Affiliation:
Department of Analytical Chemistry, University of Valencia, Research Building, 46100 Valencia, Spain
Email: Daniel.Gallart@uv.es
ORCID: https://orcid.org/0000-0001-5283-2114
Explor Foods Foodomics. 2024;2:651–658 DOI: https://doi.org/10.37349/eff.2024.00056
Received: July 31, 2024 Accepted: October 16, 2024 Published: November 12, 2024
Academic Editor: Zuhaib F Bhat, SKUAST-Jammu, India
The article belongs to the special issue Food Contaminants: Analysis, Occurrence and Risk Assessment
Edible oils are essential in daily diet due to their high contribution of fatty acids, fat-soluble vitamins, and triglycerides. During the processes of growing, processing, storage, transport, or packaging, they can be contaminated by ubiquitous phthalate esters, considered endocrine disruptors. Thus, analytical methodologies to allow tight control of their presence are mandatory. Several sample treatments have been considered in this study: microwave-assisted extraction (MAE), liquid-liquid extraction (LLE), dispersive solid phase extraction (DSPE), magnetic SPE (MSPE), solid phase microextraction (SPME), Surface-enhanced Raman spectroscopy (SERS) or its combinations. However, the selection of an analytical procedure is usually performed from an Analytical Chemistry perspective, without considering factors such as the sustainability of the selected methodology and/or its impact on the environment. In this sense, the Green Analytical Chemistry strategy can play an important role. To demonstrate this fact, six different analytical procedures have been evaluated in terms of sustainability by using several greenness evaluation tools. Procedures from MAE-gel permeation chromatography (GPC)-SPE till SERS, showing adequate analytical characteristics, have been selected. The application of Analytical GREEnness calculator (AGREE), AGREE preparation (AGREE prep), and blue applicability grade index (BAGI) tools showed that MAE-GPC-SPE was the less green analytical procedure while SERS was the greener one. The BAGI evaluation showed that headspace (HS) and SERS were the most applicable procedures.
The growing environmental conscience in Analytical Chemistry has promoted the evaluation of many of the methodologies traditionally used in the analysis of phthalates in complex matrices such as food samples, leading to the development of new methodologies designed from this point of view and taking into account its impact on the safety of environment and operators. In this sense, the development of analytical procedures including terms such as miniaturization or automation has improved the ability to isolate the analytes from complex samples using minimal reagents, reducing consumables, and decreasing energy consumption. Consequently, a clear evolution in sample treatment methodologies can be observed in recent decades. However, sample preparation steps remain the harder-working and time-spending part of the analytical processes [1, 2]. According to the literature, the analysis of ubiquitous molecules in complex samples, such as phthalates, requires an exhaustive sample treatment due to the complexity of the sample matrix, the low concentration of analytes, and the low limits allowed by legislation. In this sense, it is common to use techniques such as solid phase extraction (SPE), liquid-liquid extraction (LLE), and solid-liquid extractions to perform the clean-up step and the pre-concentration of targeted analytes to increase both, the selectivity and sensitivity of the determination. However, pre-concentration steps often require: i) a large sample amount, ii) high solvent volumes, and iii) long extraction times, being most of them non-automated methodologies [3]. According to this situation, from the last decades of the 20th century, different green metric tools have been developed for the evaluation of sustainability and impact on the environment of the analytical procedures employed. From the earliest Chemical Hazard Evaluation for Management Strategies and the National Environmental Methods Index (NEMI) [4, 5], till more complex and quantitative tools, different evaluation tools have been developed following different concepts and criteria of the Green Analytical Chemistry, presenting advantages and drawbacks. These new evaluation tools sometimes include free software and were developed considering sample treatment steps together with sample acquisition and measurements, such as Analytical Eco-scale [6], Complex Green Analytical Procedure Index (Complex GAPI) [7], and Analytical GREEnness calculator preparation (AGREE prep) [8], among others tools [9–15]. In this work, several analytical procedures for the determination of phthalates in edible oils have been evaluated in terms of sustainability using different evaluation criteria, AGREE, AGREE prep, and blue applicability grade index (BAGI). In this sense, it is important to pay attention to how can be the evaluation result different depending on the employed tool for methodologies that, a priori, are valid from the point of view of the analytical parameters obtained.
