EME | Shi et al. | 2023 | [134] | |
| Chemically modified EME membranes | Li et al. | 2022 | [135] | |
| EME, the liquid membrane, review | Hansen et al. | 2022 | [136] | |
| EME, fundamentals and applications, review | Martins et al. | 2023 | [137] | |
| HF-LPME food, environmental, forensics applications | HF-LPME, GC-MS, OP pesticides, fish | Sun et al. | 2011 | [138] |
| UAE, HF-LPME, GC/NPD, OP pesticides, baby foods | González-Curbelo et al. | 2013 | [139] | |
| Automated 3-Phase HF-LPME, HPLC/UV, herbicides, water | Tajik et al. | 2015 | [140] | |
| DES-HF-LPME, GC-MS, phenolics from beverage plastics | Afshar Mogaddam et al. | 2020 | [141] | |
| NADES, SLM, PALME, LC-MS/MS, OP nerve agents, urine | Bauchouareb et al. | 2022 | [142] | |
| HF-LPME, GC-MS, organo-tin compounds in fruit juice | González-Domínguez, Sayago | 2023 | [143] | |
| HF-LPME, HPLC, herbicides in soil | Moret et al. | 2023 | [144] | |
| HF-LPME, HPLC-FLD, fluoroquinolones, chicken liver | Moema et al. | 2023 | [145] | |
| HF-LPME, PALME, GC-MS, aromatic amines, urine | Lorenzo-Parodi et al. | 2023 | [146] | |
| NADES-HD-LPME, HPLC/UV, triazines, water, urine | Díaz-Álvarez et al. | 2023 | [147] | |
| EME food, forensics applications | EME, HPLC/UV, caffein, gallic acid, coffee | Khajeh et al. | 2017 | [148] |
| EME, HPLC/UV, melamine, infant formulae | Rezaee et al. | 2022 | [149] | |
| MAE, EME/PALME, IC, perchlorate in seafood | Nsubuga et al. | 2016 | [150] | |
| ENE-AAS determination of Cr(VI) in food samples | Goodarzi et al. | 2022 | [151] | |
| Chitosan SLM/EME/PALME/HPLC/UV, coffee, tea | Román-Hidalgo et al. | 2023 | [152] |
LPME: liquid phase microextraction; HF-LPME: hollow fiber LPME; EME: electromembrane extraction; PALME: parallel artificial liquid membrane extraction; SBME: solvent bar microextraction; GC: gas chromatography; MS: mass spectrometry; OP: organophosphorus; UAE: ultrasound-assisted extraction; NPD: nitrogen/phosphorus detector; HPLC: high performance liquid chromatography; UV: ultraviolet; DES: deep eutectic solvent; NADES: natural deep eutectic solvent; SLM: supported liquid membrane; MS/MS: tandem mass spectrometry; FLD: fluorescence detection; UV: ultraviolet; MAE: microwave aided extraction; IC: ion chromatography; UHPLC: ultra-high performance liquid chromatography
In HF-LPME extractions, the extraction solvent is contained within the lumen of a so-called porous hollow fiber, usually polypropylene, though other polymeric materials can be used. The fiber is prepared to length, as needed, and typically contains 5–50 µL of extraction solvent. This technique has been used primarily for extraction of polar or ionizable analytes from aqueous solutions. Two-phase HF-LPME is also an equilibrium process while three phase can result in nearly exhaustive extraction given long enough time, and the use of a complexing, derivatizing or pH sample, receptor solution differential to effectively make the extraction irreversible. The major deficiencies of the technique are long extraction times (45–99 minutes) and pore plugging of the fiber. The technique can also be used with a flat sheet of membrane, separating sample and receiving solution, discussed below [124–129].
An example by Sun et al. [138] illustrates the applicability of HF-LPME for determination of organophosphorus pesticides in fish tissue. Samples were first extracted with acetone, and centrifuged. The acetone extracts were rotory evaporated, and redissolved in 10 mL 5% methanol/water. After filtration, a 2-phase HF-LPME extraction with 30 µL of o-xylene for 30 min was followed by GC-MS analysis. LODs ranged from 2.1–4.5 µg kg-1 for 8 pesticides. A second example by Afshar Moggadam et al. [141] illustrates the ability of HF-LPME to concentrate trace impurities, using a solvent stir bar HF-LPME mode [referred to as solvent bar microextraction (SBME)], combined with simultaneous derivatization. The procedure involved using a fiber containing a DES extraction solvent (8-hydroxyquinoline:pivalic acid), chloroacetyl chloride derivatizing agent and an iron wire inserted into the fiber, with both ends heat sealed. The solvent bar was placed in the sample and stirred for 5 min. The derivatized extracts were analyzed by GC-MS and the procedure was validated using FDA recommendations for the determination of 12 phenols in fruit juices packaged in plastic. The technique had LOQs ranging from 29–76 ng L-1 and EFs of more than 1,000. In a third example by Gonzalez-Dominguez et al. [143] a simultaneous derivatization-extraction of organotin compounds in a solution of 1 mL fruit juices, 4 mL of buffer solution and sodium tetraethyl borate, and a 10 min 2-phase extraction with hexane contained in the lumen of the fiber. The extract was analyzed by GC-MS, with LOQs of 0.8–1.8 µg L-1. In a fourth example, Lorenzo-Parodi et al. [146] compared 3-phase HF-LPME and 3-phase PALME for the determination of aromatic amines in the urine of smokers. PALME was determined to be faster, less labor intensive, capable of automation and required much smaller volumes of sample. GC-MS analyses resulted in LODs ranging from 45–75 ng L-1 for 13 aromatic amines.
EME was originally conducted with a three phase HF-LPME system, with electrodes placed inside of the fiber lumen and the sample. A potential placed on the electrodes enabled the extraction of ionized drugs from biological fluids in much shorter timeframes than HF-LPME alone [130–137]. Both HF-LPME and EME have more recently have transposed to a 96 well system, using the PALME methodology, will be discussed in section CF, microfluidics and 96 well LPME.
Two examples illustrate the potential of EME for the analysis of foods, Rezaee et al. [149] extracted melamine adulterant in infant formulae using EME, with a fiber reinforced with nano graphine oxide (NGO). The NGO acted as an adsorbent interface, enhancing the electrical conductivity and migration of analyte into the acceptor solution, thus enhancing recoveries of polar analytes. The fiber solvent (SLM) used was 1-octanol, the sample solution was adjusted to pH 3 and the acceptor solution (20 µL) pH 2 with a 70 V potential for 10 min, followed by HPLC/UV. An LOQ of 0.1 µg kg-1 for infant formula was obtained. In the second example by Nsubuga et al. [150] seafood samples were microwave digested and the solutions subjected to pEME. pEME was performed in polypropylene envelopes containing an electrode, 120 µL of 0.1 mol L-1 NAOH and 1-hexanol as the SLM solvent. EME was conducted with 25 mL of sample solution (pH 6) at 12 V for 10 min. Following EME, the extract was analyzed with IC for an LOD of 40 µg kg-1.
