Abstract
The objective of this work was to compile data for the characterization of pistachio’s chemical composition and to analyze the benefits of their consumption in the diet. Pistachio edible seed is cultivated mainly in America, Mediterranean countries and Middle East. The geographical precedence may affect its mineral content as well as its lipidic profile and it may also influence the content of bioactive compounds. Pistachio presents a high proportion of vitamins, carotenoids, polyphenols, and flavonoids that have been associated with pistachio health benefits such as its antioxidant and anti-inflammatory activities. Pistachio intake would reduce glycemic index and control Type-2 Diabetes Mellitus. Clinical studies have also indicated that the presence of phytosterols, monounsaturated fatty acids (MUFAs) and dietary fiber from pistachio grains may reduce the risk of cardiovascular diseases (CVDs). Furthermore, the main wastes of pistachio industry [pistachio green hull (PGH) and pistachio shell (PS)] could be also considered a good source of bioactive compounds. Recent studies showed that the encapsulation of these nutraceutical compounds of PGH may be a green strategy for manufacture high-value foods within the framework of circular economy. Moreover, PS can be considered a good source of cellulose nanocrystals (CNC) that may be used for encapsulation and stabilization of oil-water emulsions.
Keywords
Anti-inflammatory activities, bioactive compounds, circular economy, encapsulation, geographical precedence, nutritional characterization, pistachioIntroduction
Nowadays the population is changing its dietary habits towards a healthy lifestyle, becoming conscious and responsible consumers of the food they eat. This scenario creates a demand for natural, unprocessed, organic, and environmentally friendly foods. Besides, this claim increases the search for healthy foods that are a source of nutrients such as dietary fiber and bioactive compounds that could reduce the risk of non-communicable diseases, improving human health [1–3]. Because of its good nutritional profile and excellent taste, pistachio is one of the most appreciated nuts that can be included in a healthy diet.
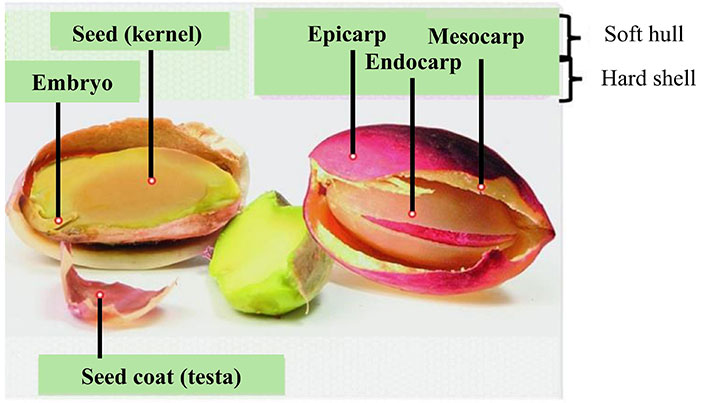
Pistachio is the fruit that is produced by the tree of the genus Pistacia, which belongs to the Anacardiaceae family, and it is native to the Middle East region of countries such as Iran, Turkey, Syria and Afghanistan [4]. There are several different species including P. atlantica, P. cabulica, P. chinensis, P. falcata, P. integerrima, P. kinjuk, P. kurdica. P. lentiscus, P. mutica, P. palaestina, P. terebinthus and P. vera. The fruits of the last of these species are edible [5]. At present, pistachio cultivation is distributed throughout the world in the Middle East (Iran, Turkey, Syria), Central Asia (China), Mediterranean region (Spain, Italy, Greece) and in American countries such as the US and Argentina. In 2021, more than 915,000 tons of pistachios were produced in the world, with the US being the largest pistachio producer, followed by Iran [6]. The pistachio fruit can be classified as a semi-dry drupe, containing a single edible seed (kernel) covered by a thin soft coat (testa), surrounded by lignified shell (endocarp), which is enveloped by a green fleshy hull (mesocarp and epicarp), depending on the degree of ripeness (Figure 1). Pistachios kernels are most commonly consumed as a snack, whether peeled, roasted and/or salted [7]. In addition, pistachios are consumed in different ways around the world, depending on the culture and customs of the people. In Sicily and Iran, the fruit is used to make liqueurs and condiments [8]. In Iran, it is used as an ingredient to make pistachio nougat, while pistachio ice cream is popular in Italy and also in Argentina. In some regions, the industry uses the discarded fraction that are not marketed as snacks (closed pistachios) to develop wafers, chocolate, butter, oil, pistachio baklava, and sausages [9, 10].
During pistachio processing, two by-products are obtained: the pistachio green hull (PGH) and pistachio shell (PS). Both are removed during the processes of de-hulling and shelling and account for > 75% of the harvested crop [11].
PGH is known to be a rich source of bioactive compounds, especially phenolic compounds (PC). However, much of this valuable by-product is discarded as waste, leading to environmental problems. Various authors have confirmed its considerable antioxidant, antimicrobial, and antimutagenic capacity [12–14].
The objective of this work was to review and compile data for the characterization of pistachio’s chemical composition and to analyze the benefits of their consumption in the diet, identifying their nutritional properties and main bioactive compounds. In addition, the chemical characterization of pistachio hull and shell is also explored together with the identification of different strategies for incorporating pistachio bioactive compounds into different matrices.
Bibliographic research
A bibliographic search was conducted in different specialized virtual platforms such as Google Scholar, Scielo and PubMed using different keywords, as listed below: pistachio, pistachio nuts, pistachio composition, Pistacia vera tree, pistachio world production, pistachio benefits, health pistachio, pistachio waste, recycling of pistachio wastes, pistachio PC, pistachio encapsulation, among others.
The information gathered on the nutritional composition of pistachio nuts was arranged and organized using tables, which allowed to compare the different origins of the nut. Daily intake recommendations from the WHO and the FAO were also taken into account in the disscusion.
A literature search was conducted on the scientific evidence of the beneficial effects commonly attributed to pistachio consumption. This allowed to know the most recent advances. Complementarily, several textbooks were used to understand more general or theoretical issues.
A revision was carried out to characterize the waste caused by pistachio industry. Articles about possible applications of bioactive compounds from pistachio waste in encapsulated systems were discussed.
Statistical analysis
When it was possible a statistical analysis was performed. The assumptions on normality and homogeneity of variance were checked. Using quantile-quantile (Q-Q) plot, Shapiro-Wilks test and Levene test (P = 0.708) it could be assumed that the fatty acids data presented a normal distribution. Then, for the analysis of the fatty acids results, a single factor one-way ANOVA was used. The Fisher’s least significant differences (LSD) were calculated by comparing the means at a 95% confidence level using the InfoStat 2018 software.
Nutritional value of pistachio from different regions
It is interesting to know the type of nutrients that pistachios can contribute to the diet in order to evaluate their possible effects on health and well-being. The range of nutritional composition of edible pistachio nuts grown in different countries is shown in Table 1. Lipids are the main component of the nut (between 47.5% and 57.0%) [15, 16] followed by protein (17.1% and 27.1%) [15, 17]. The content of dietary fiber varied between 8.6% and 15.3% [17, 18]. According to Bulló et al. [19], insoluble dietary fiber represents approximately 97% of the total dietary fiber. The kernel has a low moisture content (between 3.3% and 5.34%) [18, 20]. The total ash content was approximately 3% [15–18, 20, 21].
Minimum and maximum nutritional composition of pistachios nuts found in the bibliographic review
| Component | Lipids (%) | Proteins (%) | Total dietary fiber (%) | Moisture (%) | Ash (%) |
|---|---|---|---|---|---|
| Minimum | 47.5 [15] | 17.1 [15] | 8.6 [17] | 3.30 [20] | 2.24 [21] |
| Maximum | 57.0 [16] | 27.1 [17] | 15.3 [18] | 5.34 [18] | 3.12 [17] |
Amino acid, fatty acids profiles and minerals
Proteins are important macromolecules from a nutritional point of view. They contain essential amino acids, which cannot be synthesized by the human body and must be obtained from foods. According to Table 2 the amount of each amino acid of the pistachio nuts proteins and in an ideal protein recommended by WHO/FAO [22]. Salinas et al. [23] found that Argentine pistachios have high levels of lysine (Lys), threonine (Thr), leucine (Leu), tyrosine (Tyr), and phenylalanine (Phe), providing an amount of 50% more than that recommended by WHO/FAO [16, 22, 24]. Furthermore, proteins contain approximately 2.5 g of histidine (His), 5.5 g of valine (Val), 4.4 g of isoleucine (Ile) and 1 g threonine (Trp) per 100 g of proteins [16, 23–25]. Regarding the non-essential amino acids, it can be observed that the maximum content of aspartic acid (Asp) + glutamic acid (Glu) is about 29.69% which corresponds to Argentinean pistachio proteins [23], while in those from the US, the amount was 13.1% [25] (Table 2). Besides, pistachios proteins have a high proportion of arginine (Arg; 10.08 g per 100 proteins), which is involved in the cardioprotective and antihypertensive effects of this seed, as described in the following sections.