Green Analytical Chemistry aims to make analytical procedures more eco-friendly and safer for humans, involving a variety of criteria such as the quantities and toxicity of reagents, waste production, energy consumption, procedural steps, miniaturization, and automation. The AGREE evaluation tool, proposed by Pena-Pereira et al. [9], is a versatile and user-friendly assessment tool that provides clear and informative results using criteria based on the 12 principles of Green Analytical Chemistry [16]. In this sense, criteria applied by AGREE tools are: 1) direct analysis, 2) minimal sample size, 3) in situ analysis, 4) integrated processes, 5) automation and miniaturization, 6) avoid derivatization, 7) waste generation, 8) multi-analyte analysis, 9) energy consumption, 10) renewable sources, 11) toxicity of reagents, and 12) operator safety. The output of the AGREE tool is a coloured pictogram constituted by twelve segments coloured automatically between red (non-sustainable) and dark green (sustainable) determined by the mathematical expressions developed by the authors, showing the structure of weak and strong points of the methodology. In addition, the mathematical score assigned to each criterion is converted into a unified 0–1 score scale and colour. The final score reflects these principles and is displayed in the middle of the pictogram, indicating the overall score and the method’s performance in each criterion.
On the other side, the AGREE prep tool [8], evaluates 10 impact categories, converting them into sub-scores on a 0 to 1 scale, which are then used to compute the final assessment score. These categories include: 1) sample preparation placement, 2) hazardous materials, 3) renewability and reusability, 4) waste generation, 5) sample size, 6) sample throughput, 7) automation, 8) energy consumption, 9) configuration for analysis, and 10) operator safety. The evaluation criteria encompass the selection and usage of solvents, materials and reagents, waste generation, energy consumption, sample size, and throughput. The tool also allows for differentiating the importance of each criterion by assigning weights. The assessment process is conducted using intuitive, open-access software that produces a straightforward pictogram, detailing the overall performance and identifying potential risks. As the AGREE evaluation tool, the scores and colours assigned to each segment by the AGREE prep tool are mathematically assigned depending on the option selected from the pull-down list of the software. The main difference between AGREE and AGREE prep lies in the sample preparation step. AGREE tool takes an overview of the whole analytical procedure used, while AGREE prep gives greater weight and importance to the sample preparation stages.
Finally, the BAGI assesses the practicality of analytical methods [14]. BAGI evaluates ten key attributes: 1) the type of analysis, 2) the number of analytes determined simultaneously, 3) the sample throughput per hour, 4) the reagents and materials used, 5) the necessary instrumentation, 6) the capacity for simultaneous sample processing, 7) the need for pre-concentration, 8) the level of automation, 9) the sample preparation method, and 10) the sample volume. As in previous cases, the evaluation is performed by a free and easy to use software that mathematically assigns coloured segments to the pictogram as a function of the options selected in the software pull-down list. The assessment of these attributes, generates an asteroid pictogram with segments coloured from white (non-applicability) to dark blue (applicability) depending on the analytical procedure evaluated, along with a corresponding final score, mathematically calculated. This tool makes it easy to identify the strengths and weaknesses of a method in terms of practicality and applicability and facilitates the comparison of the performance of different analytical methods, being the threshold value for the acceptance of the practicality fixed in 60.
The metric systems employed to evaluate the greenness of the selected analytical procedures has been selected due to constitute comprehensive tools attending the Green Chemistry principles, offering flexibility through the ability to assign weights to different criteria. It is user-friendly and provides easily interpretable-coloured pictograms that highlight both strengths and weaknesses. The employed software is available for free download and allows for quick and straightforward analysis, typically completed in just a few minutes in contrast with other evaluation tools that are time-consuming and the scores are selected by the user and non-automatically. On the other side, the selected tools can be applied to the main part of the analytical methodologies, including the completely analytical procedure (AGREE), the sustainability-focused on the sample treatment stages (AGREE prep), and the applicability of the methodology (BAGI).