DLLME, in its several variations, is the most widely used LPME mode, because extraction occurs almost instantaneously or within the 2–5 min needed to form a dispersion of water insoluble solvent in an aqueous sample. DLLME reviews and food preparation applications are listed in Table 7 [153–187]. The EFs are typically in the range of 50−150, and recoveries 90–119%, and, along with the short extraction times, account for the popularity of this LPME technique [154–157]. These results are due to the huge surface area of the extraction solvent formed by dispersion formation [155, 156]. Dispersion can be achieved several ways, and the technique used depends on the nature of the sample, to some extent, but also on the preference of the analyst [165]. These techniques are illustrated below. DLLME has been used successfully, alone, in food analyses, or as a further cleanup-concentration technique for SPE and QuEChERS extractions. A downside to the technique is the tendency of experimentalists to emphasize minimizing LOD and LOQ by using relatively large volumes of sample and extraction solvent. Greener DLLME sample preparations, when possible, should limit sample sizes to 5 mL or less and solvent sizes 400 µL or less (preferably less than 50 µL) to provide an analyte concentration allowing for less sensitive analytical instrumentation and less hazardous waste production [157]. In addition, DLLME, as with all extraction techniques, requires a highly qualified analyst. Each extraction method must be rigorously developed and optimized, even when based on a published method. DLLME can in some cases be automated, but this is not commonly employed [185]. After dispersion formation, the dispersion must be broken and the sample and extraction phases separated. This can be accomplished by centrifugation, the addition of water soluble solvent or salt, with the use of temperature control, or the use of a magnetic solvent (MDES, MIL), as discussed below [66, 72, 159].
DLLME and variants, reviews and food application papers
| DLLME | Subject | Author(s) | Year | References |
|---|---|---|---|---|
| DLLME reviews | DLLME classification and terminology | Šandrejová et al. | 2016 | [153] |
| DLLME with derivatization | Sajid | 2018 | [154] | |
| DLLME applications | Teshale, Taye | 2019 | [155] | |
| DLLME, overview of reviews | Dmitrienko et al. | 2020 | [156] | |
| DLLME, modes and solvents | Kokosa | 2020 | [157] | |
| Effervescence-assisted (EA)-DLLME | Lasarte-Aragonés et al. | 2020 | [158] | |
| Magnetic DESs in microextraction techniques | Grau et al. | 2022 | [159] | |
| Coacervation, DLLME strategies in hydrophilic media | Pacheco-Fernández et al. | 2021 | [160] | |
| DLLME, HLLME with DESs, review | Ramezani et al. | 2022 | [161] | |
| Challenges in DLLME: advanced strategies | Faraji | 2024 | [162] | |
| In situ dispersion formation/decomposition in situ-DLLME | Ahmadi et al. | 2023 | [163] | |
| SS-DLLME | Ullah, Tuzen | 2023 | [164] | |
| Dispersion techniques for DLLME | El-Deen et al. | 2023 | [165] | |
| DLLME food applications: surfactant assisted (SA)-DLLME | SA-DLLME, HPLC/DAD, aromatic amines, barbequed meat | Barzegar et al. | 2019 | [166] |
| SA-DLLME-SFOc, GC/ECD, OCPs, cocoa | Mardani et al. | 2021 | [167] | |
| SA-DLLME, GC-FID, chlorpyrifos, green tea, GC-FID | Tian et al. | 2022 | [168] | |
| DLLME food applications: vortex assisted (VA)-DLLME | MDES-VA-DLLME, QTOF-MS, PFAS, edible oils | Fan et al. | 2021 | [169] |
| VA-DLLME with DES for sulfonamides in water | Mostafa et al. | 2022 | [170] | |
| DES-VA-DLLME-SFO, Vis, Fe, food, water | Zhang et al. | 2022 | [171] | |
| High density DES-VA-DLLME, HPLC/UV, herbicides, milk | Feng et al. | 2022 | [172] | |
| MDES-VA-DLLME, HPLC/DAD, fungicides, juice, vinegar | Wang et al. | 2023 | [173] | |
| DES, VA-DLLME, HPLC/DAD, flavonoids, vinegar | Bai et al. | 2023 | [174] | |
| DLLME food applications: ultrasound-assisted (UA)-DLLME | UA-DLLME, LC-MS, chloramphenicol, honey | Campone et al. | 2019 | [175] |
| DES-UA-DLLME-SFO, FAAS, NI, Co, food, water | Tavakoli et al. | 2021 | [176] | |
| DES-UA-DLLME-SFO, HPLC/DAD, antibiotics, food | Shirani et al. | 2022 | [177] | |
| UA-DLLME-SFO of propineb in water, food, water | Elik, Altunay | 2023 | [178] | |
| DLLME food applications: air-assisted (AA)-DLLME | MDES-AA-DLLME, UV, melamine, milk products | Elik et al. | 2023 | [179] |
| DLLME food applications: SS-DLLME | DES-SS-DLLME, GC-FID, phenolic antioxidants, oils | Mogaddam et al. | 2021 | [180] |
| Octanoic acid, SS-DLLME, flow-Vis, Co, food | Santos et al. | 2022 | [181] | |
| Salicylic acid, SS-DLLME, HPLC/UV, insecticides, honey | Wang et al. | 2023 | [182] | |
| DLLME food applications: in situ-DLLME | In situ-DLLME, pH induced, antibiotics in water | Ma, Row | 2021 | [183] |
| MIL-in situ-DLLME, HPLC/UV, sulfonamides in milk | Yao, Du | 2020 | [184] | |
| SUPRAS-in situ-DLLME, HPLC-FLD, PAHs, tea | Timofeeva et al. | 2021 | [185] | |
| DES-in situ-DLLME-SFO, LC-MS, antibiotic, honey | Nemati et al. | 2021 | [186] | |
| MDES-in situ-DLLME, HPLC/UV, triazine herbicides, rice | Piao et al. | 2021 | [187] |
DLLME: dispersive liquid-liquid microextraction; DESs: deep eutectic solvents; HLLME: homogeneous liquid-liquid microextraction; DAD: diode array detector; SFO: solidified floating organic droplet; OCPs: organochlorine pesticides; GC: gas chromatography; ECD: electron capture detector; FID: flame ionization detector; QTOF: quadrupole time of flight; PFAS: polyfluorinated aliphatic solvents; Vis: visible; UV: ultraviolet; FAAS: flame atomic absorption spectroscopy; MIL: magnetic ionic liquid; SUPRAS: supramolecular solvent; FLD: fluorescence detector; PAHs: polycyclic aromatic hydrocarbons; MDES: magnetic deep eutectic solvent
Note. Adapted from “Green microextraction methodologies for sample preparations” by Kokosa JM, Przyjazny A. Green Anal Chem. 2022;3:100023 (https://www.sciencedirect.com/science/article/pii/S2772577422000222?via%3Dihub). CC BY 4.0 Deed.