Amino acid profile of pistachios. Minimum, maximum and average content of amino acids in pistachio nuts
| Amino acids (g/100 g proteins) | Essential amino acids | Some non-essential amino acids | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lys | His | Thr | Val | Met | Cys | Ile | Leu | Phe + Tyr | Trp | Asp + Glu | Ser | Pro | Arg | Gly | Ala | |
| Minimum | 4.9 [24] | 2.3 [16] | 2.9 [24] | 5.2 [24] | 0.89 [23] | 0.95 [24] | 4.1 [16] | 6.8 [24] | 7.8 [16] | 0.65 [23] | 13.1 [25] | 5.37 [24] | 3.36 [24] | 8.52 [24] | 4.36 [24] | 3.70 [24] |
| Maximum | 7.30 [23] | 2.7 [24] | 4.09 [23] | 6.1 [25] | 1.8 [25] | 2.6 [16] | 4.7 [25] | 9.18 [23] | 10.53 [23] | 1.4 [16] | 29.69 [23] | 7.32 [23] | 7.31 [23] | 11.64 [23] | 5.45 [23] | 5.37 [23] |
| Average | 6.1 | 2.5 | 3.5 | 5.6 | 1.3 | 1.8 | 4.4 | 8.0 | 9.2 | 1.0 | 21.40 | 6.35 | 5.33 | 10.08 | 4.91 | 4.53 |
| Reference protein [22] | 4.5 | 1.5 | 2.3 | 3.9 | 1.6 | 0.6 | 3.0 | 5.9 | 3.8 | 0.6 | / | / | / | / | / | / |
Lys: lysine; His: histidine; Thr: threonine; Val: valine; Met: methionine; Cys: cysteine; Ile: isoleucine; Leu: leucine; Phe: phenylalanine; Thr: threonine; Asp: aspartic acid; Glu: glutamic acid; Ser: serine; Pro: proline; Arg: arginine; Gly: glycine; Ala: alanine. The symbol “/” indicate that the amino acids are not essentials
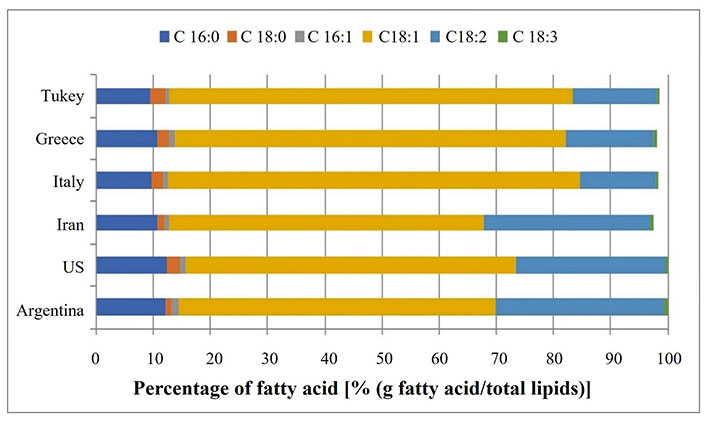
Triglycerides (TG) are the major compounds in the lipid fraction of pistachios. Oleic acid, linoleic acid and α-linolenic acid are the major unsaturated fatty acids found in the kernel [26]. Linoleic acid (18:2-ω6) and α-linolenic acid (18:3-ω3) are considered essential because the organism does not possess an enzymatic system for the synthesis, elongation, and desaturation of fatty acids beyond C-9 [27]. Both essential fatty acids are key molecules for the formation of structural lipids (skin and nerve tissue phospholipids) and are precursors of hormones such as eicosanoids-prostaglandins, which are essential for the regulation of blood pressure, kidney function, immune function, and uterine contraction [28, 29]. Pistachios had similar percentages of saturated fatty acids (SFA) such as palmitic acid (9.5% to 12.4%) and stearic acid (1% to 2.6%) and similar levels of palmitoleic acid (0.7% to 1.3%) [20, 30, 31]. The main differences were found in monounsaturated fatty acids (MUFAs) with 18 carbon atoms where the samples from Argentina, the US, Iran presented similar percentages of C18:1 (from 55.1% to 55.3%) and different from that of Italy, Greece, and Turkey, which presented the highest values of oleic acid (68.3% to 72.0%, P = 0.0004; Figure 2). These differences could be related to climatic factors such as temperature, which varies according to the area where the fruit is grown. This factor affects the activity of the enzymes (desaturases) involved in the synthesis of linoleic acid from oleic acid, increasing their activity in cold areas [32, 33]. The α-linolenic acid (18:3) content was similar in all pistachios (Figure 2).
Due to the differences in the fatty acid profile, the ω6:ω3 ratio varies. The ω6:ω3 ratio recommended in the diet is between 5:1 and 10:1 to avoid CVD risks [34]. The ω6:ω3 ratio in pistachios from Italy, Greece, and Turkey was 33, 31, and 29, respectively [31], while in pistachios from Argentina, the US and Iran it was between 48 and 59 [20, 30, 31]. This difference is an indicator that the fatty acids profile of pistachios is dependent on their high-altitude cultivation, geographical origin and the maturity of the fruit [35].
Finally, the most important minerals present in pistachios grown in Argentina [36], US, Turkey, Iran [37], Spain [38], and Italy [18] are summarized in Figure 3. In all pistachio nuts, the most important mineral was potassium (K), mainly in Iranian pistachio nuts (1,589 mg/100 g) [37] (Figure 3A); while the Na content reported is very low, ranging from 8.72 mg/100 g [36] to 26.4 mg/100 g [38] (data not shown). Both minerals are important because they regulate the membrane potential of tissue-forming cells and are involved in nerve impulse transmission mechanisms. Intracellular K regulates water-electrolyte balance, osmotic pressure and is required for enzyme activity and protein synthesis, while extracellular K is involved in muscle contractility and blood pressure regulation [28]. Therefore, in order to reduce blood pressure and the risk of CVD, a high K intake with a low sodium intake is suggested [39]. However, with a recommended dietary intake (RDI) of 2,400 mg/day, the sodium content of a 30 g serving of pistachios is negligible.
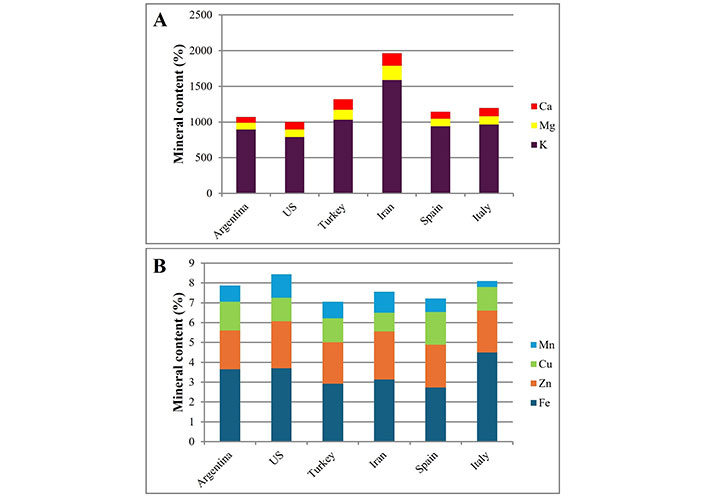
Mineral content in pistachios grown in different countries. (A) Ca, Mg and K in pistachios grown in different countries; (B) Mn, Cu, Zn and Fe in pistachios grown in different countries
Regarding calcium (Ca), Iranian pistachios had the highest Ca content (175.2 mg Ca/100 g) [37], while the phosphorus (P) content was between 416 mg/100 g and 816.4 mg/100 g (data not shown). Ca and P are essential for preventing the risk of bone fractures by providing skeletal rigidity [40], and they are present in the bone structure as hydroxyapatite [Ca10(PO4)6(OH)2] or as calcium phosphate. Calcium is also involved in the mechanisms of coagulation, contraction of muscles, and transmission of nerve impulses; also influences the permeability of cells membranes and is an activator of enzymes such as ATPase and lipases. Phosphorous is also found in DNA, RNA, phospholipids of the lipid bilayer of cell membranes, and in energy molecules such as ATP and ADP [28].
Pistachios from the Middle East (Turkey and Iran) had the highest magnesium (Mg) content, followed by those from Spain and Italy (around 115 mg/100 g). In contrast, American pistachios (Argentina and US) contained around 100 mg Mg/100 g (Figure 3A). In addition to its structural function, this mineral acts as an essential co-factor for over 300 enzymes and is involved in energy metabolism, glucose utilization, protein synthesis, fatty acid synthesis and degradation, and is necessary for stabilizing the helical structure of the DNA and RNA molecules [36, 41].
Iron (Fe), zinc (Zn), copper (Cu) and manganese (Mn) are trace minerals important for nutrition. Fe is a structural component of hemoglobin (oxygen transport proteins) and myoglobin, of the enzyme cytochrome (in oxidative metabolism), of enzymes involved in the mechanisms of thyroid hormone and bile acid synthesis, and in the control of signals between neurotransmitters such as dopamine and serotonin [28]. Pistachios from Italy, US and Argentina contain the highest amounts of Fe (3.7–4.5 mg Fe/100 g pistachios; Figure 3B). According to the recommendations (around 14 mg/day), one serving of pistachios would provide < 10% of the RDI [40]. With regard to Zn and Cu, no differences were observed in their content depending on the geographical origin, containing about 2 mg of Zn and 1 mg of Cu per 100 g of pistachios (Figure 3B). Zn is a component of enzymes involved in the synthesis and degradation of carbohydrates, lipids, proteins and nucleic acids, it stabilizes the structure of components and cell membranes, and it is involved in the immune system. The presence of Cu influences the activity of enzymes in oxidative reactions [28]. Although both minerals are present in small amounts in the pistachio fruit, based on nutritional requirements, one serving of pistachios would provide about 8% of the RDI of Zn and 48% of the RDI of Cu. In Figure 3B, it can be observed that pistachios contain approximately 1 mg Mn/100 g of pistachios, however, pistachios grown in Italy and Spain had lower amounts of this mineral, 0.3 mg/100 g [18] and 0.69 mg/100 g of pistachio [38], respectively.
Vitamins, phytochemicals, and carotenoids
Pistachios contain lipophilic vitamins (A and E) and water-soluble vitamins such as vitamin C and some of the B complex like pyridoxine (B6), thiamine (B1) and riboflavin (B2). This information is summarized in Table 3.
