Affiliation:
1School of Agriculture, Faculty of Agriculture Forestry and Natural Environment, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece
Email: elenzym@agro.auth.gr
ORCID: https://orcid.org/0009-0006-8680-0704
Affiliation:
2Department of Food Science and Technology, School of Geoscience, International Hellenic University of Thessaloniki, 57400 Sindos, Thessaloniki, Greece
ORCID: https://orcid.org/0009-0004-6276-3414
Affiliation:
3Pesticide Science Laboratory, School of Agriculture, Faculty of Agriculture Forestry and Natural Environment, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece
Email: rmenkis@auth.gr
ORCID: https://orcid.org/0000-0002-8023-2391
Explor Foods Foodomics. 2024;2:599–612 DOI: https://doi.org/10.37349/eff.2024.00053
Received: April 05, 2024 Accepted: August 29, 2024 Published: October 12, 2024
Academic Editor: Nives Ogrinc, Jožef Stefan International Postgraduate School and Jožef Stefan Institute, Slovenia
The article belongs to the special issue Metrological Aspects in the Analysis of Nutrients, Functional Compounds, Additives and Contaminants in Food and Feed
Aim: Volatile organic compounds (VOCs) are often human-made contaminants used and generated in the manufacturing of numerous products, presenting notable environmental and health hazards. Therefore, the development of sensitive and reliable analytical methods is crucial for their detection with accuracy, timeliness, and automation capabilities. The objective of this study is to demonstrate the suitability of the in-tube extraction dynamic headspace (ITEX-DHS) sampling method for the gas chromatographic/mass spectrometric (GC/MS) analysis of BTEX (benzene, toluene, ethylbenzene, and xylenes) compounds in aqueous matrices. It emphasizes the method’s metrological reliability and innovative approach to precisely determining VOCs in aqueous environments providing a tool to prevent contamination of the agrifood sector.
Methods: Following the optimization of various experimental parameters, including salt incorporation and adjustments of both dry purging and desorption conditions. The method’s performance was evaluated for repeatability, reproducibility, and robustness.
Results: Limit of detection (LOD) and limit of quantification (LOQ) were for all substances determined lower than 50 and 100 ng/L, respectively. Average relative standard deviations below 5% were achieved for all analytes, with recovery rates ranging between 93% and 101%. Subsequently, the method was applied for the determination of BTEX in one hundred groundwater samples. The findings revealed that the BTEX levels were below the LOD in 84.2% of samples. However, in the remaining samples, more than one compound was detected at concentrations higher than the LOQ.
Conclusions: The ITEX method emerges as a highly favorable alternative to both solid phase microextraction (SPME) and purge and trap (P & T) methods for determining BTEX in aqueous samples, providing significant advantages. Its strengths lie in its increased robustness, extended trap lifespan, and enhanced sensitivity, underscoring its superior performance in VOC analysis. The total analytical method allows the sensitive and robust determination of VOC.
ITEX-DHS sampling method coupled to GC/MS for the sensitive analysis of BTEX in aqueous samples. ITEX-DHS: in-tube extraction dynamic headspace; GC/MS: gas chromatographic/mass spectrometric; BTEX: benzene, toluene, ethylbenzene, and xylenes
In recent years, the rapid industrialization and urbanization processes have significantly increased the potential for water contamination by various pollutants, posing a threat to both human health and the environment [1]. Among the pollutants, benzene, toluene, ethylbenzene, and xylenes (BTEX) compounds have garnered considerable attention due to their widespread use in industrial activities and their well-documented adverse health effects [2]. BTEX compounds are considered as common environmental pollutants that are often found in water due to industrial discharges, petroleum leaks, and runoff. These contaminants can enter the food chain when contaminated water is used for irrigation or as a part of food processing. The presence of these contaminants in the waters can have significant connections to food safety and quality.
As environmental regulations become increasingly stringent, the proposed methodology holds great promise for applications in environmental monitoring, industrial hygiene, and risk assessment. Regular monitoring of BTEX levels in water sources used for agriculture and food processing is essential. By achieving heightened sensitivity and accuracy in the determination of BTEX, this research endeavors to provide a robust analytical solution that meets and exceeds the demands of contemporary analytical challenges.
Gas chromatography/mass spectrometry (GC/MS) has long been established as a powerful tool for volatile organic compound (VOC) analysis, offering exceptional sensitivity and selectivity [3, 4]. However, as the demand for higher analytical efficiency and increased sample throughput grows, there is a continuous need for optimization and validation of analytical methods to ensure the accuracy and reproducibility of results [5, 6].
Solid-phase microextraction (SPME) and liquid-liquid extraction (LLE) are commonly employed techniques for sample extraction and pre-concentration for the determination of BTEX in water matrixes [7, 8]. Also, advances in microextraction techniques, such as hollow fiber liquid-phase microextraction (HF-LPME) and stir-bar sportive extraction (SBSE), have been explored for their efficiency and reduced solvent consumption [9, 10].
Efforts to enhance extraction yields have focused on increasing the extraction phase quantity, primarily through the utilization of packed sorbent materials. Berezkin et al. [11] developed a method utilizing a sorbent bed for BTEX compound determination. Wang et al. [12] introduced the needle trap (NT), a needle-based device employing packed sorbent. Saito et al. [13] presented a similar needle extraction device for GC/MS analysis of VOCs using a copolymer bed of methacrylic acid and ethylene glycol dimethacrylate.
The introduced in-tube extraction (ITEX) technique employing Tenax TA demonstrated improvements over previous needle-based methods and brought forth numerous benefits, including the extraction of VOCs from minimal sample volumes. This characteristic makes it particularly suitable for applications with limited sample availability. Additionally, it minimizes matrix effects, enabling more accurate and reliable quantification of BTEX in complex water matrices without extensive sample pre-treatment, positioning it as a promising alternative to traditional sample preparation methods [14, 15].
Moreover, ITEX can be integrated into automated systems, facilitating high-throughput analysis of water samples. Automation of sample extraction and injection processes reduces the potential for human error and enhances reproducibility. Limited research and literature exist regarding the combined use of GC/MS and ITEX for determining BTEX compounds. The objective of this study is to enhance the effectiveness and reliability of the method by optimizing it to reduce analysis duration while upholding excellent sensitivity in detecting BTEX compounds in water samples. This optimization aims to turn the method into a valuable tool for promptly and accurately detecting BTEX compounds in aqueous samples, offering a rapid and sensitive approach for environmental monitoring and risk assessment purposes. We delve into method development details, focusing on extraction conditions, chromatographic separation, and mass spectrometric detection to achieve optimal analytical performance. Additionally, the proposed analytical approach was validated in terms of limits of detection, limits of quantitation, linearity, accuracy, and precision, following the guidelines outlined by the European action in chemistry (Eurachem).
As environmental concerns escalate and regulatory standards evolve, the outcomes of this research not only contribute to the advancement of analytical methodologies but also hold practical implications for environmental monitoring, occupational safety, and public health. While BTEX compounds are primarily a concern due to their toxicity, they can also even at low concentrations, either affect the aroma and flavor or perceived freshness of food products, impacting negatively the food quality. Establishing and enforcing stringent limits for BTEX compounds in food and water is part of ensuring food safety. Sensitive analysis methods enable the detection of even trace amounts of these contaminants, helping to protect both food quality and public health. The amalgamation of GC/MS and ITEX offers a promising avenue for achieving heightened precision and efficiency in the determination of BTEX compounds, thereby facilitating a more accurate assessment of environmental and occupational exposures [14]. Specifically, the optimized method exhibits a notable reduction in both detection and quantification limits for BTEX analysis in aqueous samples that are further used in the agri-food sector.