Solvent assisted (SA)-DLLME involves dissolving a water-insoluble extraction solvent (such as C2Cl4, 1-octanol, DES, MDES, NDES, IL, or MIL) in a water-soluble solvent (ACN, ethanol, methanol, or acetone) and injecting the solution rapidly into the aqueous sample, to obtain a dispersion [72, 156, 157]. The proper volumes of sample, extraction solvent and dispersion solvent must be obtained by experiment. Published data should be used as guides only. The dispersion is broken up by centrifugation, salt or dispersion solvent addition. Chlorinated solvents are sometimes still used, since their high density allows easy removal from the bottom of the centrifuge tube. Low density solvent may require using a thin neck tube or removing the water with a long needle syringe first [157, 165]. High melting liquids (such as undecanol, some DESs, and ILs) can be solidified and physically removed to a sample vial (DLLME-SFO) [157]. MDESs and MILs can be retrieved, sometimes without centrifugation, using a neodymium magnet, though this must be followed up by back extraction with 10–20 µL of solvent compatible with the analytical instrument. NADES and some DESs can be directly used in GC. Ionic DESs and ILs are normally chromatographed with HPLC [155–157].
In a paper by Barzegar et al. [166] barbequed meat was analyzed for aromatic amines. One gram of homogenized sample was mixed with KOH, ACN and ethanol and microwave extracted. The resulting solution was centrifuged, the pH adjusted to 3 and Carrez I and II solutions (potassium ferricyanide, ZnSO4) added to clarify the proteins and derivatize amines in the solution. After changing the pH to 11 and centrifugation, the upper liquid phase was used for DLLME. A solution of 100 µL of 1-octanol in 600 µL ethanol was injected into the prepared solution and, after centrifugation, 20 µL of the upper layer of 1octanol analyzed by HPLC/UV. LODs ranged between 0.25–0.71 µg kg-1 for 4 aromatic amines. In a second example, Mardani et al. [167] analyzed cocoa powder for organochlorine residues using a DLLME-SFO preparation procedure. Cocoa powder (1 g) was mixed with 4 mL of 5% NaCl solution, and 80 µL 1-decanol in 1 mL ethanol injected after raising the temperature to 80℃. After refrigerated centrifugation, the solidified 1-decanol extract was analyzed with GC/ECD. LOQs ranged from 0.091–0.175 µg kg-1 for 5 organochlorine pesticides (OCPs), including Aldrin.
VA-DLLME avoids the need for a dispersion solvent, which, when used in excess, can decrease the yield of extraction solvent recovery. Typically, the extraction solvent is rapidly injected by syringe into the sample, which is then immediately vortexed for 30–90 s. Vortexing does not always yield a true dispersion, which should not immediately separate into separate layers. However, vortexing still results in significant extractions. The sample is retrieved as above [157].
Total iron in water and food samples was determined Zhang et al. [171] using reductive-derivatization, followed by VA-DLLME-SFO. Solutions containing Fe (II) and Fe (III) were mixed with a DES (thymol:lauric acid) and a complexing agent (1,10-phenanthroline). The DES acted as both the extraction solvent and a reducing agent, converting Fe (III) to Fe (II), which then complexed with the 1,10-phenantroline. After VA-DLLME and cooling, the solidified DES extraction solvent was analyzed with VIS detection. The LOQ for total iron was 1.5 µg L-1. Wang et al. [173] analyzed strobilurin fungicides in water, juice and vinegar using MIL-VA-DLLME. The MDES was prepared from methyltrioctylammonium chloride, ferric chloride and heptanoic acid. Addition of 200 µL of the MDES to 5 mL of sample and vortxing for 3 min. was followed by addition of 90 mg of carbonyl iron powder (CIP) to aid in separating the MDES. The MDS, was then filtered to remove the coagulated CIP-ferric chloride, and the DES extract analyzed by HPLC/UV. The resulting LODs for 3 fungicides ranged from 1–2 µg L-1.
UA-DLLME is comparable to VA-DLLME, but usually results in a more stable dispersion system. Ultrasound should be limited to as short a time as possible, to prevent analyte degradation and possible loss of more volatile analytes. Sample retrieval is the same as above [157].
An example of DES-UA-DLLME-SFO for the determination of nickel and cobalt in broccoli, spinach and environmental water samples was demonstrated by Tavakoli et al. [176]. Food samples were digested with HNO3/H2O2 and 150 µL DES (d,l-menthol:decanoic acid) added to 50 mL of prepared sample, 2 mL pH 6 buffer and 100 µL of complexing agent [2-(5-bromo-2-pyridoazol)-5-(diethylamino) phenol] dissolved in ethanol. After ultrasonic treatment for 2 min., the sample was centrifuged and cooled in an ice bath. The solidified DES was removed, allowed to melt and diluted to 1 mL with ethanol, and the extract analyzed by FAAS. LOQs were 1.1 µg L-1and 1.3 µg L-1, for Co and Ni, respectively and the EF was 50. A second example of UA-DES-DLLME-SFO was used by Elik and Altunay [178] for propineb fungicide in water and foods, including cereal based baby foods and tomato. The procedure involved the addition of 330 µL of DES (8-hydroxyquinoline:pivalic acid) to a 5 mL sample solution (pH buffered to 4.6), 1.5 min sonication, centrifugation, solidifying the DES in an ice bath, diluting the solidified DES in 500 µL of ethanol and UV detection. The LOD was 6.1 µg L-1, and the EF was 93.
The term air-assisted (AA) is a misnomer, since dispersion is actually obtained by the shearing forces resulting from the rapid movement of sample and extraction solvent into and out of a syringe. The process works best with small volumes of sample, to ensure maximum sample-solvent interaction [157, 165].
Elik et al. [179] used a MDES-AA-DLLME procedure for the analysis of melamine in milk and milk products. Prepared solutions were extracted by drawing a solution of 260 µL magnetic DES [octanoic acid:Aliquat-336:Co (II), 3:2:1] and 125 µL acetone in and out of a the sample with a syringe 6 times. The MDES was separated from the sample solution with a neodymium magnet, the DES back extracted and the extract analyzed by UV-Vis. The LOQ was 0.9 µg L-1, with an EF of 144.
Switchable solvents (SSs) are simply water insoluble acids or bases which become soluble or insoluble, depending on the pH of the solution. As an example, sodium hexanoate added to an aqueous sample will becomes an insoluble dispersion when acid is added. Recovery is as above [157, 162].
A DES-SS-DLLME extraction was developed by Mogaddam et al. [180] for the extraction of phenolic antioxidants from edible oil samples. The antioxidants were first extracted from the 5 mL of oil at 80℃ with 1.25 mL of 1.5 mol L-1 NaOH for 5 min and centrifuged, The extracted antioxidants were concentrated for analysis by dissolving DES (tetrabutylammonium chloride:hydroquinone) in the alkaline solution, followed by dispersion formation of the DES upon acidification. After centrifugation, the DES extract was analyzed by GC-FID. The LOQs ranged from 0.36−1.41 µg L-1 with EFs averaging 400. Wang et al. [182] developed a simple SS-DLLME for on-site sample preparation of benzoylurea insecticides in water and honey samples. The water and honey samples (5 mL honey diluted to 100 mL) were filtered to remove particulates, and 15 mL added to a plugged 20 mL plastic syringe, along with 1.1 mL (100 mg mL-1) of salicylic acid solution. After addition of H2SO4 solution to acidify the sample, a salicylic acid dispersion formed. NaCl (0.5 mL, 20%) was added, the syringe plunger attached, the plug removed and replaced by a needle containing a porous fabric to retain the salicylic acid. The water was expelled from the syringe, which was then returned to the laboratory for analysis of the extract. After dissolving the salicylic acid in methanol, the solution was filtered and analyzed by HPLC/UV. The LODs for the insecticides were 1.5 µg L-1 for the water samples and 30–90 µg kg-1 for the honey samples.