Vitamin content in pistachios from different countries and treatment (roasting)
| Vitamin (µg/100 g) | NP | RP | |||
|---|---|---|---|---|---|
| US [42] | Italy [43] | Turkey [44] | Iran [19] | US [47] | |
| A (µg retinol/100 g) | 34.07 | - | 15.21 | 21.25 | 13 |
| E | |||||
| α-Tocopherol | 2,100 | 500 | 2,400 | 160 [46] | 2,170 |
| γ-Tocopherol | 30,600 | 105,400 | 37,700 [45] | 11,200 [46] | 23,400 |
| B1 | 654 | - | 740 | - | 695 |
| B2 | 447 | - | 160 | - | 234 |
| B6 | 1,032 | - | 1,400 | - | 1,120 |
| C | - | 3,480 | 1,280 | < 1,000 | 3,000 |
NP: natural pistachio; RP: roasted pistachio; B1: thiamine; B2: riboflavin; B6: pyridoxine. The symbol “-” indicates that the data was not available
Provitamin A is found in plants as carotenoids (mainly β-carotene). This vitamin is essential for maintaining good vision and influencingprotein metabolism. Additionally, it has a protective effect against the progression of chronic diseases due to the antioxidant activity of β-carotene [28]. Table 3 shows that pistachios from the US had the highest level of vitamin A (34.07 μg retinol/100 g) [42], followed by Iranian pistachios with 21.25 μg retinol/100g [19]. Vitamin E is a lipid-soluble vitamin with high antioxidant capacity that is susceptible to oxidation by high temperatures. Plants synthesize α-, β-, γ-, δ-tocopherols and α-, β-, γ-, δ-tocotrienols [48]. α-Tocopherol has a greater antioxidant activity than γ-tocopherol, which participates in enzymatic processes and plays an important role in platelet aggregation. Table 3 shows that γ-tocopherol is the predominant (30,600 µg/100 g to 37,600 µg/100 g pistachios) [42, 45, 47]. Vitamin B1 is phosphorylated to B1 pyrophosphate and acts as a coenzyme in catabolic reactions involved in the initiation of the Krebs cycle and in the synthesis of pentose, which is essential for the formation of nucleic acids [40]. This is a water-soluble vitamin that is sensitive to prolonged heat treatment. Vitamin B2 is a component of the coenzymes flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). FMN is involved in redox reactions and the respiratory chain, while FAD is involved in the oxidation of fatty acids, the formation of niacin (B3) from Try, and the reduction of folic acid (B9). It is sensitive to light and heat at pH > 7 [49]. Vitamin B6 is an enzymatic cofactor involved in metabolic reactions such as the biosynthesis and catabolism of amino acids and the biosynthesis of vitamin B3 from Try, among others [28]. Pistachios contain lower values of B1 [42, 44, 47] respect to vitamin B6 (Table 3). The amount of vitamin B2 in pistachio was between 160 μg/100 g and 447 μg/100 g. One serving of pistachios provides about 20% of the RDI of B1, 10% of B2, and 39% of B6. Ascorbic acid or vitamin C, is an antioxidant that participates as an enzymatic cofactor in hydroxylation, oxidation, and amino acid biosynthesis reactions [28]. This vitamin is thermolabile and sensitive to oxidation. Table 3 shows that the fruits may contain up to 3,480 µg/100 g, covering 6% of the RDI.
Carotenoids are pigments divided into two classes: xanthophylls and carotenes. Lutein and zeaxanthin are two xanthophyllic compounds present in pistachios (Table 4), they are responsible for the color and the antioxidant activity [42]. Pistachios from the US contain high amounts of β-carotene [42] and also of xanthophyll (lutein + zeaxanthin). Besides, Bulló et al. [19] reported lower values of lutein + zeaxanthin in Iranian pistachios. The values reported by the US Department of Agriculture (USDA) [47] for roasted pistachios (RPs) were lower, possibly due to the thermal sensitivity of these carotenoid compounds.
Carotenoids and phytosterols of pistachios grown in different countries
| Compounds | US [42, 50] | Italy [18, 51] | Turkey [19] | Iran [19, 46] | USDA [47] | Greece [15] | |
|---|---|---|---|---|---|---|---|
| Carotenoids (µg/100 g) | β-Carotene | 204 | 180 | 122.5 | 20.36 | 159 | - |
| Lutein + zeaxanthin | 2,757 | - | 728.6 | 456.1 | 1,160 | - | |
| Phytosterols (mg/100 g) | Campesterol | 10.1 | 15.43 | - | 12.4 | - | 12.24 |
| Stigmasterol | 2.3 | 4.3 | - | 2.43 | - | 2.8 | |
| β-Sitosterol | 209.8 | 241.27 | - | 210.0 | - | 224.4 | |
| Δ5-Avenasterol | 26.2 | 17.67 | - | 15.8 | - | 29.96 | |
| Total | 248.4 | 278.7 | - | 240.6 | 222 | 269.4 | |
USDA: US Department of Agriculture. The symbol “-” indicates that the data was not available
Phytosterols are plant sterols found in the lipidic fraction of plants. They are structural components of cell membranes that stabilize the lipid membranes. They are considered functional compounds because of their hypocholesterolemic effect, since they inhibit the absorption of cholesterol at the intestinal level, they reduce cholesterol re-esterification in enterocytes by inhibiting the activity of the enzyme acyl-CoA:cholesterol acyltransferase (ACAT), and finally stimulate the outflow of non-esterified cholesterol from enterocytes into the intestinal lumen [52]. According to literature, the total phytosterol content is around 250 mg/100 g for all pistachios, containing mainly β-sitosterol (83% of total phytosterols; Table 4).
Several compounds with antioxidant activity
Several antioxidant compounds are found in pistachios. Noguera-Artiaga et al. [53] evaluated the total phenolic compound content of seven different varieties of P. vera L. and reported values ranging from 500 mg to 6,065 mg gallic acid equivalent (GAE)/100 g pistachios. In addition, several authors have investigated PC and their antioxidant activity in P. vera L. variety Kerman from Argentina [36], US [54] and variety Bronte from Italy [55]. In general, all the authors found higher levels of phenolics, flavonoids and anthocyanins in the skin than in the kernel (Table 5). Italian pistachios of Bronte variety presented a higher content of PC than pistachios of the Kerman variety from Argentina and the US. Regarding flavonoids, catechin was the main flavonoid while cyanidin-3-O-galactoside is the predominant anthocyanin [36, 54, 55].
Some of the PC identified in skin and kernel of pistachios grown in different countries
| Compounds | Argentina(µg/g d.b.) [36] | Italy(µg/g d.b.) [55] | US (µg/g w.b.) [54] | ||||
|---|---|---|---|---|---|---|---|
| Skin | Kernel | Skin | Kernel | Skin | Kernel | ||
| Phenols | Gallic acid | 75 | 8 | 1,453 | 12.66 | 197.4 | 11.9 |
| Flavonoids | Procyanidin dimer | 55 | 6 | - | - | - | - |
| (–) Epicatechin | 27.53 | 3 | 104.8 | - | 229.1 | - | |
| (+) Catechin | 140 | 16 | 377.45 | 2.41 | 1,774.2 | 21.5 | |
| Eriodyctiol-O-glucoside | 3.35 | 0.4 | 365.68 | 31.91 | - | - | |
| Genistein | - | - | - | 69.15 | - | - | |
| Daidzein | - | - | - | 42.45 | - | - | |
| Quercetin | 13.7 | 2 | 17.75 | - | 144.4 | 13.0 | |
| Isoquercetin | 49.3 | 6 | - | - | - | - | |
| Myricetin | 1.6 | 0.2 | - | - | 68.2 | 140.6 | |
| Eriodictiol | 13.7 | 2 | 63.17 | 9.3 | 59.2 | - | |
| Naringenin | 1.9 | 0.2 | 11.44 | - | - | - | |
| Luteolin | 30.4 | 3 | 18.97 | - | 52.8 | 204.9 | |
| Anthocyanins | Cyanidin-3-O-galactoside | 21.14 | 0.2 | 5,865.12 | - | 876.5 | - |
| Cyanidin-3-O-glucoside | 0.55 | 0.01 | 32.56 | - | 212.7 | - | |
d.b.: dry matter basi; w.b.: wet matter basis. The symbol “-” indicates that the data was not available
Bioactive compounds and health benefits of pistachio
Pistachios contain nutrients and bioactive compounds that may work synergistically to improve health. Main studies regarding the health effects of pistachio nut consumption are summarized in Table 6.