Analytical grade methanol (HPLC-Ultra LC-MS grade) was purchased from HiPerSolv CHROMANORM (VWR Chemicals BDH, Netherlands) and was used for the preparation of stock and working solutions. Ampoule of BTEX (a mixture of benzene, toluene, ethylbenzene, p-xylene, m-xylene, and o-xylene, 200 mg/L each in methanol) standard solution (batch number 800839), and ethylbenzene D10 (batch number 781749) [as the internal standard (IS)] were purchased from Merck (Darmstadt, Germany). Sodium chloride (> 99.5%, analytical reagent grade) purchased from Chem-Lab NY (Belgium) was used to differentiate the ionic strength of the water samples. Nitrogen (99.999% purity) and Helium (99.999% purity) were used as auxiliary and carrier gas, respectively.
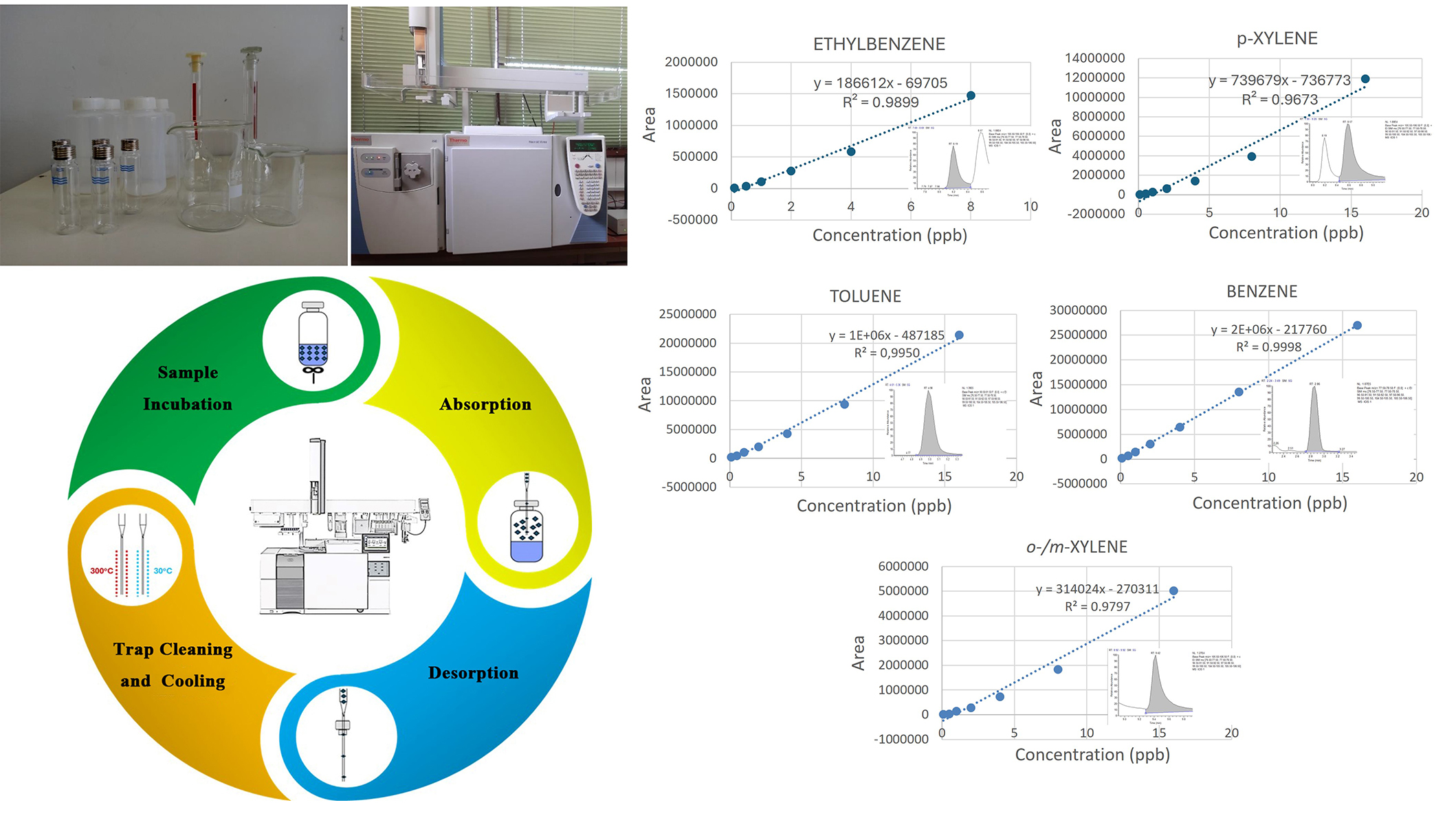
Mixed methanolic stock solutions with a concentration of 2,000 mg/L were stored at 4°C in the darkness. A standard mixture of BTEX compounds (10 mg/L) was prepared before each experiment by dilution of these primary stock solutions in methanol. The working standard solutions, with concentration levels ranging from 0.001 to 0.01 mg/L were prepared by diluting the mixed stock solutions with methanol in final volume 10 mL into 20 mL screw cap headspace vials (Thermo Fisher Scientific) and stored at 4°C in the darkness. A methanolic stock/standard solution with a concentration of 10 μg/L ethylbenzene D10 was prepared and used as an IS. Lower concentrated solutions for calibration, determination of limit of detection (LOD) and limit of quantification (LOQ), and method optimization were prepared through volumetric dilution to achieve the desired concentration levels.
All samples were analyzed using a Trace GC Ultra (batch number 620111155, Thermo Finnigan, Milan, Italy) gas chromatography coupled with an ion source quadrupole (ISQ) (batch number 120112, Thermo Fisher Scientific, Milan, Italy) single quadrupole mass spectrometer. ITEX was performed with a Triplus RSH autosampler (batch number 237688, supplied by Thermo Fisher Scientific, Thermo Finnigan, Milan, Germany). Data acquisition, processing, and evaluation were carried out using the standard software Xcalibur Data SystemVersion 4.2 (Thermo Finnigan, Austin TX, US). The analytes were separated by a Mid-polar column, OPTIMA 624, 30 mL, 0.25 mm inner diameter (ID), 1.4 µm (Macherey Nagel, Germany).
The temperature gradient employed to achieve separation of the desired compounds proceeded as follows: an initial duration of 10 min at 37°C, followed by a gradual increase of 3°C per minute until reaching 110°C, where it was maintained for 1 minute; subsequently, a rapid ramping of 30°C per minute was implemented until reaching 240°C, where it was sustained for 5 min. The entire GC program duration amounted to 43.5 min, with the transfer line and ion source temperatures set at 200°C and 220°C, respectively. A programmable temperature vaporizer (PTV) injector from Thermo Finnigan, Milan, Italy, operated in splitless mode at a base temperature of 250°C for the injection port, with a splitless duration of 0.5 min. During injection, the carrier gas flow rate was maintained at 1 mL/min. MS operated in electron impact (EI) ionization mode at 70 eV. Single ion monitoring was utilized for all measurements, including those of real samples. Quantification was based on specific quantifier ions and confirmation ions (refer to Table 1 for details). A chromatogram depicting a 500 ng/L standard under optimized conditions was generated.