In situ-DLLME, also referred to as homogeneous liquid-liquid microextraction (HLLME) most commonly involves formation of a dispersion by the in situ formation of an insoluble extractant solvent, or by temperature control to form a SUPRAS [157, 160, 163].
Three example are discussed below.
Antibacterial sulfonamides were determined in milk with MIL-in situ-DLLME sample preparation in a paper by Yao and Du [184]. The technique involved adding a water soluble MIL (containing an organic free radical, 4-OH-Tempo) to milk samples which had been denatured and filtered. KPF6 was then added to the solution, resulting in the substitution of the chloride ion in the soluble IL with the PF6-1 ion and formation of an emulsion, extracting the contaminants. The MIL was separated from the solution using a neodymium magnet, back extracted into methanol and HPLC/UV used for final analysis for 5 sulfonamides. LOQs ranged from 1.8–3 µg L-1, with EFs between 42–47. Timofeeva et al. [185] developed an automated system for the analysis of PAHs in tea infusion using SUPRAS-in situ-DLLME, based on a syringe pump system interfaced with HPLC/fluorescence detection (FLD). The procedure involved first: the formation of a SUPRAS emulsion by mixing hexanoic acid with NaOH in a 1:4 molar ratio. This was followed by addition of 1.2 mL of the emulsion to 3.8 mL of tea sample in the syringe, with stirring. Stirring was then stoped to allow separation of the phases. The upper phase (50 µL) was then transferred from the syinge directly to the HPLC for analysis. The syringe was automatically cleaned during the HPLC run for a another sample. The LODs for PAHs ranged from 0.02−0.04 µg L-1, and EFs ranged from 38−46. As a final DLLME example, Piao et al. [187] developed a MDES-in situ-DLLME sample preparation for the analysis of triazine herbicides in rice. The procedure involved extraction of 1 g of powdered rice with 4 mL hexane and centrifugation. Addition of 250 µL DES (ethylene glycol: tetrabutylammonium chloride), 40 mg iron chloride, and 90 mg of CIP to the hexane was followed by removal of the generated MIL/CIP. Separation of the MIL/CIP with a neodymium magnet extracted the herbicides from the hexane. The MIL/CIP was then back extracted with 5 mL of diethyl ether, the ether evaporated, the residue dissolved in 100 µL of ACN, and 20 µL of the extract analyzed by HPLC/UV. LOQs ranged from 5–10 µg kg-1.
Due to the reduction in sample and extraction solvent sizes, LPME lends itself to CF, microfluidic and 96 well techniques. However, very little research or application has been published related to food sample analyses using them. A list of relevant review and application papers are listed in Table 8 [188–201], as a guide to those wishing to explore this area. Also included is one application paper using microfluidic with EME extraction conditions. CF has been used for DI-SDME, HS-SDME, HF-LPME and microfluidic extractions [188–194]. Microfluidic techniques are most frequently used with membrane extraction (PALME, EME). MIL and MDES held to a neodymium rod allows multiple DI-SDME extractions with 96 well devices. Membrane PALME and pEME have been used with 96 well devices as well. PALME involves the use of a 96 well system with a flat membrane to perform extractions, with two and three phase HF-LPME and EME emulations [188–194]. The 96 well plate uses orbital motion for mixing of up to 96 sample wells, therefore allowing multiple simultaneous extractions, and also using less sample than traditional HF-LPME and EME. Sample and extraction solvent volumes of 250 µL or less are commonly used. Disposable conductive chambers have also been used for the 96 well pEME applications, which eliminates disposal or cleaning of sample plastic or metal sample chambers. Since the sample and receiver chambers are similar in volumes, the EFs for these systems are close to 1. The advantage, however, is the increased sample throughput resulting from the simultaneous processing 30 or more samples, as well as the cleanup resulting from the use of a membrane system. microfluidic devices have been used most successfully for membrane extractions, similar to PALME, but under CF conditions. Microfluidics are still a developing area for LPME, and applications are essentially centered on biological fluids, as with PALME [192–194].
CF, microfluidics and 96 well LPME reviews and key application papers
| Recent LPME techniques | Subject | Author(s) | Year | References |
|---|---|---|---|---|
| Reviews | LPME and EME in microfluidic devices | Ramos-Payán | 2019 | [188] |
| Flow based methods and applications in chemical analysis | Tomofeeva et al. | 2021 | [189] | |
| Nanofluidic devices for biological analyses | Yamamoto et al. | 2021 | [190] | |
| Conductive vial EME | Schüller et al. | 2023 | [191] | |
| Microfluidic devices in sample extraction overview | Alidoust et al. | 2021 | [192] | |
| Lab-on-a-chip systems in molecular diagnosis | Cunha et al. | 2022 | [193] | |
| EME with microfluidic devices | Hansen et al. | 2022 | [194] | |
| Key application papers | MIL-DI-SDME with 96 well device | Mafra et al. | 2021 | [195] |
| NADES-SLM-SDME, HF-LPME 96 well system | Morelli et al. | 2020 | [196] | |
| SLM-SDME, HF-LPME 96 well system | Lopes et al. | 2022 | [197] | |
| Automated DES-VA-DLLME, 96 well system | Ju et al. | 2023 | [198] | |
| 3-Phase HF-LPME 96 well system, extraction time | Schüller et al. | 2022 | [199] | |
| Microfluidic, microextraction and analysis on a chip | Santigosa-Marillo et al. | 2023 | [200] | |
| EME, microfluidic system, derivatization, HPLC/UV, biogenic amines, food | Zarghgampour et al. | 2018 | [201] |
LPME: liquid phase microextraction; EME: electromembrane extraction; MIL: magnetic ionic liquid; DI: direct immersion; SDME: single drop microextraction; NADES: natural deep eutectic solvent; SLM: supported liquid membrane; HF-LPME: hollow fiber liquid phase microextraction; DES: deep eutectic solvent; VA: vortex assisted; DLLME: dispersive liquid-liquid microextraction HPLC: high performance liquid chromatography; UV: ultraviolet
Morelli et al. [196] developed a method for the extraction and analysis of 11 contaminants of concern in environmental water, including pesticides and hormones. The sample preparation involved extraction with DES impregnated in the pores of 1 cm long hollow fibers, placed over the pins of a 96 well sampler. The authors refer to this as HF-microporous membrane liquid-liquid microextraction (HF-MMLLME). A less cumbersome and more descriptive name which will be used here is DES-SLMME (DES-SLM microextraction). In effect, this technique is actually a variation of SDME, with the solvent held in the pores of the fiber polymer, rather than at the tip of a syringe. The procedure involved immersion of the fiber in 400 µL of DES (Thymol:Camphor, 1:1) for 10 min., immersion of the SLM into 1.5 mL of water sample for 50 min, and desorption with 300 µL of acetone:methanol (3:1) and analysis of 20 µL of the extract with HPLV/UV. The LODs ranged from 0.3–6 µg L-1. The maximum EFs for this technique can be no higher than 5, due to the final ratio of sample and final desorption volumes, but the procedure allows automation of at least 30 samples simultaneously.