Summary of research supporting pistachio’s health benefits
| Health benefits | Type of study | Principals results | References |
|---|---|---|---|
| Antioxidant and anti-inflammatory potential | RCT: parallel-design study in healthy individuals, n = 44G1: regular diet, n = 22G2: regular diet + pistachios, n = 22 | ↑: AOP↓: MDA | [56] |
| RCT: prospective study in healthy young men, n = 32 | ↑: SOD↓: TOS, MDA, LOOH, IL-6=: CRP, TNF-α | [57] | |
| In vitro assay in macrophages cell line J774-A1 stimulated with LPS (from Escherichia coli 1.0 µg/mL) | ↓: TNF-α, IL-1β, NO, ROS, MDA, iNOS, COX-2 | [58] | |
| In vivo assay with Sprague-Dawley male rats with paw edema induced by CAR, n = 30CAR: n = 10CAR + NP: n = 10CAR + RP: n = 10 | With NP↓: MPO, edema volume, number of inflammatory cellsNormal appearance of muscle fibersWith RPNo significant changes | [58] | |
| Cardioprotective and antihypertensive effects | RCT: parallel-design study in healthy individuals. n = 44G1: regular diet, n = 22G2: regular diet + pistachios, n = 22 | ↑: HDL↓: TC=: LDL, TG | [56] |
| RCT: prospective study in healthy young men, n = 32 | ↑: BAFMVD↓: LDL, TC, TG, CT/HDL, LDL/HDL=: HDL, blood pressure | [57] | |
| RCT: parallel-design study in individuals with hyperlipemia, n = 56G1: regular diet, n = 27G2: regular diet + pistachios, n = 29 | ↑: HDL, BAFMVD↓: LDL, TC/HDL, PWV | [59] | |
| In vitro assays: ACE enzyme inhibitory activity by pistachio peptides | ↓: confirmation of ACE inhibitory activity | [60] | |
| Glycemic control and T2-DM | RCCT: Crossover clinical trial in prediabetic patients, n = 54 | ↓: glycemia, insulin, HOMA-IR, IL-18=: HbA1c | [61] |
| RCT: Parallel-design study in individuals with hyperlipemia, n = 56G1: regular diet, n = 27G2: regular diet + pistachios. n = 29 | ↓: glycemia | [59] | |
| RCCT: double-blind, placebo-controlled trial in prediabetic individuals, n = 48 | ↓: glycemia, HbA1c=: HOMA-IR | [62] | |
| RCT: prospective study in healthy young men, n = 32 | ↓: blood glucose | [57] | |
| RCT: patients with metabolic syndrome, n = 10 | ↓: glycemic index | [63] | |
| In vitro assay: α-amylase and α-glucosidase enzymatic activity | ↓: α-amylase and α-glucosidase activity | [53] | |
| Weight control | RCT: patients with obesity, n = 52 | ↓: BMI, body weight | [64] |
| RCCT: Double-blind, placebo-controlled trial in prediabetic individuals | ↓: BMI | [62] | |
| Gut microbiota modulation and antimicrobial effect | Animal’s trial: healthy rats and with T1-DMG1: Healthy animals + control diet, n = 6G2: Healthy animals + pistachio diet, n = 6G3: diabetic animals + control diet, n = 6G4: diabetic animals + pistachio diet, n = 6 | ↑: fecal lactobacilli and bifidobacterial irmicutes population in healthy animals, Bifidobacterium genus in diabetic rats↓: Bacteroidetes levels in healthy animals | [65] |
| Animal’s trial: mice fed a HFDG1: control animals + standard diet, n = 8G2: obese animals + HFD, n = 8G3: obese animals + HFD+pistachio, n = 8 | ↑: Parabacteroides, Dorea, Allobaculum, Turicibacter, Lactobacillus, Anaeroplasma, Oscillospira, Desulfovibrio, Coprobacillus, Bilophila↓: Firmicutes/Bacteroidetes | [66] | |
| RCCT: randomized cross-over, n = 16 | ↑: LAB=: Bifidobacterium | [67] | |
| In vitro: antimicrobial potential of phenolic extracts from NP and RP. ATCC strains | ↑: bactericidal activity on L. monocytogenes, Staphylococcus (S.) aureus and Methicillin-resistant S. aureus strains (NP > RP) | [68] |
↑: increase; ↓: decrease; =: remains unchanged; n: number of individuals. RCT: randomized controlled trial; RCCT: randomized crossover clinical trial; AOP: antioxidant potencial; MDA: malonaldehyde; SOD: superoxide dismutase; TOS: total oxidant status; LOOH: lipid hydroperoxides; IL-6: interleukin-6; CRP: C-reactive protein; TNF-α: tumor necrosis factor-α; NO: nitric oxide; ROS: reactive oxygen species; iNOS: inducible NO synthetase; COX-2: cyclooxygenase-2; NP: natural pistachio; RP: roasted pistachio; LPS: lipopolysaccharide; CAR: carrageenan; MPO: myeloperoxidase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; TC: total cholesterol; TG: triacyclglycerols; BAFMVD: brachial artery flow-mediated vasodilation; PWV: pulse wave velocity; ACE: angiotensin-converting enzyme; HOMA-IR: homeostasis model assessment of insulin resistance; HbA1c: glycated hemoglobin A1c; BMI: body mass index; HFD: high-fat diet; ATCC: American Type Culture Collection; LAB: lactic acid bacteria; T2-DM: type 2 diabetes mellitus
Antioxidant and anti-inflammatory Potential
Inflammation and oxidative stress must be controlled because they play an important role in the pathogenesis and progression of CVD and other pathologies such as atherosclerosis. Oxidative stress is the imbalance between oxidant and antioxidant components in which reactive oxygen species (ROS) predominate. Several clinical and animal studies have shown that pistachio consumption helps to promote the antioxidant status and the anti-inflammatory balance (Table 6). Kocyigit et al. [56] conducted a randomized controlled trial (RCT) in healthy individuals (n = 44) who were divided into two groups: one group consumed a regular diet containing foods from all groups without nuts or nut by-products, and the other group consumed a regular diet supplemented with pistachios (65–75 g/day). They evaluated the antioxidant potential (AOP) and malonaldehyde (MDA) levels in plasma, both parameters indicative of oxidative status. MDA is one of the secondary products of lipid peroxidation of polyunsaturated fatty acid (PUFA) and is studied as an indicator of this process. After three weeks, the pistachio consumption group had an 85% increase in plasma AOP levels and a decrease in MDA levels [56]. Sari et al. [57] conducted a prospective study with 32 young men who consumed a Mediterranean diet rich in vegetables and fish but limited in red meat, fatty foods and eggs for the first 4 weeks. Then, RPs were included as a replacement to the MUFA for the next 4 weeks. Total oxidative status (TOS), MDA levels, lipid hydroperoxides (LOOH), and superoxide dismutase (SOD) enzyme activity were determined on blood samples at the beginning and the end of each dietary period. These authors found that the pistachio diet decreased LOOH, TOS and MDA levels and increased the SOD enzyme activity from 0.42 to 1.55 IU/mL. In addition, Sari et al. [57] studied the anti-inflammatory effect by measuring high-sensitivity C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in serum and plasma samples. CRP is a circulating plasma protein that increases in response to inflammation, and its expression is regulated by IL-6 and IL-1 and TNF-α [69]. IL-6 is a cytokine with anti-inflammatory and pro-inflammatory activity secreted by macrophages, T cells, endothelial cells and fibroblasts. The release of IL-6 is induced by IL-1 and is increased in response to TNF-α [70]. TNF-α is also a pro-inflammatory cytokine secreted by the immune system and by adipocytes [71]. Sari et al. [57] reported a significant reduction in serum IL-6 levels in participants consuming a pistachio diet, without significant changes in CRP and TNF-α levels. Paterniti et al. [58] analyzed the potential anti-inflammatory and antioxidant properties of polyphenolic extracts of natural pistachios (NPs) and RPs, inducing the inflammatory process in an in vitro assay using a culture of lipopolysaccharide (LPS)-stimulated macrophage cells. These authors found that phenolic extracts of NP reduced the degradation of inhibitors of nuclear kappa B and both (NP, RP) reduced the TNF-α and IL-1β production in a dose-dependent manner. This indicated that the inflammatory process was controlled. Bagheri-Hosseinabadi and Abbasifard [72] attributed the inhibition of the transcription NF-κB to pistachio flavonoids. In terms of oxidative power, these authors found that phenolic extracts significantly reduced the cellular expression of cyclooxygenase-2 (COX-2) and the inducible nitric oxide (NO) synthetase (iNOS) enzymes and decreased NO production. As a result, the PC prevented the generation of an oxidative environment and attenuated cellular damage [73]. Paterniti et al. [58] also performed in vivo studies on rats after a subplantar injection of carrageenan (CAR). The histological examinations showed that CAR treatment disorganized edema muscle fibers in shape and size, lost normal muscle architecture, together with an accumulation of inflammatory cells and an increase in myeloperoxidase (MPO, marker of neutrophilic infiltration) enzyme activity. However, after the treatment with the NP phenolic extract, the researchers observed a normal appearance of the muscle fibers, with a decrease in infiltrating inflammatory cells, MPO activity, and in edema volume.
Cardioprotective and antihypertensive effects
The endothelium is the inner lining of blood vessels and the lymphatic system and is therefore in direct contact with the blood. Its functions include regulation of vascular tone, maintenance of blood fluidity and coagulation, and the production of cytokines and regulation of vascular inflammatory function. Chronic inflammation and oxidative stress are associated with an increase in endothelial dysfunction, an event that precedes the process of atherosclerosis and CVD. Atherosclerosis is characterized by narrowing of the arteries and the formation of atheroma due to inflammation, lipid accumulation, cell death and fibrosis [74, 75].
In general, total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and TG in the blood are parameters that indicate the risk of CVD. High levels of TC, TG and/or LDL indicate an increased risk of atherogenesis, while high levels of HDL are desirable as they are responsible for lowering blood cholesterol [74].
Several authors investigated the cardioprotective effects of pistachios. In this regard, Kocygit et al. [56] and Sari et al. [57] showed that addition of pistachio in the diet increased HDL content and decreased plasma TC, LDL and TG (Table 6). This may be due to the presence of phytosterols and MUFAs in pistachio.
Endothelial functionality is usually studied using non-invasive methods, such as brachial artery flow-mediated vasodilation (BAFMVD) and arterial stiffness measurement, such as arterial pulse wave velocity (PWV), which assess endothelium-dependent vasodilation as an indicator to predict CVD risk. A high BAFMVD and a low PWV are associated with a high degree of vasodilation.
Sari et al. [57] found an increase in BAFMVD (Table 6) with an improvement in vasodilation in participants who consumed pistachios. Relaxation of the vascular endothelium may be impaired by decreased NO synthesis. NO is a free radical produced by the enzyme endothelial NO synthetase (eNOS) from the amino acid L-Arg, and is an endogenous vasodilator compound whose deficiency produces arterial hypertension. Some factors can decrease the expression of eNOS, such as high levels of oxidized LDL. Pistachios have high levels of L-Arg (Table 2) and a high proportion of antioxidants (Table 5), which contribute to the proper function of the endothelium and the maintenance of a normal blood pressure [76].