Retention times and selected ions for the analysis of the target compounds
| Compound | Retention time (min) | Target ion (m/z) | Quantified ion (m/z) |
|---|---|---|---|
| Benzene | 12.52 | 78 | 77 |
| Toluene | 20.66 | 91 | 92 |
| Ethylbenzene C8H10 | 27.38 | 91 | 105, 106 |
| p, m-Xylene | 27.95 | 91 | 105, 106 |
| o-Xylene | 29.66 | 91 | 105, 106 |
| Ethylbenzene C8D10 | 27.05 | 98 | - |
-: no data
Before extraction, the upper section of the needle body was filled with 25 mg of Tenax TA (80/100 mesh). The samples underwent incubation at 70°C for 10 min with static stirring at 500 rpm. Meanwhile, the ITEX trap was preconditioned by heating it to 300°C and flushing it with nitrogen for 10 min. Once the trap temperature returned to 30°C, the sample was extracted through 40 cycles of 1.25 mL each, with a flow rate of 50 µL/s. Thermal desorption occurred in the PTV injector, where the trap was heated to 300°C and injected at a rate of 100 µL/s. Subsequently, a post-conditioning phase of 10 minutes, following the same procedure as the preconditioning, was carried out. The decision to conduct both pre- and post-conditioning was based on the overall sample preparation time, ensuring that conditioning could be performed simultaneously with other tasks. The entire sample preparation process for each sample, including incubation, preconditioning, extraction, desorption, and post-conditioning, takes approximately 38 min and is conducted concurrently with GC analysis. Consequently, the GC temperature program serves as the determinant factor for the overall analysis time.
The effect of the following experimental parameters on the ITEX extraction efficiency, including agitator temperature, number of extraction cycles, trap temperature, desorption temperature, incubation time, extraction volume, and extraction speed was studied. All experiments were performed in triplicate and the means of the results were used for optimization. The statistical analysis for the comparison of the parameters under consideration was carried out according to the Τukey Method and 95% confidence level.
Agitator temperature was confirmed in increments of 10°C from 40°C to 90°C. For this evaluation, the trap extraction temperature was 45°C. The extraction flow rate remained constant at 100 μL/s, while the extraction volume for each cycle was maintained at 1,000 μL. Thirty extraction cycles were carried out. Before the extraction process the incubation, time was set to 15 min. The desorption volume and desorption temperature were set to 1,000 μL and 250°C respectively. The optimal extraction temperature was 70°C, which is illustrated in Figure 1. In the case of BTEX, the difference in the extraction efficiency at different incubation times (5, 10, 15, and 20 min) was not significant (P > 0.05). Thus, in the parameter set of the optimized method, the used incubation time was 10 min.
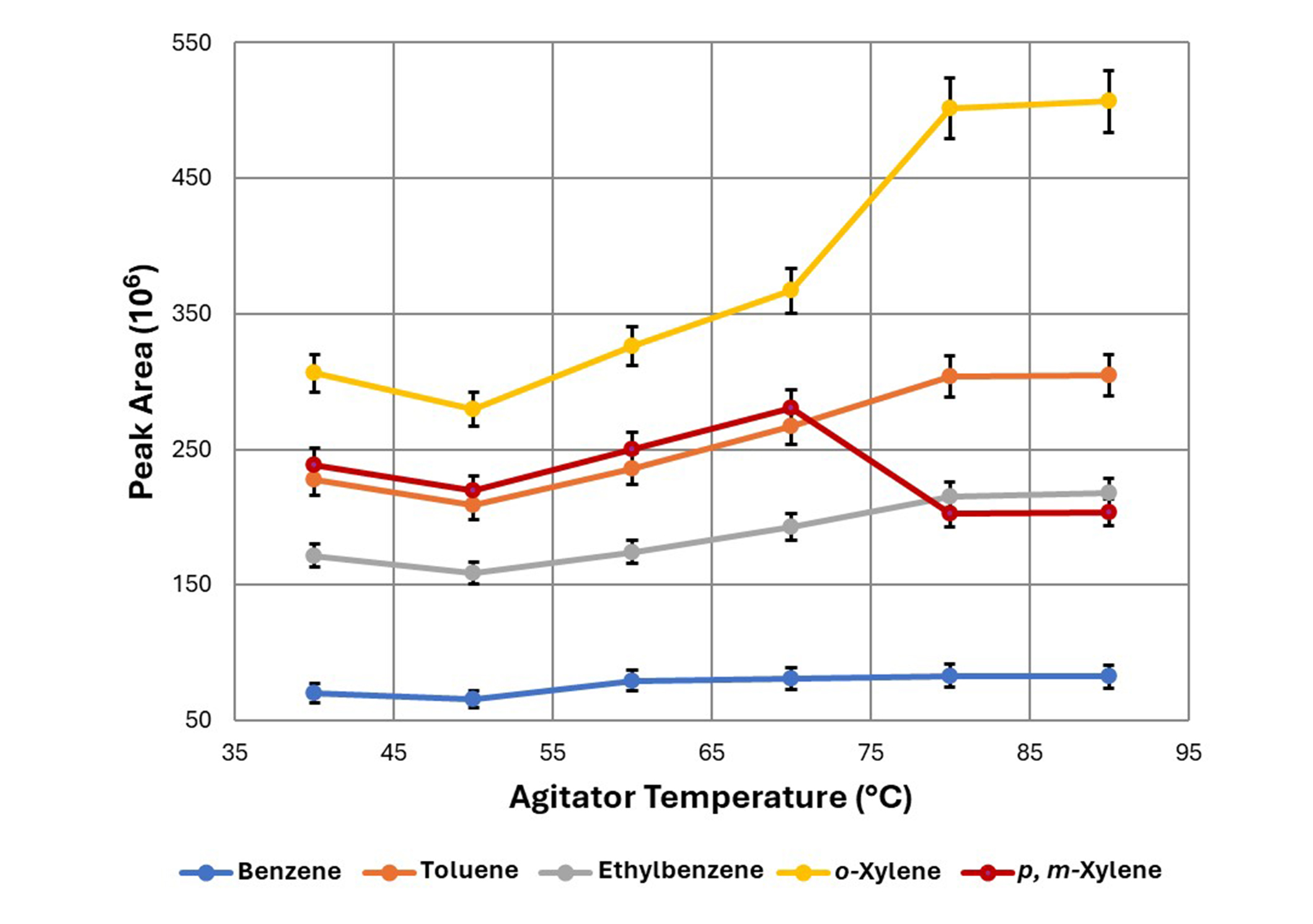
The effect of agitator temperature on the extraction efficiency of BTEX compounds. Each point represents the mean value (± st. error). BTEX: benzene, toluene, ethylbenzene, and xylenes; st.: standard
To increase the method sensitivity the number of extraction cycles was investigated within a range between 5 up to 50 as shown in Figure 2. Before extraction, the samples were equilibrated for 15 min at 70°C. During the extraction process, the trap extraction temperature was set at 45°C. The extraction flow rate and extraction volume were set to 100 μL/s and 1,000 μL, respectively. In order to ensure an adequate extraction time, a fixed value of 40 extraction cycles was chosen for the optimized method.