An EME sample preparation for 5 biogenic amines in foods, using a CF microfluidic device, was developed by Zarhgampour et al. [201]. Samples (1 g), including sausage, were treated with trifluoroacetic acid, vortexed, filtered and the resulting solution diluted to 5 mL. The SLM for the microfluidic was a 3 mm × 4 mm porous polypropylene sheet, which was impregnated with 2-nitrophenyl octyl ether containing 10% 2-(2-ethylhexyl) phosphate. The receiving solution was 50 µL of 0.09 mol L-1 HCl. A total volume of 2 mL of the sample was passed through the microfluidic device, and the receiving solution then treated with dabsyl chloride derivatizing agent, to allow analysis and detection by HPLC/UV. LODs ranged from 0.3–8 µg L-1 for this semiautomated CF microfluidic system.
The original QuEChERS procedure was developed to meet the needs of laboratories monitoring agricultural products for pesticide contaminants [6]. Several hundred pesticides, ranging from very polar, to very nonpolar, were the potential contaminants of high-water content fruits, vegetables and processed products. To add to the requirements of the task, the samples contained very complex matrices, including sugars, lipophilic components and pigments. Due to the massive scale of the monitoring requirements, a simple, fast, and reliable method was needed to simultaneously test for more than 100 potential pesticides which could be present.
The method centered on two crucial preparation steps. First, a 10 g (mL) homogenized sample was treated with 10 mL of ACN, followed by addition of a mixture of MgSO4 and NaCl to the extract to remove most of the water from the ACN, while extracting contaminants (salting out and extraction). The second step involved the use of a mixture of additional MgSO4 and primary-secondary amine (PSA) sorbent to remove remaining water and matric interferences, including carboxylic acids. Addition of small amounts of 0.05–0.1% acetic acid was added when base sensitive pesticides were present, and graphitized carbon black (GCB) addition removed chlorophyl from green leafy vegetable samples and C18 absorbent removed additional lipid material.
PSA is a diamine containing primary and secondary amines, bonded to silica: (Si-CH2CH2CH2NHCH2CH2NH) and is added to the ACN extract as a “dispersive solid phase extraction (d-SPE)”, which is easily separated from the extraction solvent after centrifugation [6].
This initial work was followed up by two independent validation regimens [202, 203], which were the basis for the resulting published QuEChERS procedures used as official methods for multiclass pesticide sample preparations for agricultural foods and food products [204, 205]. The method procedures have been further modified during the last 2 decades to enable agricultural, dairy, meat and fish produce monitoring for pesticides, PAHs, and harmful biological contaminants [8]. The QuEChERS methodology has also been further modified by Lehotay [9], to enable a wider array of analyte polarities to be analyzed. The terms “efficient and robust” were added to QuEChERS (QuEChERSER) to distinguish this variation. The four classical papers covering the developments of QuEChERS [6, 9, 202, 203] are recommended reading requirements for analytical scientists working in the fields of environmental, forensics, industrial and foods analyses for the development of validated analytical methodology. They coherently present the scope, purpose, advantages and limitations of sample preparation methodology, and the development of validated analytical methods.
The original QuEChERS methodology relied on a reliable, rugged and relatively inexpensive single quadrupole benchtop GC-MS instrument for the final pesticide analysis [6]. The development of more sophisticated and sensitive instrumentation (GC-MSMS, UHPLC-MS/MS) allowed a wider range of more than 100 multiclass analytes, especially the polar pesticides, to be analyzed without the need for derivatization. This advantage is also a major disadvantage, since the required instrumentation is very expensive to purchase and, importantly, to maintain and operate, making them practical only for major high-throughput regulatory or research laboratories. In addition, while QuEChERS removes most of the matrix contaminants from the analytical sample, enough remains that can damage or contaminate the instruments, requiring increased maintenance [9]. An additional disadvantage of QuEChERS is that the technique produces large amounts of hazardous waste, since solvents, extracts, and solid wastes in contact with hazardous materials must in turn be treated as contaminated materials [4, 12, 13, 24]. One solution to this last limitation is to reduce the quantities of sample, solvents, and reagents required for sample preparation. This approach has led to the development of the µ-QuEChERS methodology.
While the original QuEChERS method developers foresaw the possibility of using smaller amounts of sample, solvent and reagents, the approved methods used 10–15 g of sample, to provide the concentrations of pesticides in the final extracts to allow LOQ in the low ppb or high ppt ranges [204, 205]. An attempt to reduce sample requirements for QuEChERS was published in 2015 by Porto-Figueira et al. [11]. Their approach for monitoring zearalenone, a fungus aflatoxin in cereal grains, used 0.3 g of cereal sample (maize, corn) 0.7 mL ACN and 0.2 g of QuEChERS salt for the first step, and 75 mg MgSO4, 12.5 mg of C18 12.5 mg of PSA for the cleanup. The final extracts were filtered, evaporated to near dryness and diluted with HPLC mobile phase. The samples were then analyzed using UHPLC-FLD.
Seven applications using the µ-QuEChERS approach are included in Table 9 [6–9, 11, 202–211]. The methodology has been used for pesticides, aflatoxins, alkaloids and polyphenols analysis in a variety of foods, food products and water runoff from washed foods. The reduced sample sizes, however, continue to require the use of highly sensitive chromatographic and detection instrumentation in the final analyses, though hazardous waste, analysis time and costs are reduced, while potentially increasing sample throughput and improving the green nature of the total methods.