Other authors have also investigated the effect of pistachios on endothelial function. Kasliwal et al. [59] performed an RCT with 60 volunteers with dyslipidemia (altered blood lipid levels) divided into two groups, one group consumed a regular diet, while the others consumed the regular diet supplemented with 40 g/day of pistachio for 12 weeks. The results showed favorable changes in endothelial function (> BAFMVD and < PWV) in individuals who consumed pistachios. In addition, the participants had an increase in HDL level and a reduction in the fasting glucose levels, LDL and TC/HDL ratio [59]. This improvement in the lipid profile of dyslipidemia individuals may be a consequence of the lower amount of SFA along with a higher proportion of MUFAs, PUFAs, and dietary fiber in pistachios (Figure 2).
Regarding the blood pressure, Sari et al. [57] observed no differences in this parameter in healthy young men during the period of pistachio consumption. However, Dumandan et al. [60] demonstrated the inhibitory activity of total soluble protein hydrolysates from pistachios on angiotensin-converting enzyme (ACE) in in vitro studies (Table 6). This enzyme converts angiotensin I to angiotensin II, which increases blood pressure [77]. Therefore, more studies should be conducted in order to elucidate the real effect of pistachio consumption on blood pressure.
Impact on glycemia and type 2 diabetes mellitus
Hernández-Alonso et al. [61] conducted a randomized crossover clinical trial (RCCT) in prediabetic patients (n = 54, aged 25 to 65) with a body mass index (BMI) < to 35 kg/m2. All participants followed a balanced diet and physical activity for 15 days before the study. They were then divided into two groups: those who followed a diet without pistachios (D1) and those who followed a diet supplemented with 57 g/day of pistachios (D2) for the next 4 months. At the end of the first phase, each person continued on a balanced diet for 15 days, and then the diets were reversed for a further 4 months. These authors observed a notable reduction in basal blood glucose and fasting insulin levels in individuals adhering to D2, as well as a decrease in pro-inflammatory cytokines and indicators of insulin resistance. Nonetheless, the glycated hemoglobin A1c (HbA1c) parameter, a biomarker that reflects the average blood glucose level over the past 3 months, remained unaltered between participants on the D1 and D2 diets (Table 6). The authors confirmed the beneficial impact of pistachios on individuals with type 2 diabetes mellitus (T2-DM). Parham et al. [62] conducted a RCCT with patients diagnosed with T2-DM and found that consuming 25 g of pistachios twice per day over 12 weeks resulted in a prolonged reduction in HbA1c and basal glycemia.
Kendall et al. [63] evaluated the glycemic response (postprandial glycemia vs. time) on 10 young overweight volunteers (around 48 years old and BMI of 28 kg/m2) after consumption of different portions of pistachios (28 g, 56 g and 84 g). Twice a week, blood samples were collected from participants after a 10–14 h of food abstinence. Subsequently, the subjects consumed the corresponding food, and blood samples were taken again at 15 min, 30 min, 45 min, 60 min, 90 min, and 120 min. Postprandial blood glucose levels were notably reduced after the consumption of the three doses of pistachio alone in comparison to the control (white bread). The authors repeated the trial, mixing each dose of pistachio with white bread, to assess the impact of pistachio dose on glycemic response. Results showed a decrease in glycemic response as the amount of pistachio consumption increased [63].
The enzymes responsible for carbohydrate digestion are α-amylase (salivary and pancreatic) and α-glucosidase (found in the brush border of intestinal epithelial cells). Noguera-Artiaga et al. [53] assessed the inhibitory impact of various pistachio cultivars on α-amylase and α-glucosidase enzymes. Their findings indicated that the Kerman cultivar had the highest inhibitory effect on α-amylase [with a mean 50% inhibitory concentration (IC50) equal to 5.39 mg dry pistachio/mL] and α-glucosidase (IC50 < 0.05 mg pistachio/mL, respectively). According to these authors the flavonoids present in pistachios, such as quercetin-3-O-rutoside, genistein, isoquercetin, rutin, and quercetin, act as inhibitors of α-amylase and α-glucosidase enzymes [53].
Effect on weight control
Pistachios diet could have effects on the body weight of consumers. Li et al. [64] conducted a study with 52 obese patients who followed an isocaloric diet to reduce weight. The patients were divided into two groups: the first group consumed a daily snack of 53 g of salted pistachios as part of their dietary plan, while the second group consumed a non-pistachio snack of 56 g. Li et al. [64] evaluated weight gain, BMI, TC, HDL, and TG levels in blood. The researchers observed that BMI and body weight decreased in both groups, but the decrease was more pronounced in the patients who consumed pistachios. Parham et al. [62] reported that diabetic participants who consumed pistachios had a lower BMI. They attributed the anti-obesity effect to mechanisms involving starch blockade, appetite suppression, and inhibition of fat absorption, which were due to pistachios’ low energy density and also to the inhibition of α-amylase and α-glucosidase enzymatic activity.
Pistachios contain dietary fiber, including insoluble fiber, which helps regulate energy intake and contribute to maintaining or reducing body weight and increasing the satiety. It is important to note that these benefits are only achievable through the moderate consumption of pistachios as part of a balanced diet [78].
Gut microbiota modulation and antimicrobial effect
The intestinal microbiota comprises microorganisms that colonize the walls of the human intestine. Several authors have evaluated the impact of pistachio nut diets on the composition of animal’s intestinal microbiota. The findings reveal changes in the microbiota composition as a result of consuming pistachio nuts (Table 6). Yanni et al. [65] performed a study with healthy and sick-with T1-DM-animals. They divided them into two groups: one fed a control diet and the other a diet supplemented with pistachios. The results showed an increase in Lactobacillus and Bifidobacteria in the intestinal segments of diabetic animals. In contrast, both groups showed an increase in Bifidobacteria and Lactobacillus and a decrease in Enterococci bacteria in the feces after the pistachio feeding period. Additionally, the healthy pistachio-fed animals showed an increase in Firmicutes, Bifidobacterium, Lactobacillus, Turicibacter, and Romboutsia, and a decrease in Bacteroidetes. In diabetic animals they found an increase in Actinobacteria [65].
Terzo et al. [66] studied if the chronic intake of pistachio prevents obesity-associated inflammation and dysbiosis in a high-fat diet (HFD)-fed mice. For this purpose, these authors used obese mice that were fed with three diets: a control diet, a HDF, and a HFD supplemented with pistachio (HDF-P) for 16 weeks. In HDF-P mice, Phylum Firmicutes/Bacteroidetes ratio was reduced with respect to the HFD group. A pistachio diet significantly increased the abundance of the group of microorganisms belonging to the phylum Tenericutes, bacteria that are positively associated with the modulation of the immune system and contribute to intestinal integrity. Moreover, they evidenced a decrease in the group of bacteria belonging to the Proteobacteria, indicators of obesity-related metabolic disorders, in humans and rodents.
Animals fed with pistachios showed an increase in bacteria of the genus Lactobacillus, Parabacteroides, Dorea, Allobaculum, Turicibacter and Anaeroplasma, which are associated with positive effects on the health of the host. In addition, Allobaculum and Dorea are among the major producers of butyrate. Instead, bacteria associated with inflammation, such as Desulfovibrio, Coprobacillus, Oscillosphira and Bilophila, decreased in animals fed with pistachio. A decrease in Desulfovibrio favors the production of short chain fatty acids (SCFA). Ukhanova et al. [67] evaluated the effect of pistachios and almonds on the microbiota in an in vivo human study. For this purpose, they conducted a randomized controlled three-crossover study with a total of 16 subjects. During the three periods, the participants consumed a low-fiber diet. The first group did not consume pistachios, in the second group 42.5 g of pistachios were added to the diet, and the third group incorporated a portion of 85 g of pistachio. Fecal samples were collected at the beginning and end of each period. The analysis of bacterial DNA extracted from feces, amplified and then analyzed by electrophoresis with a denaturing gradient, showed that individuals who consumed pistachios did not change the profile of Bifidobacterium, but increased the lactic acid bacteria (LAB). In addition, pistachio consumption had a greater effect on the fungal microbiota than did almond consumption.
The antimicrobial effect of NP and RP extracts rich in polyphenols against Gram-negative bacteria, Gram-positive bacteria [American Type Culture Collection (ATCC) strains, food and clinical isolates], yeasts and the fungi Aspergillus niger was studied by Bisignano et al. [68]. Using the broth microdilution method, they determined the minimum inhibitory concentration, minimum bactericidal concentration, and minimum fungicidal concentration of phenolic extracts on gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Pseudomonas mirabilis); gram-positive bacteria (Listeria monocytogenes, Enterococcus hirae, Enterococcus faecium, Bacillus subtilis, Staphylococcus epidermidis, Staphylococcus aureus); yeasts (Candida albicans and Candida parapsilosis); and fungi. They have been tested on ATCC strains, Listeria (L.) monocytogenes isolated from food and S. aureus from clinical isolates. They reported that pistachio phenolic extracts were effective only against gram-positive microorganisms. These authors demonstrated a bactericidal effect of both phenolic extracts of pistachio on the pathogens S. aureus and L. monocytogenes, which was more pronounced with NP extracts. A bacteriostatic effect was observed for the rest of the Gram-positive strains. Since the heat treatment of roasting affects the content of compounds of phenolic nature, NP extracts were more effective in antimicrobial activity than RP extracts because compounds such as catechin, epicatechin and isoquercetin were in higher concentration [68].
Natural dietary fiber and phytochemicals that reach the proximal colon, such as those present in various nuts, provide substrates for the maintenance of healthy and diverse microbiota. The antimicrobial activity of flavonoids is attributed to various mechanisms such as damage to the cell cytoplasmic membrane through H2O2 production, inhibition of nucleic acid synthesis, and inhibition of energy metabolism [68]. The bactericidal activity of pistachio extracts could be used to control the growth of some microorganisms in foods to improve safety and may find application as a topical treatment for S. aureus.
So far, the beneficial effects of pistachio consumption on health have been detailed and discussed. However, some bioactive compounds such as fiber and phenols extracts remain in the discarded shells. This may have a negative impact in the industry activity and could lead to environmental problems. Therefore, it is useful to know which compounds can be used as active ingredients in foods and/or drugs.