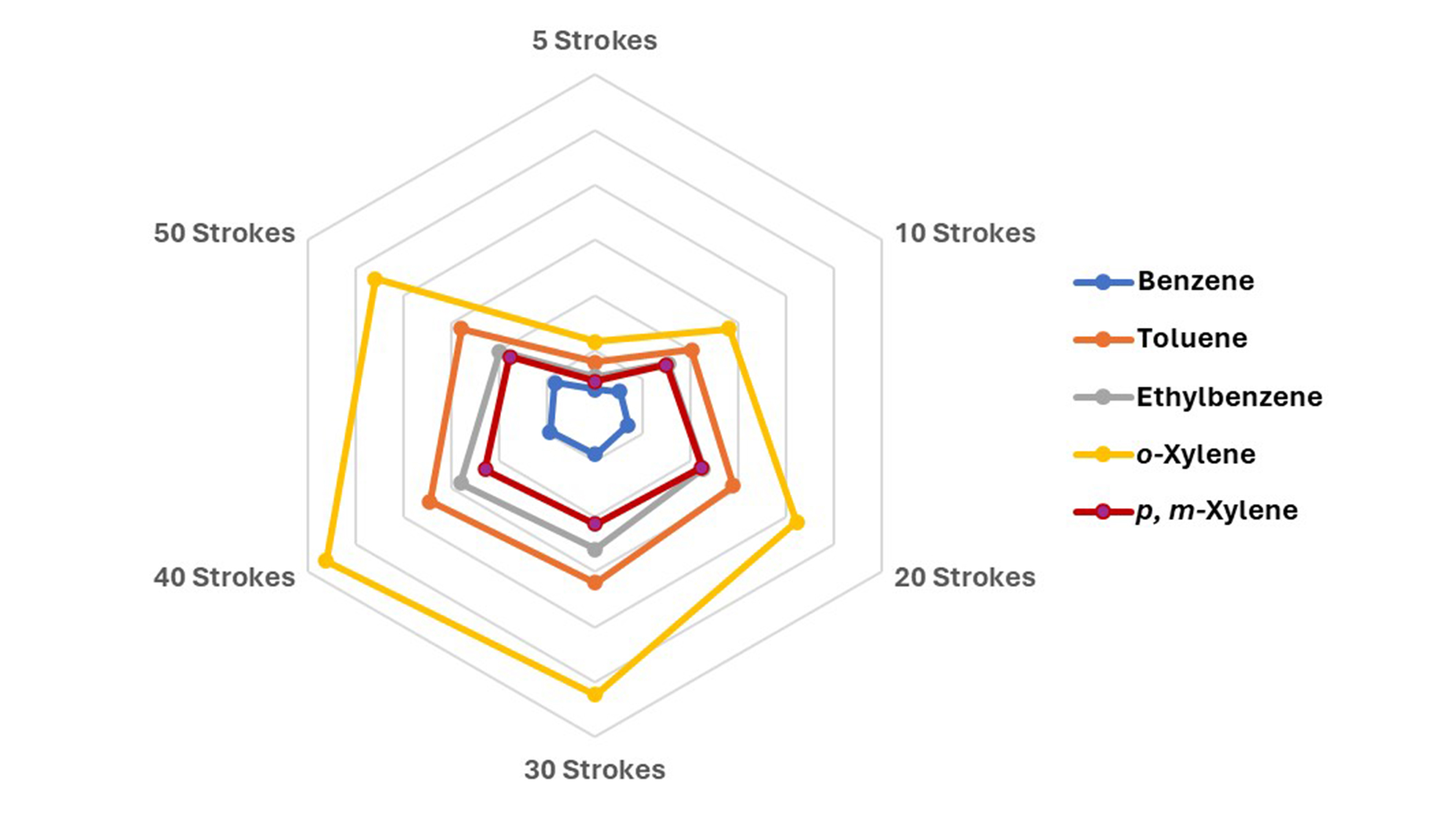
The effect of the extraction cycle on the extraction efficiency of BTEX compounds. BTEX: benzene, toluene, ethylbenzene, and xylenes
The following volumes of extraction were tested: 250, 500, 750, 1,000, and 1,250 μL. The highest efficiency of extraction for almost all compounds was achieved for the volume of 1,250 μL (Figure 3A). In the case of o-xylene seems that it still has a trend of increase, and the plateau is not reached yet. Considering that there is a limit of the syringe at 1,300 μL and at least 10% less volume should be used, the highest extraction volume used for this experiment was 1,250 μL.
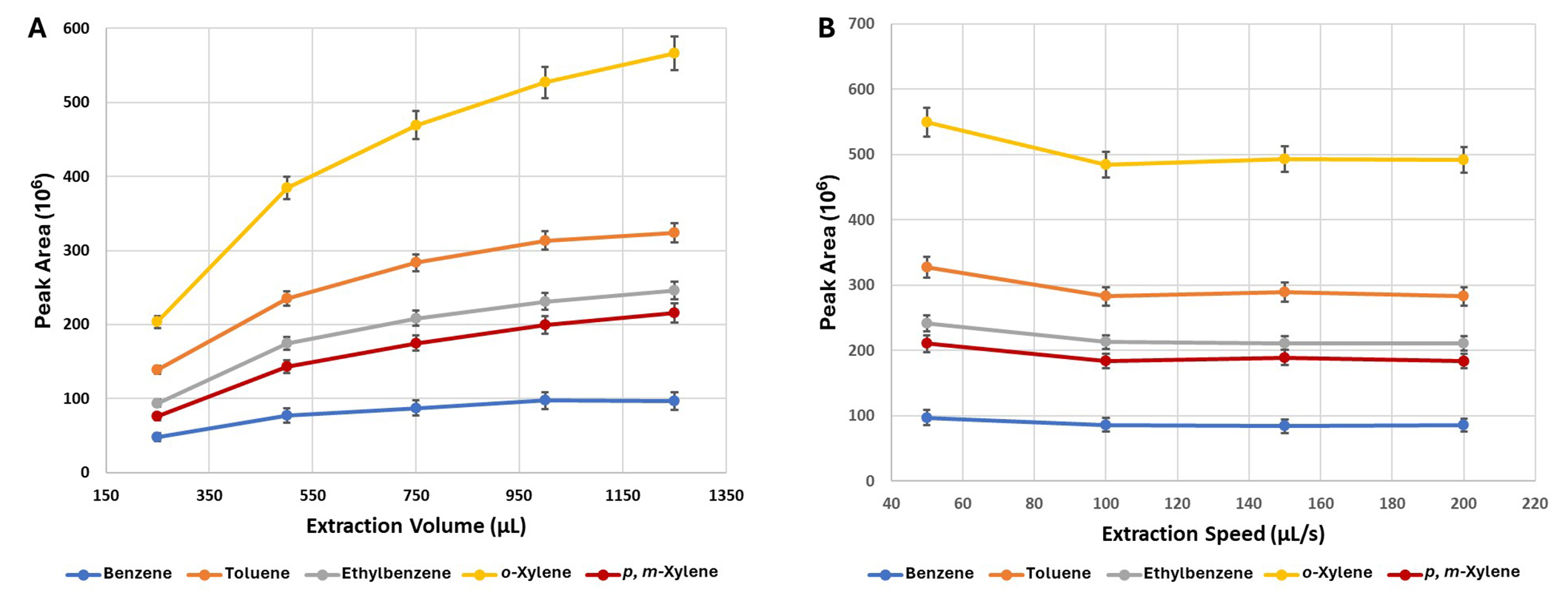
The effect of extraction volume (A) and extraction speed (B) on the extraction efficiency of BTEX compounds. Each point represents a mean value (± st. error). BTEX: benzene, toluene, ethylbenzene, and xylenes; st.: standard
Extraction speed consists of aspirate and dispense speed. The first is related to the speed of raising the syringe plunger, and the second with the speed of lowering the syringe plunger during extraction cycles. The extraction speed was varied between 50 μL/s and 200 μL/s and no significant (P > 0.05) differences were observed for the different extraction speeds (Figure 3B).