Micro-quick, easy, cheap, effective, rugged, and safe (µ-QuEChERS)
| QuEChERS | Subject | Author(s) | Year | References |
|---|---|---|---|---|
| QuEChERES and QuEChERSER methodologies, reviews | Original QuEChERS methodology | Anastassiades et al. | 2003 | [6] |
| QuEChERS (AOAC) methodology | Lehotay | 2007 | [202] | |
| AOAC official method 2007.01 | AOAC | 2007 | [203] | |
| (CSN EN 15662) QuEChERS methodology | Payá et al. | 2007 | [204] | |
| BS EN 15662 EU Committee for Standardization, QuEChERS | BS EN 15662 | 2018 | [205] | |
| QuEChERSER methodology review | Lehotay | 2022 | [9] | |
| QuEChERS applications review 2020-2023 | Santana-Mayor | 2023 | [8] | |
| QuEChERS review | Varela-Martínez | 2020 | [7] | |
| µ-QuEChERS applications | µ-QuEChERS method, cereals | Porto-Figueira et al. | 2015 | [11] |
| µ-QuEChERS, polyphenols in baby foods | Casado et al. | 2018 | [206] | |
| µ-QuEChERS, alkaloids in oregano | Izcara et al. | 2020 | [207] | |
| µ-QuEChERS, alkaloids in vegetables | González-Gómez et al. | 2022 | [208] | |
| µ-QuEChERS, patulin in apple juice | Câmara et al. | 2023 | [209] | |
| µ-QuEChERS, pesticides in food washing | García-Cansino et al. | 2023 | [210] | |
| µ-QuEChERS, capsaicinoids in red peppers | Rodrigues et al. | 2023 | [211] |
QuEChERS: quick, easy, cheap, effective, rugged, and safe; AOAC: Association of Official Analytical Chemists (AOAC International); QuEChERSER: QuEChERS, efficient, robust; µ-QuEChERS: micro-QuEChERS
Two recent examples illustrate the scope of the µ-QuEChERS sample preparation technique. González-Gómez et al. [208] developed a µ-QuEChERS procedure for determination of tropane alkaloids in leafy vegetables, with GC-MS/MS analysis. The procedure reduced reagent and solvent requirements 10 fold, compared to QuEChERS. The leafy vegetables were lyophilized to remove water, and 0.5 mL of water and 1 mL of ACN added to 0.1 g of the powder for the initial extraction. After vortexing, 0.65 g of partitioning salts and citrate buffer were added with vortex and ultrasound mixing. After centrifugation, the upper layer was treated with MgSO4 (150 mg) and PSA (25 mg), vortexed and centrifuged. Internal standards were added, and the supernatant blown dry, redissolved in ACN:water, (1:1), filtered and analyzed by UHPLC-MS/MS. The LOQs for the method were < 2 µg kg-1. The presence of alkaloids like scopolamine in samples illustrates the co-dependence and connection between environmental and food analyses, since these alkaloids could have arisen either through co-harvesting weeds containing the alkaloids, or by uptake from the soil containing residual alkaloids. The second procedure by Câmara et al. [209] involved the analysis of patulin in apple juices using µ-QuEChERS, followed by UHPLC-MS/MS. The µ-QuEChERS extraction involved the addition of 1 mL of ACN/1% acetic acid to 100 mg of sample, salting out with 0.65 g partitioning salts, ultrasound mixing and centrifugation. The supernatant was filtered and subjected to HPLC-MS/MS analysis. LOQ for patulin was 1.2 µg kg-1. These results should be compared to the similar results achieved by Li et al. [113] (section DSDME) using LLLME.
As illustrated in sections from Single drop microextraction (SDME) modes to CF, microfluidics and 96 well LPME, LPME sample preparations purify and concentrate analytes so that greener, less sensitive, and much less expensive instrumentation can be used for analyses. This section lists reviews applications (Table 10) for LPME, used in conjunction with other sample preparation techniques, including SPE and QuECHERS for further purification and concentration of samples, and increasing the green nature of sample preparations for food, environmental, biological and other analytical sample preparations which contain complex matrices [1, 10, 212–229].
LPME coupled to other extraction techniques, reviews and applications
| LPME with other techniques | Subject | Author(s) | Year | References |
|---|---|---|---|---|
| Combined extraction technique reviews | LPME combined with other extraction techniques for food analysis | Moreda-Pineiro et al. | 2019 | [1] |
| LPME and coupled green extraction techniques for food analysis | Moreda-Piñeiro et al. | 2023 | [10] | |
| SPE-DLLME application | SPE-DLLME, HPLC/UV, carbamates, fruits and vegetables | Zhou et al. | 2012 | [212] |
| EME-DLLME application | EME-DLLME, GC-MS/MS, biogenic amines in non-alcoholic beer | Kamankesh et al. | 2023 | [213] |
| µ-QuEChERS-DLLME application | µ-QuEChERS-DLLME, GC-MS, PAHs in coffee, tea, water | Kamal El-Deen et al. | 2021 | [214] |
| d-SPE-DSDME application | d-SPE-DSDME, GC-MS/MS, multiple residue pesticides, tea | Li et al. | 2020 | [215] |
| QuECHERS-DLLME applications | QuEChERS-DLLME-SFO, GC/ECD, OCPs, fish | Wang et al. | 2017 | [216] |
| QuEChERS-IL-DLLME, LC-MS, pesticides, fruits, vegetables | Lawal et al. | 2018 | [217] | |
| QuEChERS-DLLME, GC-MS, ethion, bifenthrin, palm dates | Abdel Ghani et al. | 2018 | [218] | |
| QuEChERS-DES-UA-DLLME, GC-FID, pesticides, tomato | Farajzadeh et al. | 2019 | [219] | |
| QuEChERS-DLLME-H2SO4, GC-MS/MS, halo-organics, fish | Nagyová, Tölgyessy | 2019 | [220] | |
| QuECHERS-EMR-Lipid-DLLME, GC-MS, PAHs, smoked foods | Slámová et al. | 2020 | [221] | |
| QuEChERS-DLLME, GC-MS, 4-PAHs, roasted cocoa beans | Agus et al. | 2020 | [222] | |
| QuEChERS-DLLME, GC-MS, multiclass pesticides, yoghurt | Szarka et al. | 2020 | [223] | |
| QuEChERS-DLLME-H2SO4, LC-MS, Br-fire retardants, fish | Okšová, Tölgyessy | 2020 | [224] | |
| QuEChERS-DLLME, LC-MS, neonicotinoid pesticides, grains | Ma et al. | 2020 | [225] | |
| QuEChERS-low density DLLME, GC-MS, Fiprinil, eggs | Zhao et al. | 2021 | [226] | |
| QuEChERS-IL-DLLME, LC-MS, multiclass pesticides, vegetables | Lawal, Low | 2021 | [227] | |
| MNP-QuEChEERS-DLLME, GC-MS, OC-pesticides, vegetables | Yu et al. | 2021 | [228] | |
| QuEChERS-DLLME, LC/QTOF-MS/MS, pesticides in fruits | Sel et al. | 2023 | [229] |
LPME: liquid phase microextraction; SPE: solid-phase extraction; DLLME: dispersive liquid-liquid microextraction; HPLC: high performance liquid chromatography; UV: ultraviolet; µ-QuEChERS: micro-quick, easy, cheap, effective, rugged, and safe; GC-MS: gas chromatography-mass spectrometry; PAHs: polycyclic aromatic hydrocarbons; d-SPE: dispersive solid phase extraction; DSDME: directly suspended deoplet microextraction; SFO: solidified floating organic droplet; ECD: electron capture detector; OCP: organochlorine; DES: deep eutectic solvent; UA: ultrasound-assisted; FID: flame ionization detector; EMR: enhanced matrix removal; MNP: magnetic nanoparticle; QTOF: quadrupole time of flight; MS/MS: tandem mass spectrometry
Many multiresidue sample preparation methods for food sample analyses are based on QuEChERS or SPE, and most combined sample preparations use DLLME for final extract purification, solvent-instrument compatibility and concentration. DLLME is a natural fit in this combination since the final extraction solvent for these methods is oftern ACN, which then can serve as the dispersion solvent for SA-DLLME. However, all LPME modes can potentially be used in conjunction with other extraction methods, including SPME, pressurized hot water extraction (PHWE), pressurized liquid extraction (PLE), and ultrasonic-assisted extraction (UAE) [3]. Table 10 lists 2 recent reviews [1, 10] for combined sample preparation techniques, an example of SPE combined with DLLME for carbamates in food and environmental samples [212], a combined EME-DLLME method for biogenic amines in beer samples [213], a µ-QuEChERS/DLLME method for PAHs in coffee and tea [214], and a modified QuECHERS method combined with DSDME for a multiple residue of pesticides in tea [215]. Additionally, 14 QuEChERS and modified QuEChERS methods, combined with DLLME, are listed for the analyses of halogenated compounds, pesticides and PAHs in a variety of fruits, vegetable, fish and milk products [216–219]. Most of these procedures were developed with the goals of increasing EFs, decreasing matrix interferences and changing the final solvent to one compatitable with the final analytical instrumentation. However, these methods, as well as most published research and application papers, should always be viewed as works in progress requiring scrutiny and the potential for improving methodology. As examples, in several of these procedures, the LPME extraction solvent was CHCl3 or another halogenated solvent, rather than a more recently developed green solvent. In other cases, the final extraction solvent was evaporated and changed to one compatible with UHPLC-MS/MS, when GC-MS may have been just as suitable for analysis. In several of the QuECHERS/DLLME methods, dispersion (or pseudo-dispersion) was achieved by adding water to the ACN, followed by addition of the extraction solvent, contrary to standard conditions for SA-DLLME, VA-DLLME or UA-DLLME. Thus, this area of research needs additional systematic examination of more effective approaches for combined extraction techniques.