In this study, six analytical procedures employed for the determination of phthalates in edible oils have been compared from the Green Analytical Chemistry point of view: the reference ISO methodology based on the use of LLE and dispersive SPE (DSPE); a methodology based on the use of QuEChERS, two procedures based on the use of magnetic SPE (MSPE) or solid phase microextraction (SPME). On the other hand, a procedure based on the use of microwave-assisted extraction (MAE) followed by gel permeation chromatography (GPC), and a procedure based on the use of Surface-enhanced Raman spectroscopy (SERS) were compared.
Table 1 indicates experimental details of the sample treatment, analytical techniques for the detection, and analytical parameters obtained for the determination of phthalates in edible oils by employing the selected analytical procedures. For this purpose, an intensive bibliographic search has been performed to ensure that the selected analytical procedures provide similar analytical characteristics. As it can be seen, regardless of the procedure applied, the analytical parameters provided by these methodologies are quite similar, showing comparable limit of detection (LOD) and limit of quantification (LOQ) and accuracy values in the same order of magnitude. Considering this situation, the selection of these analytical methodologies can be quite complicated. However, the use of greenness evaluation tools can allow the discrimination between them by taking into account their sustainability. Figure 1 shows the green evaluation of the selected methodologies by employing the AGREE, AGREE prep, and BAGI evaluation tools.
Experimental details, detection, and analytical parameters of procedures included in this study
| Method | Extraction | Determination | LOD* | LOQ* | Accuracy (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 0.5 g sample, 5 mL methanol MAE extraction.Extract evaporated in a rotary evaporator.Reconstituted in 10 mL ethyl acetate: n-hexane (1:1, v:v).GPC clean-up: ethyl acetate: n-hexane (1:1, v:v).SPE clean-up: eluted with 5 mL methanol: dichloromethane (1:1, v:v).Dried and reconstituted in 2 mL n-hexane. | HRGC-MS/MS | 0.2–1.4 | 0.7–4.5 | 94–105 | [17] |
| 2 | 1 g sample, 5 mL acetonitrile, LLE in vortex. The procedure was repeated 3 times. Supernatant vaporized and reconstituted in 1 mL acetonitrile.Synthesis of magnetic nanoparticles: 1.35 g FeCl3·6H2O, 3.85 g NH4OAc, 0.48 g sodium, 70 mL ethylene glycol. Stirring for 1 h. Heated at 200°C for 16 h. Cooling to room temperature, rinsed with deionized water, and dried at 60°C.MSPE procedure: 10 mL of sample solution extracted with magnetic nanoparticles in vortex 20 min. Eluted with 1 mL acetonitrile. | LC-DAD | 0.5–0.9 | 1.8–3.1 | 80–103 | [18] |
| 3 | Nanocomposite preparation: 100 mg graphene, 15 mg PVC, 10 mL THF.10 mL sample was exposed to the nanocomposite fiber, rinsed, and desorbed in the HS system. | GC-FID | 0.1–0.2 | 0.3–0.6 | 87–112 | [19] |