The effect of trap temperature on extraction efficiency was studied in increments of 15°C from 30°C and 120°C and the results are presented in Figure 4. All the compounds showed optimal extraction yield at 30°C up to 60°C. Also, higher temperatures decreased the extraction yield as shown in Figure 4. To prevent water condensation in the syringe during sampling, the syringe temperature was set slightly above the trap temperature (50°C). Although the deviation in peak areas from 30°C to 60°C was not statistically significant (P > 0.05), the trap temperature of 45°C was chosen because the extraction yield of p, m-xylene decreased by rising temperature to 60°C.
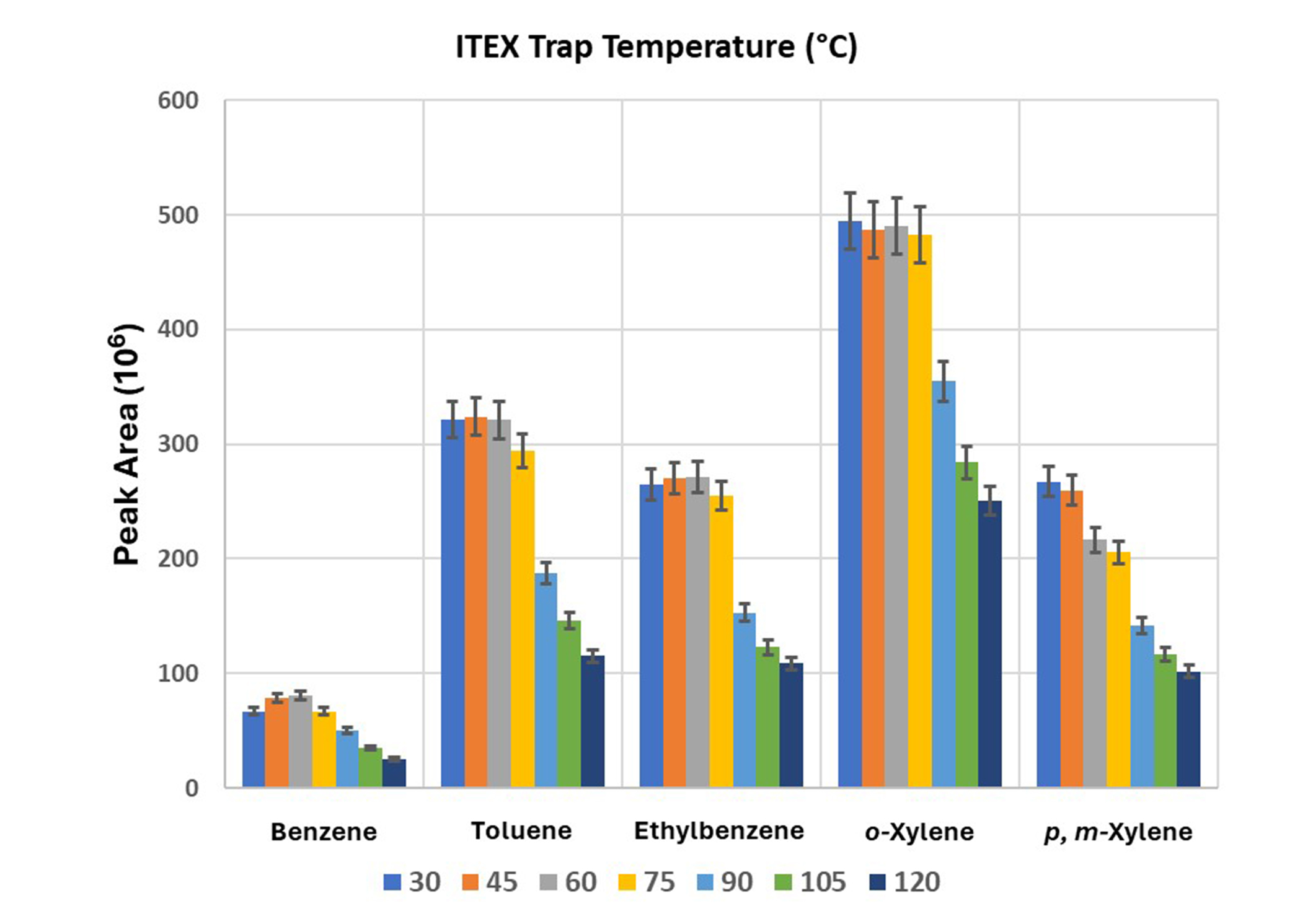
The effect of ITEX trap temperature on the extraction efficiency of BTEX compounds. Each bar represents the mean value (± st. error). ITEX: in-tube extraction; BTEX: benzene, toluene, ethylbenzene, and xylenes; st.: standard
Sensitivity can be affected by desorption temperature. As shown in Figure 5A the desorption temperature showed a strong influence on the extraction yield. Different desorption temperatures were investigated from 150°C up to 300°C. A significant influence (P < 0.05) on the extraction yield was observed at 200°C.
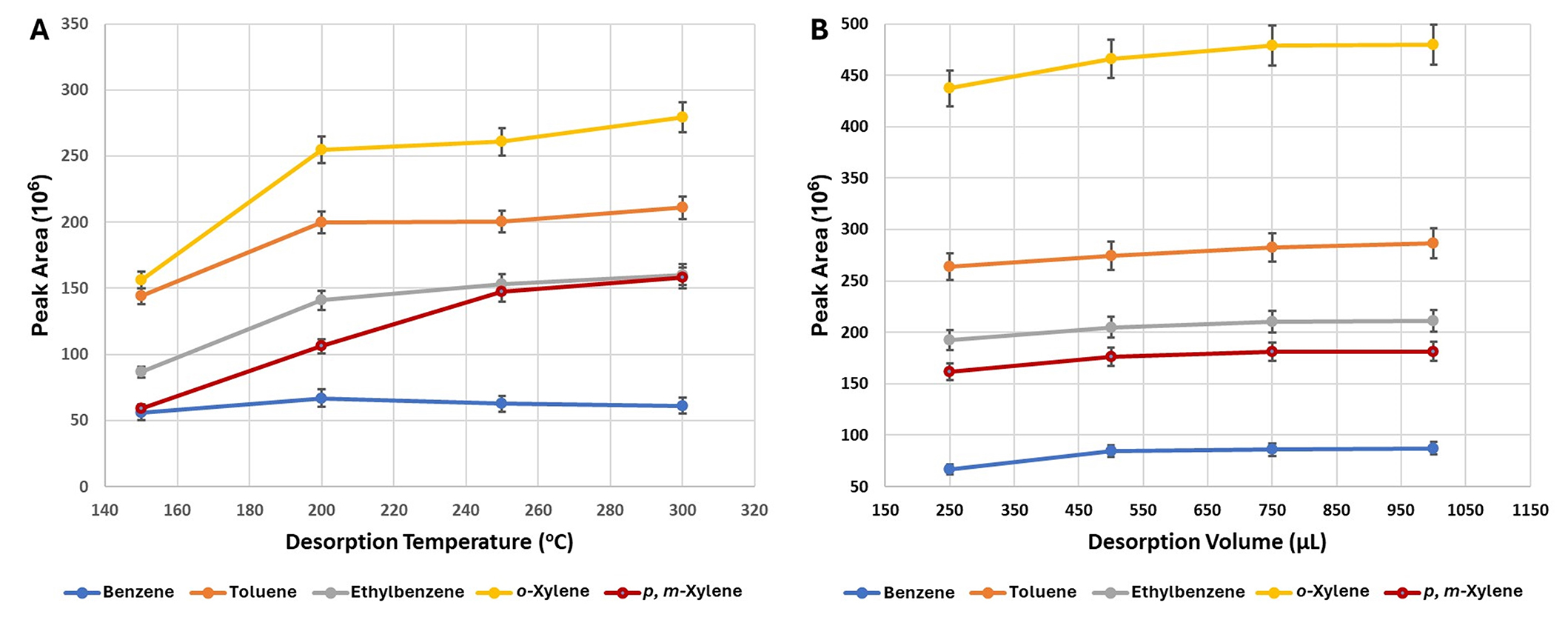
The effect of desorption temperature (A) and desorption volume (B) on the extraction efficiency of BTEX compounds. Each point represents the mean value (± st. error). BTEX: benzene, toluene, ethylbenzene, and xylenes; st.: standard
Desorption volumes of 250, 500, 750, and 1,000 μL were also tested. No significant influence (P > 0.05) on the desorption efficiency was observed (Figure 5B). A slightly increase in peak areas from 250 μL to 500 μL was observed for all compounds. This observation is in agreement with similar results reported in the literature [14, 15]. Therefore, a desorption volume of 750 μL was used.