Characterization of pistachio industry waste and the applications in encapsulated systems
PS
PS is a lignocellulosic waste with important industrial applications [79]. It is mainly composed of cellulose (38.1–54.0%), hemicellulose (15.2–31.4%) and lignin (25.2–29.4%) [80] and it is a water absorbent material, effective against certain pollutant species. According to a recent review by Igwegbe et al. [81], most research interest in pistachio adsorption has come majorly for heavy metals and dyes and its removal efficiency has been demonstrated to be up to 90% in most of the cases. Moreover, PS is also valued as a biomass feedstock for bioenergy. It has a low moisture content (3.6–5.7%), high volatile matter (79.8–83% dry matter basis) and low ash content (0.4–1.9% dry matter basis) required for bioenergy generation [79, 82].
Application of PS in foods is limited, nevertheless it is a potential source of natural bioactive compounds. Cardullo et al. [83] proposed an extraction procedure based on microwaves irradiation and identified in the extracts through high-performance liquid chromatography/electrospray ionization-tandem mass spectrometry (HPLC/ESI-MS/MS), gallic acid, pentagalloylglucose and kaempferol as the main phenolics. Their results showed that the latest would be the responsible of the high antioxidant activity found in PS. Moreover, other researchers have indicated that kaempferol and its glycosides present several health benefits such as cardiovascular, neuroprotective, anti-inflammatory and anti-diabetic effects, making PS a promising material for pharmacological studies [83]. Furthermore, Jadhav et al. [84], used subcritical water and a Ni-Graphene catalyst at 240°C to depolymerize lignin extracted from PS. Their results suggest that PS could be a good source of phenolic monomers such as vanillin and syringaldehyde.
PS has also been proposed as a potential candidate for xylan isolation and production of xylooligosaccharides. The interest in these compounds relies in their prebiotic activity and the induction of selective growth of the beneficial microflora such as Bifidobacterium. Hesam et al. [85] optimized the conditions for xylan extraction from PS using an alkaline solution of hydrogen peroxide followed by an enzymatic hydrolysis. This procedure generated a potential prebiotic mixture from xylan which contained xylobiose and xylotriose that could be applied in the development of functional foods and in the pharmaceutical industry. In line with this, Hazal et al. [86] demonstrated that xylo-oligosaccharides and xylose could also be extracted from the hemicellulose fraction of PS through microwave-assisted high-pressure CO2/H2O hydrolysis. These authors found that in the optimum conditions the extraction yield could reach up to 60% of xylose.
PGH
PGH is the external green colored mesocarp and epicarp of the pistachio fruit. This fraction is separated from the seed by the processing industry and it represents about 35–40% of the weight of the fruit [79]. PGH is primarily discarded, but due to its large amount of water (˃ 70%) it is prone to microbial contamination and it should be dried immediately [87, 88]. Thus, this waste product represents an important environmental issue.
According to Hamed et al. [89] dry PGH of Tunisia is largely composed of carbohydrates (39.70% ± 1.04%), lipids (20.41% ± 0.24%), proteins (11.23% ± 0.24%), water (10.46% ± 0.52%) and ash (14.74% ± 0.12%). Similar results were found by Moghaddam et al. [90] in PGH of Iran who reported 13.1% ± 1.2% of proteins, 9.67% ± 0.12% of lipids and 13.1% ± 0.24% of ash; while PGH from Turkey present 11.40% ± 0.41% ash; 8.54% ± 0.37% proteins [91]. The amount of ash in PGH, highlighted by Behgar et al. [92], was higher than in cotton seed hull (2.8% ash); sunflower hull (1.6% ash) and ground peanut hull (4.0% ash). The mineral content of PGH analyzed by Behgar et al. [92] was 560 mg of Ca and 3,680 mg of K per 100 g of product; more than twice the amount of Ca and K present in the pistachio nut (Figure 3). The amount of P in the PGH was found to be 240 mg/100 g, slightly lower than in the kernel [92].
Although lipids are the second most abundant macrocomponent in PGH, its characterization is scarce. Grace et al. [93] evaluated on a non-polar extract the fatty acid profile of PGH of American pistachio, and indicated that the unsaturated fatty acids comprised 87% of the total. Besides, their results showed that the polyunsaturated linoleic acid (18:2) accounts for 50% of total fatty acids content and the oleic acid (18:1) 36.5 %. In line with this result, Mahoney et al. [94] founded in the lipid fraction from PGH collected over the 2011 in California, that linoleic acid (18:2) was the predominant fatty acid. On the contrary, other authors evaluated PGH from Turkey and Iran and suggested that oleic acid (18:1) was the most abundant in their samples. Therefore, as well as in the kernel, this result would indicate that the fatty acid profile of the hull is dependent on the geographical origin.
It has been also indicated that PGH is a good source of non-digestible carbohydrates [95]. Several authors reported for different pistachio varieties values of neutral and acid detergent fiber [92, 96, 97] in the range of 18.25–22.49% and 14.3–18.29%, respectively.
The prebiotic effect of PGH water soluble polysaccharides was evaluated by Akbari-Alavijeh et al. [98]. According to their findings, the polysaccharides were predominantly composed of xylose, glucose, arabinose, and fructose (molar ratio of 1.00:2.50:19.67:28.81) and the dominant fraction (83.2%) had a molecular weight of 3.71 × 106 Dalton. Moreover, the in vitro digestion process showed that 94.3% of this structure remained undigested, and it could stimulate the growth of prebiotic bacteria such as L. plantarum PTCC 1896 and L. rhamnosus GG. Therefore, these authors suggested that PGH polysaccharides can be proposed as potential prebiotics in foods and could positively modify the gut microbiota while improving the host health.
Hamed et al. [99] extracted and identified from pistachio external hull a type II arabinogalactan and proved that these heteropoly-saccharide exhibited an immunomodulatory activity by stimulating B lymphocytes in vitro and could be considered an immunoregulatory agent in health therapeutics.
Moreover, PGH has been considered a potential promising source of pectin that could be used to deal with the high demand of this ingredient [100]. According to Arjeh et al. [88] the amount of pectin in this waste is around 18–19%, similar to other well-known sources of pectin such as apple pomace (15%) and orange peel (18%). Therefore, different studies have been conducted in order to optimize the extraction of PGH-pectin. Chaharbaghi et al. [101] found that the maximum acidic extraction of pectin (22.1% ± 0.5%) could be reached at 90℃ during of 30 min with a very acid solution (pH = 0.5) and a liquid/solid ratio of 50 v/w. Kazemi et al. [100] found a similar result (18.13% of extraction yield) with a microwave-assisted procedure with a power of 700 W, irradiation time of 165 s, and pH 1.5. According to these authors, the extracted pectin was low-methoxyl (about 12.1%) a molecular weight of 1.659 kg/mol and the emulsifying activity of 58.3%. PGH pectin has been used for food applications. In a recent publication, Kazemi et al. [102] formulated low protein and low-Phe cookies for phenylketonuria patients with an extract of PGH pectin and PC. Their results indicated that this addition enhanced the quality of samples, positively affected the overall acceptability and improved the antioxidant activity, while it did not have an impact on the Phe content of the biscuits. Fathi et al. [103] explored the development of a new biodegradable film with PGH-pectin poly vinyl alcohol and different curcumin levels. The latest showed that this novel material could be employed as an intelligent packaging for fatty foods and showed antibacterial activity against Escherichia coli and Staphylococcus aureus.
Bioactive compounds
It has been suggested that PGH has an important nutraceutical potential and various food applications associated to their high proportion of PC [79]. Polyphenols extracted from PGH have attracted special attention because of their antioxidant, anti-inflammatory, and other biological activities [96]. According to Erşan et al. [104] the phenolic content of PGH ranged from 6,120 mg/100 g ± 45 mg/100 g to 10,000 mg/100 g ± 48 mg/100 g of dry matter depending on the variety. This is much higher than the values found in the kernel (Table 5). Nevertheless, some authors found lower amounts of PC (2,130–3,930 mg/100 g) [12, 105]. A wide variety of PC were identified and determined in PGH such as hydroxybenzoic acid and its derivatives, like p-hydroxybenzoic, vanillic, dihydroxybenzoic, gallic, protocatechuic and syringic acids. According to many authors, gallic acid is one of the main PC in PGH [93, 104–110]. However, its extraction rate is strongly dependent on the extraction solvent and the conditions used. Rajaei et al. [13] evaluated the effect of different solvents on PC extraction yield. Their results indicate that water was the most adequate extraction solvent followed by an ethanol/water mixture, methanol and ethanol. In agreement, a high amount of gallic acid [5,480 µg/g dry weight (d.w.)] was determined by Seifzadeh et al. [109] in an aqueous extract of PGH, while Özbek et al. [105] found a lower amount of gallic acid (3,072.9 µg/g d.w.) through an ethanol/water (50:50) microwave-assisted extraction. These last authors determined high quantities of others PC like p-hydroxybenzoic acid (2,054.2 µg/g d.w.) and protocatechuic acid (314.9 µg/g d.w.) and lower amounts of syringic acid (19.0 µg/g d.w.) [105].
Garavand et al. [111] determined cinnamic acid derivatives in PGH. Their results indicate the presence of sinapic acid (26.5 µg/g), and p-coumaric acid (13.9 µg/g) using a microwave-assisted extraction with water. Similar amounts of p-coumaric acid (17.1 µg/g) were reported in microwave-assisted ethanol:water extracts, together with caffeic acid (11.8 µg/g) by Özbek et al. [105]. Another derivate of cinnamic acid found in PGH was caffeic acid-O-hexose (150.9 µg/g d.w.) [109].