Three following examples illustrate the utility of combining LPME with other sample preparation techniques, for the further purification and concentration of samples in food analyses. Zhou et al. [212] developed a method involving SPE, followed by DLLME for the HPLC/UV analysis of carbamates of fruit and vegetables. Homogenized samples were treated with NaCl and MgSO4, and ACN. After sonication and filtration, the liquids were rotary evaporated, and reconstituted in water and filtered. Portions of the filtrates were subjected to SPE, using C18 packing, washing with water to remove polar matrix interferences and eluted with 1 mL ACN. For DLLME, 35 µL of chloroform was added to the ACN extract and rapidly injected into 5 mL of water. After centrifugation, the CHCl3 was removed with a syringe and evaporated. The residue was dissolved in 25 µL of methanol and 5 µL analyzed by HPLC/UV. The LODs ranged from 5–60 pg kg-1 for 3 carbamates. A second example by El-Deen and Shimizu [214] coupled µ-QuEChEERS to an AA-DLLME sample preparation for the analysis of PAHs in coffee, tea and environmental waters. Water samples were prepared by direct AA-DLLME for GC-MS analysis. The coffee and tea samples were powdered and 200 mg samples were extracted with 1 mL each of water and ACN. After vortexing, 200 mg of salts were added, and the sample vortexed and centrifuged. A mixture of MgSO4, PSA and C18 (200 mg) was added to 800 µL of the supernatant, the mixture vortexed, centrifuged and 600 µL of the extract evaporated to dryness and redissolved in 5 mL of water. AA-DLLME was performed by adding 500 µL of diethyl carbonate and drawing the mixture in and out of a syringe 6 times. The sample was then centrifuged, the diethyl carbonate (300 µL separated, and 1 µL analyzed by GC-MS. In addition to further concentrating the analytes, the solvent was changed to one compatible with GC chromatography and caffein interference was significantly reduced. LODs for the procedure were 0.01–2.1 µg kg-1 for 15 PAHs. In the last example, Yu et al. [228] used a modified QuEChERS procedure for the extraction of OCPs from green leafy vegetables, followed by a further cleanup/concentration of the extract with DLLME. The QuEChERS procedure used fairly standard methodology, reduced in scale, for 2 g of the homogenized samples, which were first extracted with 4 mL of ACN, followed by the addition of 0.8 g of NaCl, but with no added MgSO4. After shaking and centrifugation, 2 mL of the ACN extract was treated with 10 mg of PSA, 20 mg of carbon black (rather than GCB), and 20 mg of Fe2O3 magnetic nanoparticles (MNPs). The MNPs were used as an additional cleanup aid, and to facilitate separating the cleanup material from the extract using a magnet. For the DLLME procedure, 1 mL of the ACN extract was added to 4 mL of water and the mixture added to 40 µL of chloroform, the mixture briefly subjected ultrasound mixing, centrifuged, and the CHCl3 dried with a small amount of MgSO4. One µL of the CHCl3 was used for GC-MS analysis of 8 OCPs. The LOQs ranged from 0.15–0.32 µg kg-1, and EFs ranged from 2–3.
A few final comments need to be presented concerning this last example, which may also pertain to questions concerning other similar published procedures. The authors used granular carbon black, rather than the much more expensive commercial GCB to decolorize the extract, with no observable reduction in extract yield. This may be a useful approach for reduced sized QuEChERS methods, but needs further investigation. The authors also use MNPs as a cleanup aid, and to facilitate removal of PSA and carbon black from the extract, rather than centrifugation. It is unknown whether this method would work on a standard QuEChERS sized extraction, but is an interesting application and should also be further investigated. Most unusual, and completely unexplained, however, is the DLLME procedure. Rather than using standard SA-DLLME conditions (addition of CHCl3 to ACN and then dispersion formation by addition of the ACN to water), the authors added the ACN directly to water and then added the mixture to the CHCl3, and then used UA-DLLME to form a dispersion. This unexplained anomaly can also be seen in some of the other examples in Table 10. This unusual modification of DLLME should be compared to SA-DLLME conditions to determine whether it is an acceptable methodology, or not, before further use of this technique for sample preparations. As a final comment, several of the combined sample preparation procedures use chlorinated solvents for extractions. For situations involving a few sample preparations, these solvents may be acceptable, but for large scale operations, even the small amounts of chloroform or other chlorinated solvents should be replaced with greener solvents, since all aqueous and organic solutions, and solids in contact with chlorinated solvents should be treated as hazardous waste, requiring expensive remediation [40].
There are many factors which must be considered before choosing a food sample preparation method involving liquid extraction—with or without using LPME. In general, these include, but are not limited to: the sample characteristics, the extraction solvent, green requirements, available instrumentation, and whether the analyses are undertaken in the field or in a laboratory. There may also be more than one appropriate sample preparation choices for a specific sample. A simplified example of method choice is illustrated in Figure 3.