The addition of salt to the aqueous sample on the headspace BTEX determination was studied by saturating a sample with NaCl, to improve the extraction of analytes because the increase in ionic strength influences the partition coefficient between the gas and the liquid phase [16, 17]. In this study, the influence of NaCl was investigated by adding various amounts of NaCl in a series of concentrations (0%, 5%, 10%, 20%, 30%, w/v) to spiked water samples. The results showed that the peak area increased and furthermore, better stability was observed during the desorption and extraction process when 10% (w/v) NaCl was added to the aqueous sample.
The effects of agitator temperature, incubation time, the number of extraction cycles, extraction volume, extraction speed, trap temperature, desorption temperature, and desorption volume on the profile of the BTEX extraction were examined by principal component analysis (PCA) using the peak area data for each detected compound. The results of the PCA analysis (Figures S1–S5) indicated that each compound exhibited a distinct correlation pattern among the variables. In the case of benzene, PCA analysis shows that extraction volume was negatively related to syringe temperature, while extraction speed was also negatively related to strokes. A positive relationship was observed between extraction volume and agitator temperature and between trap temperature and incubation time. For toluene, agitator temperature was strongly associated with incubation time but negatively correlated to syringe and trap temperature. Extraction speed was negatively related to sample volume. In the case of ethylbenzene, the incubation time was positively correlated to extraction speed and negatively related to syringe and trap temp, while extraction volume was also correlated to desorption temperature. A strong relationship was observed among extraction volume, desorption temperature, and sample volume for p, m-xylene. Besides, trap temperature was positively correlated with syringe temperature. Finally, in the case of o-xylene trap temperature was negatively related to desorption temperature and extraction volume.
The method was evaluated for its linearity, detection, and quantitation limits, intermediate precision, accuracy, and repeatability. Comprehensive statistical analyses and performance assessments were carried out for the method.
Six different aqueous calibration standard solutions of BTEX prepared in mineral water at 50, 100, 250, 500, 1,000, and 2,000 ng/L with a constant concentration of IS (500 ng/L) were analyzed using the optimal ITEX conditions and chromatography separation procedures. The values of correlation coefficient (r2), and calibration curve equation (y = ax + b) from BTEX are illustrated in Table 2. The calibration curves were found to be linear over an analytical range of 50–2,000 ng/L for benzene, toluene, and o-xylene while for ethylbenzene and p, m-xylene were 1,000 to 2,000 ng/L (Figures S6–S10). The linear regression equation was calculated by the least squares method using Minitab Statistical Software.
Validation data of ITEX-GC-MS method
| Compound | r2 | Calibration curve equation | LOD (ng/L) | LOQ (ng/L) | Recovery (%) n = 3 | RSD (%) n = 9 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 ng/L | 250 ng/L | 500 ng/L | 100 ng/L | 250 ng/L | 500 ng/L | |||||
| Benzene | 0.9999 | y = 1.59 × 105x – 3 × 106 | 11.60 | 35.16 | 99 | 97 | 99 | 3.43 | 3.01 | 2.58 |
| Toluene | 0.9998 | y = 3.32 × 105x + 6.4 × 105 | 10.05 | 30.47 | 99 | 96 | 95 | 3.65 | 3.42 | 3.09 |
| Ethylbenzene | 0.9995 | y = 7.40 × 104x – 88.09 × 105 | 28.29 | 85.72 | 101 | 93 | 97 | 3.49 | 3.16 | 2.59 |
| p-mXylene | 0.9997 | y = 1.96 × 105x – 3 × 106 | 28.75 | 87.13 | 94 | 97 | 99 | 2.58 | 2.08 | 1.85 |
| o-Xylene | 0.9998 | y = 9.33 × 104x – 2 × 106 | 15.35 | 46.51 | 97 | 97 | 99 | 2.98 | 2.62 | 2.15 |
ITEX-GC-MS: in-tube extraction-gas chromatographic-mass spectrometric; RSD: relative standard deviation; LOD: limit of detection; LOQ: limit of quantification
The sensitivity of the developed method was examined by the determination of LOD and LOQ values. The LOD and LOQ were calculated from the following equations: LOD = 3σ/S and LOQ = 10σ/S where σ is the standard deviation and S is the slope of the standard curve. The results of LOD and LOQ are given in Table 2. The LOD values for the BTEX were found to be in the range of 10 ng/L and 29 ng/L and the ranges of LOQ for BTEX were obtained from 30 to 88 ng/L.
The accuracy of the analytical method was examined using the recovery test. Drinking water samples were spiked with three different known concentrations of the analyte 100, 250, and 500 ng/L. The spiked samples were prepared in three replicates. The recovery of each analyte was calculated by using the following formula: Recovery = [(amount found – amount sample)/amount standard spiked × 100]. The recovery of each analyte is shown in Table 2. The recoveries ranged from 93 to 101%, and relative standard deviation (RSD) values were obtained below 5%. The precision was determined as RSD and was assessed as intra-day and inter-day precision. The intra-day precision was studied by analyzing nine spiked samples in three different concentration levels (100, 250, 500 ng/L) on the same day. The inter-day precision was estimated by analyzing nine times on different days at three concentration levels (100, 250, 500 ng/L) and RSD values for the intra-day precision and inter-day precision were calculated below 5%. All RSD values are considered acceptable for this parameter according to the validation guideline and therefore the precision of this method is satisfactory.
The validated method was applied for the determination of BTEX in groundwaters. One hundred water samples collected from the industrial area of Thessaloniki, Central Macedonia, were properly analyzed. No BTEX compounds were detected in 84.2% of analyzed samples. In the rest of the samples, more than one compound was detected at concentration higher than LOQ. Specifically, benzene, toluene, ethylbenzene, p, m-xylene, and o-xylene were found in 15.8%, 1.8%, 4.4%, 1.8%, and 2.6% of the examined samples, respectively, as shown in Figure 6.