PGH is also a source of natural flavonoids such as as quercetin-3-O-rutinoside, quercetin 3-O-galactoside, quercetin 3-O-glucuronide and quercetin-3-O-glucoside (Table 7). The maximum amount of quercetin-3-O-rutinoside and quercetin-3-O-glucoside was 1,280 µg/g d.w. and 2,030 µg/g d.w., respectively according to Seifzadeh et al. [109] while the minimum amount was reported in ethanol extracts by Barreca et al. [106]. Another flavonoid found in PGH was eriodictyol-7-O-glucoside, and the maximum concentration was informed in methanolic extracts of PGH (1,333.73 µg/g d.w.) by Seifzadeh et al. [109].
Bioactive compounds in pistachio hull
| Main phenolic compounds | Others phenolic compounds | Type of Extraction and determination | Associated health benefits | Reference | ||
|---|---|---|---|---|---|---|
| Name | Concentration (µg/g DM) | Name | Concentration (µg/g DM) | |||
| Gallic acid | n.d. | Galloylshikimic acid | n.d. | Acidified (0.05% trifluoroacetic acid) methanolic (80%) extractDichloromethane extractHPLC-IT-TOF-MSn | Antioxidant and anti-inflammatory | [93] |
| Quercetin | n.d. | Digalloyl quinic acid | n.d. | |||
| Myricetin-galloyl hexoside | n.d. | (Epi)catechin hexoside | n.d. | |||
| Quercetin-galloyl glucoside | n.d. | Digalloyl hexoside | n.d. | |||
| Quercetin-3-glucuronide | n.d. | Hexagalloyl hexoside | n.d. | |||
| Quercetin-3-glucoside (isoquercetin) | n.d. | |||||
| Myrcitin-3-glucoside | n.d. | |||||
| Luteolin | n.d. | |||||
| Luteolin glucuronide | n.d. | |||||
| Luteolin hexoside | n.d. | |||||
| anacardic acids | n.d. | |||||
| Quercetin-3-O-rutinoside | 340.01 | Isorhamnetin-3-O-rutinoside | 42.74 | Methanol extractReverse-phase-HPLC-DAD-FLU | Antioxidant and cytoprotective | [106] |
| Eriodictyol-7-O-glucoside | 73.29 | Isorhamnetin-3-O-glucoside | 26.74 | |||
| 4-Hydroxybenzoic acid | 72.09 | Quercetin-3-O-glucoside | 16.35 | |||
| Catechin | 60.64 | Protocatechuic acid | 10.89 | |||
| Gallic acid | 57.20 | Naringin | 9.73 | |||
| Vanillic acid | 4.46 | |||||
| Eriodictyol | 2.80 | |||||
| Quercetin-3-O-galactoside | 2.51 | |||||
| Naringenin | 2.41 | |||||
| Kaempferol | 2.22 | |||||
| Luteolin | 1.27 | |||||
| Epicatechin | 0.88 | |||||
| Quercetin | 0.77 | |||||
| Apigenin | 0.11 | |||||
| Eriodictyol-7-O-glucoside | 40.26 | Protocatechuic acid | 3.82 | Ethanol extractReverse-phase-HPLC-DAD-FLU | Antioxidant and cytoprotective | [106] |
| Isorhamnetin-3-O-glucoside | 17.54 | 4-Hydroxybenzoic acid | 3.97 | |||
| Quercetin-3-O-rutinoside | 137.25 | Vanillic acid | 0.06 | |||
| Catechin | 31.64 | Eriodictyol | 0.72 | |||
| Gallic acid | 14.50 | Naringenin | 0.20 | |||
| Naringin | 7.77 | |||||
| Quercetin | n.d. | |||||
| Kaempferol | n.d. | |||||
| Quercetin-3-O-glucoside | 7.95 | |||||
| Quercetin-3-O-galactoside | 1.14 | |||||
| Isorhamnetin-3-O-rutinoside | 1.11 | |||||
| Luteolin | 0.47 | |||||
| Apigenin | n.d. | |||||
| Epicatechin | 0.37 | |||||
| β-Glucogallin | 4,720 | Galloyl shikimic acid | 842.5 | Ultrasound-assisted methanol/water/formic acid (80/19/1, v/v/v) extractHPLC-DAD-ESI–MSn | - | [104] |
| Gallic acid | 3,102.5 | Protocatechuic acid | 482.5 | |||
| Penta-O-galloyl-β-D-glucose | 3,247.5 | Kaempferol hexoside | 475 | |||
| Galloyl quinic acid | 1,437.5 | Cyanidin-3-O-β-D-galactopyranoside | 425 | |||
| Quercetin-3-O-galactoside/glucuronide | 1,902.5 | Digalloyl hexose | 326.6 | |||
| Quercetin-3-O-glucoside | 1,900 | Myricetin-O-hexoside | 290 | |||
| Quercetin galloyl hexoside | 2,307.5 | Myricetin3-O-galactoside | 280 | |||
| Anacardic acids | 63,475 | |||||
| Catechin | 53.6 | p-Coumaric acid | 13.9 | Microwave-assisted aqueous extractHPLC-DAD-FLD-MS | - | [111] |
| Naringenin | 49.9 | Gallic acid | 11.2 | |||
| Sinapic acid | 26.5 | Vanillic acid | 10.5 | |||
| Phloroglucinol | 4.7 | |||||
| Gallic acid | 5,243.87 | Quercetin-3-O-rutinoside | 426.86 | Methanolic (75%) extractHPLC-DAD-ESI-MS/MS | - | [107] |
| Eriodictyol-7-O-glucoside | 1,333.73 | Naringenin | 245.19 | |||
| Catechin | 1,906.55 | Protocatechuic acid | 206.87 | |||
| Quercetin | 169.39 | |||||
| Eriodictyol | 123.47 | |||||
| Naringenin | 117.50 | |||||
| Epicatechin | 19.50 | |||||
| Luteolin | 3.35 | |||||
| Phloroglucinol | 9,810 | Protocatechuic acid | 13.5 | Aqueous extractReverse-phase-HPLC-DAD | α-Amylase and α-glucosidase inhibition | [108] |
| Gallic acid | 783 | Cinnamic acid | n.d. | |||
| Vanillic acid | 345 | Catechin | 0.006 | |||
| Eriodictyol-7-O-glucoside | 99 | 4-Hydroxybenzoic acid | 0.003 | |||
| Naringin | 27 | |||||
| Gallic acid | 5,480 | Quercetin-O-galloyl-O-hexoside I | n.d. | Aqueous extractUHPLC–MSn | - | [109] |
| Galloyl shikimic acid | 5,670 | Quercetin-O-galloyl-O-hexoside II | 370 | |||
| Theogallin | 4,560 | Luteolin | n.d. | |||
| Quercetin-O-hexoside | 2,030 | Kaempferol-O-galloyl-O-hexoside | n.d. | |||
| Pyrogallol | 1,840 | Luteic acid | n.d. | |||
| Quercetin-3-O-rutinoside | 1,280 | Dihydroxybenzoic acid-O-hexoside | 240 | |||
| Quercetin | n.d. | Galloyl-O-hexoside I | 190 | |||
| Methylgallate | 560 | Protocatechuic acid | 180 | |||
| Digalloylquinic acid | 490 | Caffeic acid-O-hexoside | 150 | |||
| Quercetin-3-O-glucuronide | 430 | Myricetin-O-hexoside | 120 | |||
| Galloyl-O-pentoside | 100 | |||||
| Gallic acid | 3,072.9 | Syringic acid | 19.0 | Microwave-assisted ethanol:water (50:50) extractHPLC-PAD | - | [105] |
| p-Hydroxybenzoic acid | 2,054.2 | p-Coumaric acid | 17.1 | |||
| Protocatechuic acid | 314.9 | Quercetin | 13.5 | |||
| Epicatechin | 286.9 | Caffeic acid | 11.8 | |||
| Gallic acid | 362.1 | Epicatechin | 35.6 | Methanolic (80%) extractHPLC | Antioxidant and anti-candida | [110] |
| Eriodictyol-7-O-glucoside | 127.1 | Eriodictyol | 17.2 | |||
| Catechin | 93.5 | Naringenin | 3.3 | |||
| Cyanidin-3-O-galactoside | 1431.1 | Naringin | 27.1 | |||
| Quercetin | 4.4 | |||||
| Kaempferol | 0.15 | |||||
| Luteolin | 4.6 | |||||
HPLC: high-performance liquid chromatography; DAD: diode array detector; FLD: fluorescence detector; MS/MS: tandem mass spectrometry; IT: ion trap; TOF: time-of-flight; MSn: multistage mass spectrometry; ESI: electrospray ionization; UHPLC: ultra-high-performance liquid chromatography; MS: mass spectrometry; PAD: pulsed amperometric detection; n.d.: not detected; -: not studied
Also, some representative compounds from the tannin family have been found in PGH. Gallotannins and galloyl were identified by Grace et al. [93] through mass spectrometry. Gallotannins are characterized by the presence of galloyl groups linked to a sugar molecule. Luteic acid, theogallin, galloyl-O-hexoside (penta-O-galloyl-β-D-glucose), β-glucogallin, galloyl quinic and shikimic acids were found by Seifzadeh et al. [109]. Moreover, these authors found a higher amount of galloyl shikimic acid (5,670 µg/g d.w.) through an aqueous extraction than Erşan et al. [104] (842.5 µg/g d.w.) who used a methanolic extraction.
Some of the previous authors not only determined the quantities of the PC in PGH, but they also carried out in vitro experiments to elucidate their beneficial effects. Grace et al. [93] demonstrated the antioxidant and anti-inflammatory properties of a polar PGH extract which inhibited the release of ROS and NO. Also, they reported positive effect on the gene expression of biomarkers responsible of inflammatory response.
Barreca et al. [106] demonstrated the radical scavenging activity of PGH extracts against 1,1-diphenyl-2-picrylhydrazyl (DPPH), superoxide anion and 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+). This work evaluated the cytoprotective effect of ethanolic and methanolic extracts of PGH, through their ability to inhibit erythrocyte membranes lipid peroxidation and their potential to protect the blood lymphocytes from cytotoxicity.
