8 results in Exploration of Immunology
Latest
Sort by :
- Latest
- Most Viewed
- Most Downloaded
- Most Cited
Open Access
Perspective
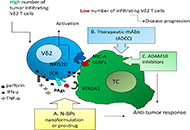
Anti-cancer γδ T lymphocytes: contradictory past and promising future
Alessandro Poggi, Maria Raffaella Zocchi
Published: April 28, 2022 Explor Immunol. 2022;2:220–228
This article belongs to the special issue Interplay of γδ T cells and Tumor Cells
Open Access
Review
A proposed new paradigm for an anti-AIDS tolerogenic vaccine
Christine Jacomet
Published: April 24, 2022 Explor Immunol. 2022;2:211–219
This article belongs to the special issue Old and New Paradigms in Viral Vaccinology
Open Access
Original Article
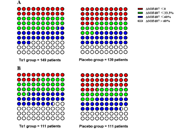
Thymosin alpha 1 therapy alleviates organ dysfunction of sepsis patients: a retrospective cohort study
Fei Pei ... on behalf of the China Critical Care Immunotherapy Research Group
Published: April 22, 2022 Explor Immunol. 2022;2:200–210
This article belongs to the special issue The Sepsis induced Immune Conundrum
Open Access
Original Article
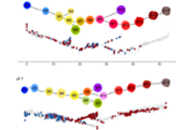
Single-cell differentiation trajectories define early stages of a human cutaneous T-cell lymphoma
Juan-Pablo Cerapio ... Jean-Jacques Fournie
Published: April 15, 2022 Explor Immunol. 2022;2:185–199
This article belongs to the special issue Interplay of γδ T cells and Tumor Cells
Open Access
Review
Paradigms in HIV vaccine research
Marc H.V. Van Regenmortel
Published: March 17, 2022 Explor Immunol. 2022;2:180–184
This article belongs to the special issue Old and New Paradigms in Viral Vaccinology
Open Access
Review
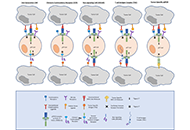
Augmenting human gamma delta lymphocytes for cancer therapy with chimeric antigen receptors
Gabrielle M. Ferry, John Anderson
Published: March 17, 2022 Explor Immunol. 2022;2:168–179
This article belongs to the special issue Interplay of γδ T cells and Tumor Cells
Open Access
Review
The role of γδ T cells in the context of allogeneic stem cell transplantation
Rupert Handgretinger ... Manon Queudeville
Published: March 16, 2022 Explor Immunol. 2022;2:157–167
This article belongs to the special issue Interplay of γδ T cells and Tumor Cells

Open Access
Review
High avidity of vaccine-induced immunoglobulin G against SARS-CoV-2: potential relevance for protective humoral immunity
Georg Bauer
Published: March 16, 2022 Explor Immunol. 2022;2:133–156
This article belongs to the special issue Vaccine-induced Immune Responses Against SARS-CoV-2 Infections
Journal Information
 Previous
Previous