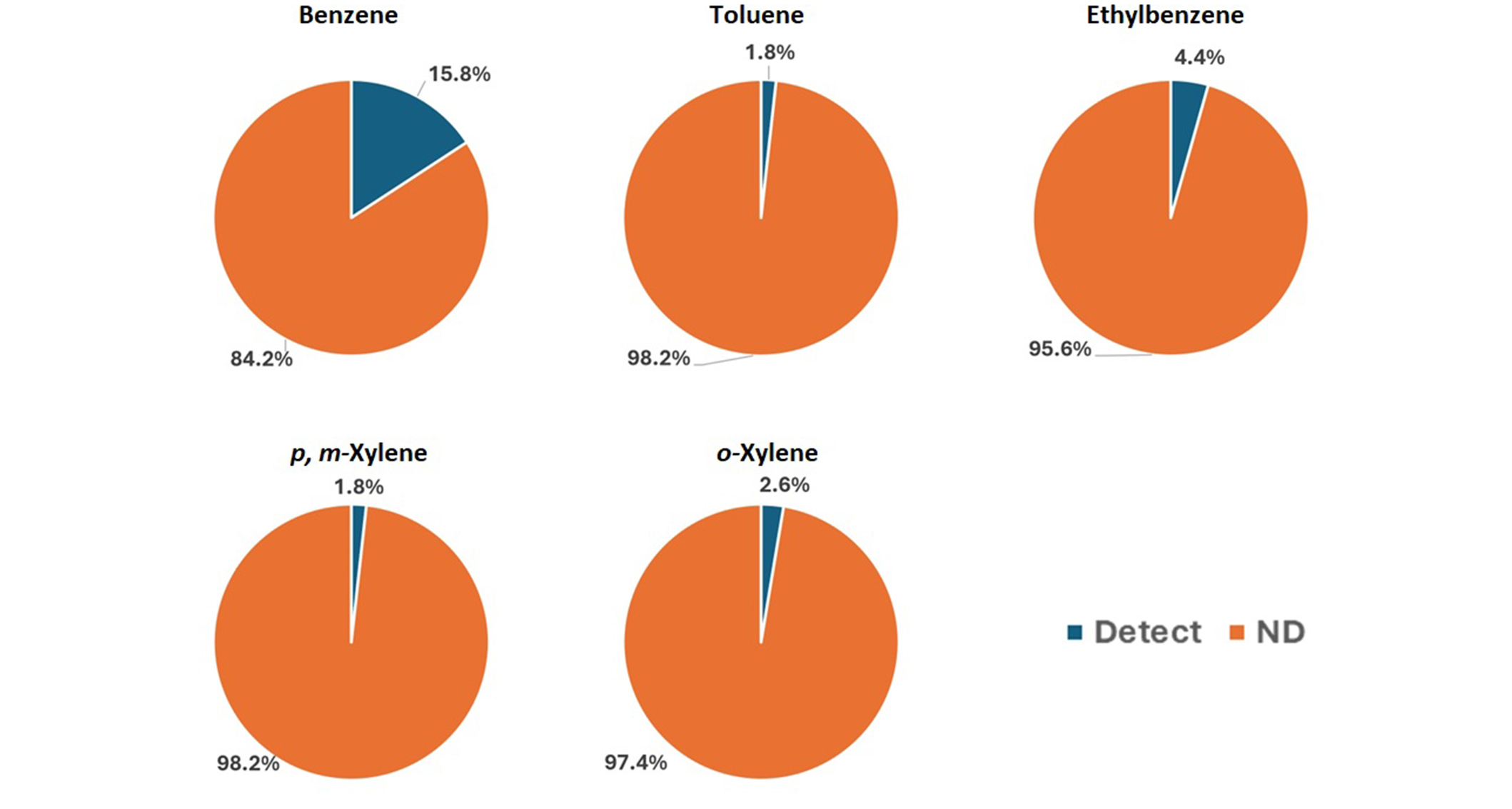
The concentrations of benzene, toluene, ethylbenzene, p, m-xylene, and o-xylene were expressed as a percentage in the samples. ND: no data
Το further confirms these results, a sample was spiked with the analytes at 500 ng/L and 1,000 ng/L concentration to assess matrix effects in triplicate. The relative recoveries in the spiked real sample were above 95% with the RSD values of less than 7.5% for all the target analytes. The chromatograms obtained by GC/MS analysis of unspiked real samples and that of spiked real samples extracted using the developed method at optimum conditions are shown in Figure 7. The findings indicated that the method was minimally influenced by the sample matrices.
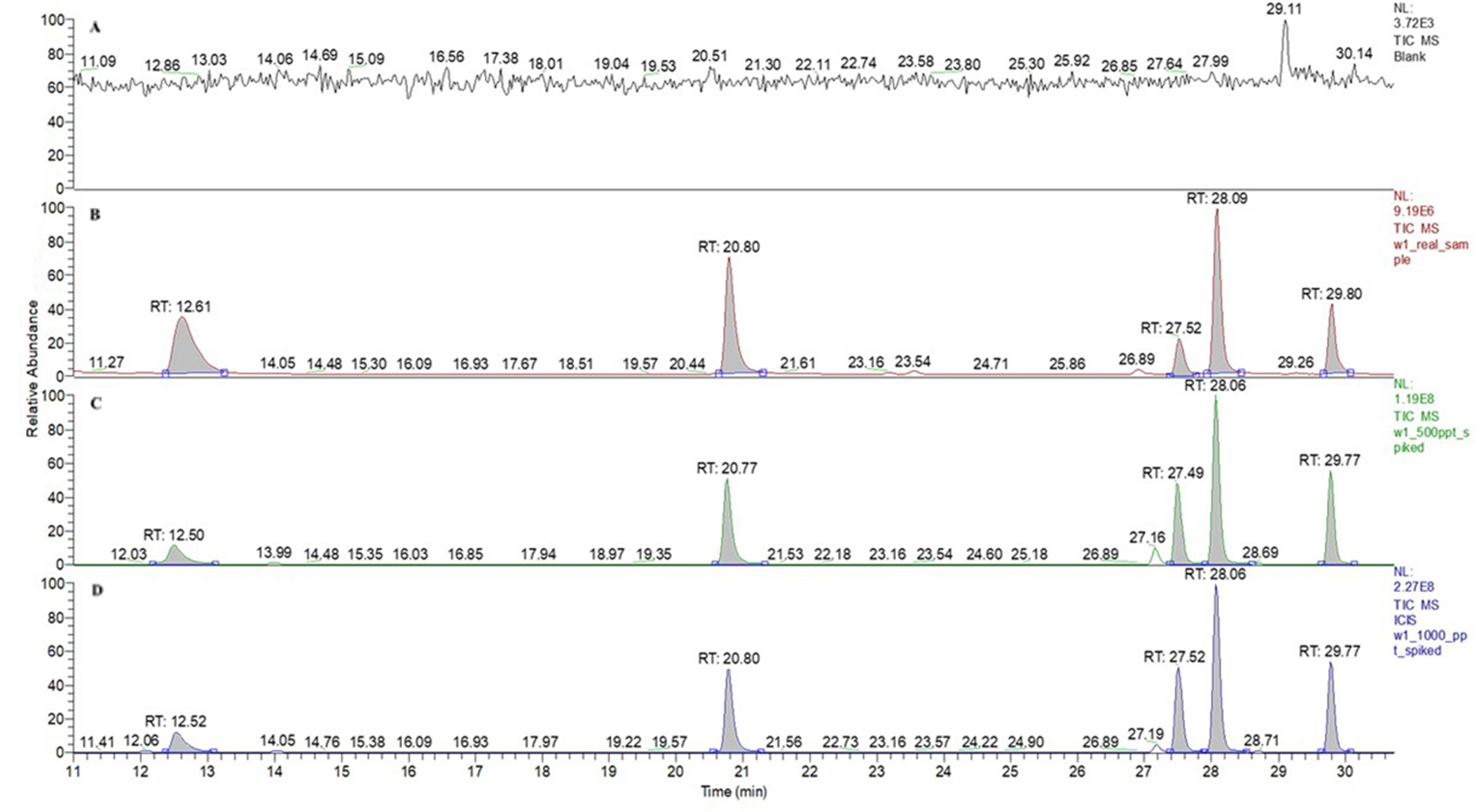
SIM chromatograms of BTEX obtained by GC/MS under optimized conditions. (A) Blank sample; (B) real groundwater sample, exhibiting benzene and ethylbenzene at concentrations 114 ng/L and 55 ng/L respectively. Toluene, p, m-xylene, and o-xylene were detected at concentrations below the LOD; (C) spiked real groundwater sample (500 ng/L); (D) spiked real groundwater sample (1,000 ng/L). RT: retention time; SIM: selected ion monitoring; BTEX: benzene, toluene, ethylbenzene, and xylenes; GC/MS: gas chromatographic/mass spectrometric; LOD: limit of detection
This study delved into the metrological intricacies of the analytical approach, focusing on optimizing parameters that influence the extraction efficiency of BTEX compounds from water samples using the ITEX-DHS sampling technique coupled with GC/MS. Independently exploring and refining extraction conditions, chromatographic separation, and mass spectrometric detection aimed to achieve impeccable analytical performance. As shown in Figure 1, the amount of extracted BTEX increased with temperature, owing to the heightened partitioning coefficient between the headspace and the sample. Notably, a plateau was observed for toluene, ethylbenzene, o-xylene, and p-xylene at 80°C, while a decrease in extraction efficiency was noted for benzene. Considering benzene’s classification as a priority pollutant in water, as per Council Directive 98/83/EC, an extraction temperature of 70°C was chosen, aligning with the established maximum contaminant level of 1 μg/L in drinking water.