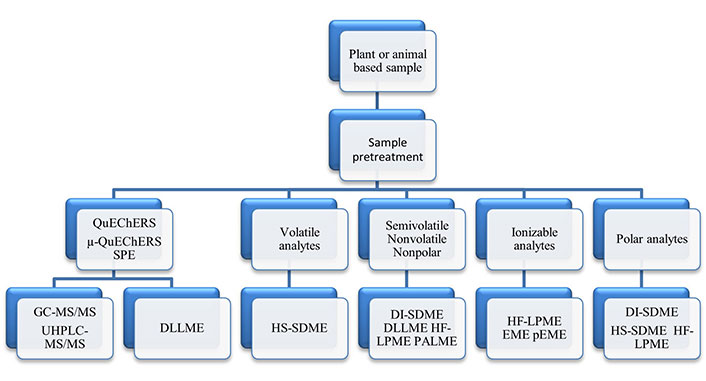
Choosing an LPME mode for plant and animal-based sample preparations. QuEChERS: quick, easy, cheap, effective, rugged, and safe; µ-QuEChERS: micro-QuEChERS; SPE: solid-phase extraction; GC-MS/MS: gas chromatography-tandem mass spectrometry; UHPLC: ultra-high performance liquid chromatography; DLLME: dispersive liquid-liquid microextraction; HS-SDME: headspace-single drop microextraction; DI-SDME: direct immersion SDME; HF-LPME: hollow fiber liquid phase microextraction; PALME: parallel artificial liquid membrane extraction; EME: electromembrane extraction; pEME: parallel electromembrane extraction
Nearly all food analyses require some sample pretreatment, ranging from filtration or centrifugation, to denaturization, lyophilization, or acid degradation. Same analytes, such as elements, aldehydes or acids may also need to be derivatized. Some samples, such as commercial fruit juices or tea effusions, can be analyzed directly by techniques such as SDME or DLLME. This depends on the complexity or the sample matrix, including the presence of fats, proteins, sugars, pigments and other components which might be co-extracted and potentially interfere with the analysis or damage the analytical instrumentation. After a pretreatment step, the number of potential analytes and their physical and chemical characteristics need to be considered, including: are the analytes volatile, or non-volatile, polar or non-polar, ionizable or ionic, organic or inorganic, liquid, oil or solid. The QuEChERS, procedure has the advantages of being suitable for a very wide variety of food samples, providing sample cleanup of fats, proteins and sugars. The downside of the methodology is that little or no analyte concentration enrichment is obtained, necessitating, in most cases, very sensitivive, expensive to purchase, and very expensive to maintain analytical instrumentation. SPE and other traditional sample preparation procedures may overcome some of these deficiencies, but generally are more time and labor intensive. These factors, in addition to the large volumes of hazardous waste potentially resulting from these methodologies, make these techniques most suitable for large volume commercial or governmental analytical laboratories. LPME methods, on the other hand, are simple to use, requiring only standard laboratory supplies, and result in a 10 to more than 100 fold increase of the concentration of target analytes, in addition to sample cleanup, and often allow the use of less sensitive and much less expensive analytical instrumentation, such as GC, GC-MS, HPLC, IC or AAS. The scheme presented in Figure 3 is very general, and omits some protentional sample preparation choices. More detailed and specific selection guides for solvents, sample types and analyte families, as well as the metrics for calculating the relative greenness of an analytical method, are presented in the tables included in this review.
Food samples require extensive sample preparations for instrumental analyses due to the complex matrices involved. Food safety regulatory agencies also require sample preparation procedures that are accurate, sensitive, robust, and, above all, fast, to handle the requirements for determining the safety of the massive amounts of foods and food products needed for human, pet, and livestock consumption. Food contamination can occur at farms or fisheries, industrial food production plants, from food packaging and handling and transport, and finally at local markets. There is also an inseparable interconnection between environmental, agricultural, forensic, cosmetic and industrial analytical chemistry involved in this requirement, and advances in analytical methodology are simultaneously applicable to all of these realms. As a response to these needs, Anastassiades et al. [6] developed a multiclass method for the accurate, sensitive, robust, and, fast analysis of agricultural products potentially containing any of several hundred potential food contaminants. The resulting official QuEChERS sample preparation methods [203, 205] have devolved into hundreds of variants, to meet the need of specific samples and specific analytes to be analyzed, but the original QuEChERS methodology remains the basis for regulatory procedures for large scale analyses of food samples containing a wide variety of possible contaminants. These complex samples, containing trace amounts of contaminants within a complex matrix, require very expensive, very sensitive, final analytical instrumentation, which require highly trained operators and continual maintenance. While suitable for high-throughput, regulatory and university research laboratories, the expense of these requirements may not be necessary or possible for smaller regulatory and field laboratories, requiring sample preparation procedures for only a limited number of specific pesticides, metals, PAHs or other contaminants.
This is the role of LPME in food sample preparations and analysis. As the examples presented here illustrate, LPME, individually or in combination with other sample preparation techniques, such as QuEChERS and SPE, can meet the needs for very sensitive and accurate analyses of specific analytes, even in the very complex matrices found in food, environmental, cosmetic, forensic and industrial samples. This is due to the ability of microextraction techniques to cleanup and concentrate samples to levels allowing the use of greener, less expensive and low maintenance final determination analytical instrumentation, including GC, GC-MS, HPLC, UV-Vis, FAAS, and IC, rather than much more expensive, high maintenance instrumentation, such as UHPLC-MS/MS or high-resolution GC-MS/MS.
The tabulated lists of of food sample preparations included here will provide the reader good starting points for developing validated green procedures for food, environmental or other samples containing simple or complex analyte analyses. This is a rich area for research and development in analytical chemistry.
µ-QuEChERS: micro-quick, easy, cheap, effective, rugged, and safe
AA: air-assisted
AAS: atomic absorption spectroscopy
ACN: acetonitrile
CF: continuous flow
CIP: carbonyl iron powder
DAD: diode array detector
DESs: deep eutectic solvents
DI: direct immersion
DLLME: dispersive liquid-liquid microextraction
DSDME: directly suspended droplet microextraction
d-SPE: dispersive solid phase extraction
EFs: enrichment factors
EME: electromembrane extraction
FAAS: flame atomic absorption spectroscopy
FID: flame ionization detector
GAC: green analytical chemistry
GC: gas chromatography
GCB: graphitized carbon black
GSP: green sample preparation
HF-LPME: hollow fiber liquid phase microextraction
HPLC: high performance liquid chromatography
HS: headspace
IC: ion chromatography
ILs: ionic liquids
LLE: liquid-liquid extraction
LLLME: liquid-liquid liquid microextraction
LODs: limits of detection
LOQs: limits of quantification
LPME: liquid phase microextraction
MDESs: magnetic deep eutectic solvents
MILs: magnetic ionic liquids
MNPs: magnetic nanoparticles
MS/MS: tandem mass spectrometry
MS: mass spectrometry
NADESs: natural deep eutectic solvents
OCPs: organochlorine pesticides
PAHs: polycyclic aromatic hydrocarbons
PALME: parallel artificial liquid membrane extraction
pEME: parallel electromembrane extraction
PSA: primary-secondary amine
QuEChERS: quick, easy, cheap, effective, rugged, and safe
SA: solvent assisted
SBME: solvent bar microextraction
SDME: single drop microextraction
SFO: solidified floating organic droplet
SLM: supported liquid membrane
SPE: solid phase extraction
SPME: solid phase microextraction
SS: switchable solvent
SUPRASs: supramolecular solvents
UA: ultrasound-assisted
UAE: ultrasonic-assisted extraction
UHPLC: ultra-high performance liquid chromatography
UV: Ultraviolet
VA: vortex assisted
Vis: visible
JMK: Conceptualization, Investigation, Visualization, Writing—original draft, Writing—review & editing.
The author declares that he has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