Moreover, Lalegani et al. [108] evaluated the in vitro capacity of PGH to inhibit α-amylase and α-glucosidase, carbohydrate digestion enzymes, by colorimetric methods. They found an inhibitory effect of different concentrations of PGH extract against porcine pancreatic α-amylase. Their results indicate a PGH extract (610 μg GAE/mL) dose dependent inhibition. They concluded that the α-amylase inhibitory activity was related to the tannin fraction of the PGH extract.
Finally, antifungal property against Candida species was evaluated by Gharibi et al. [110]. Their results indicated that pistachio extracts had a lower minimum inhibitory concentration against C. albican, C. glabrata and C. auri, than that of fluconazole treatment. These authors attribute this property to cyanidin-3-O-galactoside as the mayor polyphenol. However, they pointed out that probably the complete polyphenol profile contribute to the observed result. All this information related to polyphenol bioactivity is summarized in Table 7.
Application in encapsulated and water/oil systems
Pistachio wastes are important due to there are a source of bioactive compounds that can be extracted and incorporated in small particles and also they could also be used as encapsulation materials.
The CNC extracted from PS are excellent candidates as a carrier for encapsulation and for stabilization of oil-water emulsions. Kasiri and Fathi [112] extracted and purified CNC from PS (diameter 36.6 nm ± 8.9 nm) and evaluate its capacity to encapsulate peppermint oil. Their results showed that diffusion transport was the main mechanism for the release of peppermint oil from CNC while no significant differences in the crystallinity of CNC occurred after encapsulation. Furthermore, Kasiri and Fathi [113] evaluated the emulsifier ability of CNCs as a pickering agent in oil in water emulsions. These authors found that by increasing CNC concentration from 0.1% to 1.5%, the size of the emulsion droplets significantly decreased from 17.31 µm to 2.34 µm while the stability of the emulsions against temperature, stresses and storage time were enhanced. Therefore, they could conclude that the potential application of CNC from PS in the food industry is remarkable.
Moreover, pectin extracted from PGH is a promising encapsulation material. Its use has several benefits, such as being an emulsion stabilizer, it has gelling properties and it is generally considered a safe ingredient. Barış et al. [114] evaluated the potential use of the extracted pectin as an emulsifying agent in an oil-in-water emulsion.
As previously explained, PGH is a good source of bioactive compounds, but their application in food has several limitations. The PC may be lost during the food processing, storage time or digestion process, and in addition, they would leave to unpleasant taste in the products. Besides, the PC may present with low bioavailability in the food matrix because of their interaction with other components [115]. Therefore, recent reports have indicated that the encapsulation of bioactive compounds from pistachio waste would be a possible solution to overcome these problems. Ghandehari et al. [116] achieved the encapsulation of PGH PC by spray-dryer using maltodextrin as a carrier? and they showed that the microencapsulation improved the thermal and storage stability of PC. Moreover, Rafiee et al. [115] prepared nanoliposomes of PGH PC with lecithin (1%; particle size 92.05 nm ± 0.59 nm). These nonoliposomes were considerable stable during 2 months at 4℃, remaining approximately 75% of the phenolics in the nanoliposomes after that period with a slightly increase (3.85% increase) in the particle size. Finally, Oskoueian et al. [117] extracted PGH PC with ethyl acetate, incorporated them in nanoliposomes (96.3 nm ± 7.18 nm) and analyzed their antioxidant activity against free radicals. However, these authors did not evaluate their addition into a food material, but indicated their potential application in the pharmaceutical industry.
Other authors analyzed the incorporation of encapsulated PGH phenolics in food products such as mayonnaise [118], soybean oil [14], ice cream [119] and as a preservative for fruits [120]. A strategy for preventing lipid oxidation is the incorporation of antioxidant compounds. The food industry uses antioxidants molecules such as tert-butyl hydroquinone (TBHQ), butylated hydroxyl anisole (BHA), and butylated hydroxyl toluene (BHT); although the actual tendency is to search natural antioxidants. As it was previous mentioned, PGH is a good source of polyphenols with antioxidant properties. Therefore, Rafiee et al. [118] encapsulated PC from PGH extract into nanoliposome systems using a suspension of lecithin and water. The purpose was to incorporate them in mayonnaise and evaluate the physicochemical characteristics and microbial properties during four months of storage at 25°C. The encapsulated PC acted as antioxidants by reducing peroxide and thiobarbituric acid values. In this food system, encapsulated PGH extracts demonstrated an important inhibitory effect on microbial growth; some of the PC presented antimicrobial effect. The access and release of PC is facilitated by the affinity of liposomes for the cell membrane. This mayonnaise presented higher taste score and overall acceptability by sensory evaluation with semi-trained panelist.
Good results were also found when incorporating these encapsulates in soybean oil [14]. Nanoliposomes containing 500 ppm of PC from PGH allowed to obtain a stable soybean oil with antioxidant activity during storage demonstrated by the low peroxide and thiobarbituric acid value, primary and secondary products of lipid oxidation. They attributed this benefit to the control release of phenolic compound from liposomes, depending on the type of bounds between phenolics and phospholipids.
It was feasible to produce an ice cream enriched with microcapsules of pistachio peel extract (MPPE) [119]. Different percentages of microcapsules (0–2%) were added before the ice cream pasteurization stage. The microcapsules were made with maltodextrin. Textural parameters were evaluated, finding that viscosity, hardness and stickiness increase when the microcapsules of pistachio are incorporated. The authors concluded that MPPE improved the melting resistance, antioxidant activity and total phenolic content of the ice cream, without affecting sensorial properties.
The encapsulation of essential oil from Pistacia atlantica subsp. into chitosan nanoparticles allowed to demonstrate in vivo antifungal effect on strawberry fruit against Botrytis cinereal [120]. On the eighth day of storage the strawberry fruit immersed chitosan nanoparticles showed symptoms of spoilage, compare to the spoilage revealed on the control at the fourth day. Growth retardation of Botrytis (B.) cinereal was also demonstrated on the treated fruit. These results promise an alternative to extend the shelf-life of strawberries.
Future perspectives for the pistachio industry
Although pistachio trees show good adaptability to a variety of climate conditions, the quality of the product can decline because of intense optical radiation and high temperatures [121]. Therefore, the development of new techniques to determine the most suitable sites for pistachio cultivation is of major importance. Everest [122] evaluate the requirements for pistachio cultivation and transferred the data to a geographic information system (GIS). With overlay analysis, these authors were able to determine the most suitable places for pistachio production. Similar methodologies were used to identify the optimal area for medicinal plants through the analytic hierarchy process (AHP) and GIS [123]. Moreover, the population growth increases the need for land for non-agricultural proposes and intensifies the competition for territory. In order to overcome these problems, Aliabad et al. [124] developed a new method for monitor of new constructions in urban areas, using imaging by unmanned aerial vehicle (UAV) and the time series of Sentinel-2 images. Therefore, image analysis could be a good option to analyze the best location for pistachio production. Future studies should analyze the association between the geographical and climate characteristics and the nutritional profile of the pistachio nut.
Conclusions
Pistachio is a highly beneficial whole food for human health. It is as an excellent source of protein, dietary fiber, and essential fatty acids. It also provides high amounts of phosphorus and Cu, along with a low sodium content. Besides the presence of bioactive compounds (vitamins, carotenoids, polyphenols, and flavonoids) MUFAs, phytosterols, bioactive peptides and dietary fiber have been associated to the antioxidant, anti-inflammatory and antihypertensive properties that pistachio has, together with its potential benefits for glycemic control, diabetes prevention and weigh maintenance.
Pistachio wastes like PGH and PS could also be considered potential source of bioactive compounds. In vitro assays revealed the antioxidant and anti-inflammatory potential of PGH extracts, and their capacity to reduce oxidative stress. Besides, the pistachio bioactive components present in the wastes may be incorporated in encapsulated and emulsion systems extending their shelf-life.
Abbreviations
| ACE: | angiotensin-converting enzyme |
| AOP: | antioxidant potential |
| ATCC: | American Type Culture Collection |
| B1: | thiamine |
| B2: | riboflavin |
| B6: | pyridoxine |
| BAFMVD: | brachial artery flow-mediated vasodilation |
| BMI: | body mass index |
| CAR: | carrageenan |
| CNC: | cellulose nanocrystals |
| COX-2: | cyclooxygenase-2 |
| CRP: | C-reactive protein |
| CVDs: | cardiovascular diseases |
| HbA1c: | glycated hemoglobin A1c |
| HDL: | high-density lipoprotein |
| HFD: | high-fat diet |
| IL-6: | interleukin-6 |
| LDL: | low-density lipoprotein |
| LOOH: | lipid hydroperoxides |
| MDA: | malonaldehyde |
| MPO: | myeloperoxidase |
| MUFAs: | monounsaturated fatty acids |
| NO: | nitric oxide |
| NP: | natural pistachios |
| PC: | phenolic compounds |
| PGH: | pistachio green hull |
| PS: | pistachio shell |
| PWV: | pulse wave velocity |
| RCCT: | randomized crossover clinical trial |
| RCT: | Randomized Controlled Trial |
| RDI: | recommended dietary intake |
| ROS: | reactive oxygen species |
| RPs: | roasted pistachios |
| SOD: | superoxide dismutase |
| T2-DM: | type 2 diabetes mellitus |
| TBHQ: | tert-butyl hydroquinone |
| TC: | total cholesterol |
| TG: | triglycerides |
| TNF-α: | tumor necrosis factor-α |
| TOS: | total oxidative status |
Declarations
Author contributions
JRG: Conceptualization, Investigation, Writing—original draft. MP: Conceptualization Investigation, Writing—original draft, Supervision. MCP: Writing—review & editing, Supervision. MVS: Conceptualization, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was financially supported by Ministerio Nacional de Ciencia, Tecnología e Innovación (MINCyT); Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); and Universidad Nacional de La Plata (UNLP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2024.