| 4 | 1 mL sample, 6 mL acetonitrile, LLE performed in the vortex.DSPE extraction was performed with the supernatant 400 mg C18, and 400 mg PSA.Evaporation at 40°C until 0.5 mL volume. | GC-MS | - | - | - | [20] |
| 5 | LLE: 2 g sample, 10 mL n-hexane saturated in acetonitrile. Vortex 2 min, ultrasound bath 50°C for 20 min.Freezer –20°C for 8 h, centrifugation, obtention of supernatant.DSPE clean up: supernatant, 250 mg C18-Z-Sep+, vortex for 2 min. | GC-MS | 0.5 | 2 | 77–111 | [21] |
| 6 | Synthesis of AuNs particles: 10 μL of chloroauric acid solution, 500 μL of AuNPs solution, 220 μL of 1% sodium citrate solution, 4 mL of 30 mM hydroquinone solution, 100 mL of deionized water. Stirred for 30 min at room temperature. Centrifuged at 8,000 rpm for 20 min. The supernatant was discarded, and the remaining solution was stored at 4°C until use.500 µL of sample and 500 µL AuNSs were added to 5 mL beaker, mixed, sonicated, and shaken for 10 min; 10 µL solution was dropped onto the silicon wafer and dried at room temperature for SERS measurements. | SERS | 0.7 | 2 | - | [22] |
HRGC-MS: high-resolution gas chromatography mass detection; DSPE: dispersive solid phase extraction; GC-FID: GC flame ionization detection; GPC: gel permeation chromatography; HS: headspace; LC-DAD: liquid chromatography diode-array detector; LLE: liquid-liquid extraction; MAE: microwave-assisted extraction; MSPE: magnetic SPE; SERS: Surface enhanced Raman spectroscopy; PVC: polyvinyl chloride; THF: tetrahydrofuran; PSA: primary secondary amine; LOD: Limit of detection, LOQ: limit of quantification; *: expressed as µg Kg-1
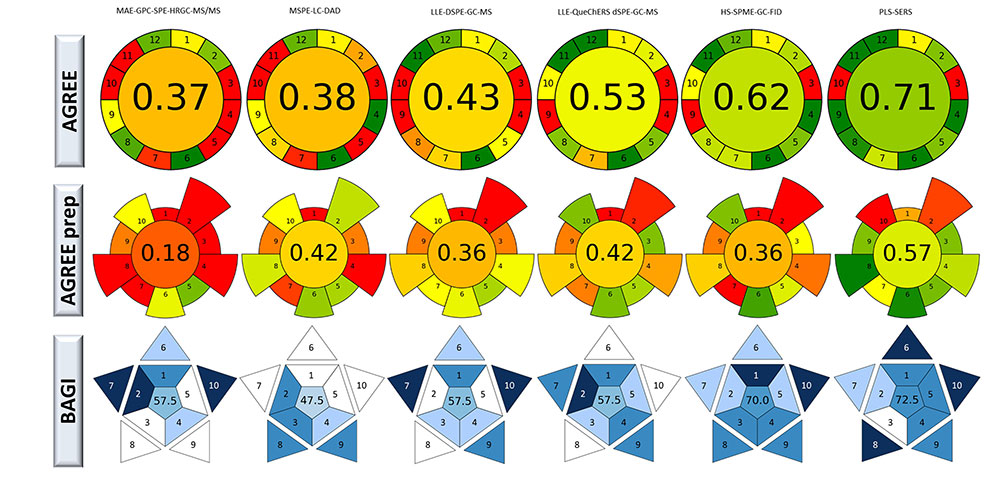
Green evaluation of the selected analytical procedures for the determination of phthalates in edible oils employing the Analytical GREEnness calculator (AGREE), AGREE preparation (AGREE prep), and blue applicability grade index (BAGI) evaluation tools. MAE-GPC-SPE-HRGC-MS: microwave-assisted extraction gel permeation chromatography solid phase extraction high-resolution gas chromatography mass detection; MSPE-LC-DAD: magnetic SPE liquid chromatography diode array detector; LLE-DSPE-GC-MS: liquid-liquid extraction dispersive SEP GC-MS; HS-SPME-GC-FID: headspace solid phase microextraction GC flame ionization detection; PLS-SERS: partial least squares-Surface-enhanced Raman spectroscopy
As can be seen, attending the AGREE evaluation, all the methodologies are penalized by the position of the analytical device, while the reagents toxicity penalizes the first three techniques compared (Table S1). On the other hand, the number of steps involved in the analytical procedure decreases the score of the MAE-GPC high-resolution gas chromatography mass detection (MAE-GPC-HRGC-MS)/MS, LLE-DSPE-GC-MS, and QuEChERS-based methodologies. On the other side, the use of direct SERS analysis together with chemometrics [partial least squares (PLS)] provided the highest green score, followed by the use of headspace-SPME (HS-SPME). In this sense, those methodologies that involve the use of high solvent volumes or the use of several non-miniaturized sample treatment stages provided the lowest green evaluation scores.