The extraction cycle emerged as a critical factor influencing analyte yield in the ITEX technique. Our findings indicated that increasing the extraction cycle led to a decrease in BTEX extraction efficiency. This observation corroborates with previous research by Kupska et al. [18], emphasizing the risk of syringe leaks with a higher number of strokes during subsequent analyses. This relationship is depicted in Figure 2. Additionally, while increasing aspiration and dispense speed reduced extraction cycle time, it correspondingly decreased extraction yield, consistent with prior findings by Kupska et al. [18], attributing this phenomenon to heightened pressure within the syringe leading to potential leaks.
Testing the effect of desorption volumes on peak areas yielded minimal impact on extraction efficiency, although a slight increase in peak areas from 250 μL to 500 μL was observed for all compounds, aligning with similar results reported in the literature [14, 15].
Validation of the developed method demonstrated satisfactory linearity, recoveries, accuracy, and precision within acceptable limits. Consistent with analogous studies, a linear regression with a correlation coefficient of 0.999 or higher is typically required to assess the linearity range [19]. Moreover, comparable LOD and LOQ values for BTEX analysis in water samples were reported in relevant literature [4, 20]. Specifically, the LOD and LOQ values presented in this study align with those developed by gas chromatography analysis in various water samples [21]. However, it is important to notice, that some VOCs studied here exhibited lower LOD and LOQ values compared to previous literature reports for VOC analysis in water samples [22]. All RSD values were deemed acceptable, indicating satisfactory precision according to validation guidelines. Furthermore, consistent recovery values for BTEX reported in the literature reaffirm the method’s reliability and suitability for accurate analysis [23, 24]. Finally, employing the optimized method for determining BTEX in groundwater samples showcased its efficacy in sensitively and reliably detecting trace levels of BTEX in routine analyses of water samples. Consequently, the optimization of the method has led to a notable reduction in both detection and quantification limits for BTEX analysis in aqueous samples when compared to existing methods documented in the literature, which use similar sample preconcentration techniques. Furthermore, the sensitive analysis of BTEXs in aqueous samples is directly relevant to the food industry due to the potential for contamination and the health risks associated with these compounds. By employing advanced analytical methods, food producers can ensure the safety and quality of their products, protecting consumer health and complying with regulatory standards.
In this study, a simple and rigorously validated quantitative analytical approach employing ITEX-GC/MS was devised to accurately quantify trace amounts of BTEX in aqueous samples, ensuring compliance with rigorous regulatory thresholds. Experimental parameters governing the efficiency of ITEX extraction, including agitator temperature, number of extraction cycles, trap and desorption temperatures, incubation time, extraction volume, and speed, were thoroughly optimized.
The validation of this optimized method revealed exceptional linearity, accuracy, and precision. Calibration curves exhibited correlation coefficients exceeding 0.999 for all analytes, demonstrating robust linearity. Moreover, the method exhibited impressive sensitivity, with LOQ ranging from 30 to 88 ng/L and LOD ranging from 10 to 29 ng/L for BTEX compounds. These values underscore the method’s high sensitivity, enabling accurate detection even at extremely low concentrations. Furthermore, the method demonstrated satisfactory recovery rates for all analytes (93% to 101%), with RSD values below 5% for each analyte, indicating excellent precision. These results are in line with certified values, highlighting the method’s reliability and accuracy.
The results of this study underscore the substantial contribution achieved by optimizing the operational parameters of ITEX. This optimization has led to a notable reduction in both analysis time and limits of detection and quantification for BTEX analysis in aqueous samples when compared to existing methods documented in the literature, which utilize similar sample preconcentration techniques. Overall, this validated method provides a practical and efficient tool for environmental monitoring and risk assessment purposes related to the analysis of BTEX in water samples.
BTEX: benzene, toluene, ethylbenzene, and xylenes
GC: gas chromatographic
IS: internal standard
ITEX: in-tube extraction
LOD: limit of detection
LOQ: limit of quantification
MS: mass spectrometric
PCA: principal component analysis
RSD: relative standard deviation
VOC: volatile organic compound
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/101053_sup_1.pdf.
The authors would like to thank the Interdisciplinary Agri‐Food Center at Aristotle University of Thessaloniki (KEAGRO-AUTH), for providing access to the equipment of the unit.
EZ: Conceptualization, Project administration, Methodology, Investigation, Writing—original draft, Visualization, Writing—review & editing, Validation, Supervision. NA: Methodology, Investigation, Software, Visualization. UMS: Conceptualization, Writing—review & editing, Resources, Funding acquisition, Validation, Supervision. All authors read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available upon request from the corresponding authors (email: elenzym@agro.auth.gr, rmenkis@auth.gr), without undue reservation, to any qualified researchers.
This research was funded by the Region of Central Macedonia through the program “Restoration of subsoil and subground water in the area of the municipalities of Thessaloniki, Ambelokipon, Menemenis and Delta”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3870
Download: 282
Times Cited: 0
Stella A. Ordoudi, Maria Z. Tsimidou
Nikolaos Nenadis, Maria Z. Tsimidou
David Heath ... Nives Ogrinc
Alexandros Nakas ... Andreana N. Assimopoulou
Gabriel Mustatea, Elena L. Ungureanu
Florinda Artuso ... Fabio Pollastrone
Sandra Gueifão ... Inês Coelho
Despoina Langari, Fani Th. Mantzouridou
Arianna Latini ... Patrizia Galeffi
Aggeliki Kalogeropoulou ... Triantafyllos Albanis
Pierpaolo Di Bitonto ... Sabina Tangaro
Maria Z. Tsimidou ... Claudia Zoani