On the other side, the evaluation using the AGREE prep tool shows that all the methodologies studied are penalized due to the sample preparation placement, while the use of hazardous materials is a weak point of all except the MSPE liquid chromatography diode array detector (MSPE-LC-DAD) procedure, see Table S1. On the other side, the waste generation penalizes the MSPE-LC-DAD procedure while the automation and the energy consumption penalizes the MAE-GPC-SPE-HRGC-MS/MS procedure. The use of spectroscopy direct analysis (SERS) provided the highest sustainability score due to the low preparative requirement of the samples. The lowest score was achieved by the methodology characterized by the use of MAE followed by GPC and SPE, due to the high volume of solvents employed in those steps. Regarding, the remaining analytical procedures studied, the scores provided values spanning a range between 0.36 and 0.42 due to miniaturized sample treatment steps.
Finally, the BAGI practicality evaluation showed that the studied methodologies provided values lower than the threshold established, with the exception of the use of HS-symposium on materials processing and microstructure evolution GC flame ionization detection (HS-SMPME-GC-FID) and PLS-SERS, see Table S2. The highest value was achieved by the use of SERS and chemometrics, due to the type of analysis, the number of analytes determined simultaneously, the sample throughput per hour, the reagents and materials used, the necessary instrumentation, and the level of automation.
The use of evaluation tools seems to be required to minimize environmental impact and promote sustainability of the selected procedure, taking into account the reduction of the number of steps, analysis time, solvents, waste generation, and multianalyte determinations, following the Green Analytical Chemistry principles. The use of this type of evaluation can be useful to select and discriminate between the different analytical procedures applied to the determination of phthalates in edible oils according to sustainability criteria. In this sense, the proposed green metrics are characterized by different concepts and criteria, and provide information to perform a comparison. Attending to the obtained results it can be seen that by using green evaluation tools, the analytical procedures can be discriminated without sacrificing the analytical characteristics such as LOD and LOQ or accuracy. In this sense, from the analytical procedures studied for the determination of these analyte types in edible oils, the use of SERS followed by chemometric treatment of data provided the highest green scores, due to it being a practically direct analysis, only penalized by the synthesis of the required particles. On the other side, the use of miniaturized sample treatment steps, such as SPME or the DSPE increases the sustainability of the sample treatment steps, while the classical sample preparation steps are the most penalized methodologies.
In the future, green metrics should be considered for the analytical procedure selection and development, being these metrics included on a regular basis in industries and research laboratories. However, it should be enhanced with economic, time, and cost evaluations to obtain a holistic vision of the procedures.
AGREE perp: Analytical GREEnness calculator preparation
AGREE: Analytical GREEnness calculator
BAGI: blue applicability grade index
DSPE: dispersive solid phase extraction
GC: gas chromatography
GPC: gel permeation chromatography
HS: headspace
LLE: liquid-liquid extraction
LOD: limit of detection
LOQ: limit of quantification
MAE: microwave-assisted extraction
MSPE: magnetic solid phase extraction
MSPE-LC-DAD: magnetic solid phase extraction liquid chromatography diode array detector
PLS: partial least squares
SERS: Surface-enhanced Raman spectroscopy
SPE: solid phase extraction
SPME: solid phase microextraction
The supplementary tables for this article are available at: https://www.explorationpub.com/uploads/Article/file/101056_sup_1.pdf.
DGM: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing.
The author declares there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Financial support of these studies from Gdańsk University of Technology by the DEC-5/2021/IDUB/II.1.3 grant under the Aurum Supporting International Research Team Building—“Excellence Initiative-Research University” program is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Olga Pardo, Francesc A. Esteve-Turrillas
Clara Ochoa-Esteso ... María Jesús Lerma-García
Gabriel Mustatea ... Elena L. Ungureanu
