Affiliation:
1Department of Hematology Laboratory, Iwate Prefectural Chubu Hospital, Kitakami 024-8507, Japan
2Department of Clinical Laboratory, Iwate Prefectural Chubu Hospital, Kitakami 024-8507, Japan
Email: iekomNo2020@gmail.com
Affiliation:
3Department of Internal Medicine, School of Dentistry, Health Sciences University of Hokkaido, Ishikari 061-0293, Japan
Affiliation:
4Departmrnt of Clinical Laboratory, Health Sciences University of Hokkaido Hospital, Sapporo 001-8071, Japan
Affiliation:
4Departmrnt of Clinical Laboratory, Health Sciences University of Hokkaido Hospital, Sapporo 001-8071, Japan
Affiliation:
2Department of Clinical Laboratory, Iwate Prefectural Chubu Hospital, Kitakami 024-8507, Japan
Affiliation:
1Department of Hematology Laboratory, Iwate Prefectural Chubu Hospital, Kitakami 024-8507, Japan
Affiliation:
1Department of Hematology Laboratory, Iwate Prefectural Chubu Hospital, Kitakami 024-8507, Japan
Affiliation:
3Department of Internal Medicine, School of Dentistry, Health Sciences University of Hokkaido, Ishikari 061-0293, Japan
Affiliation:
5Department of Molecular Patho-Biochemistry, Yamagata University School of Medicine, Yamagata 990-9585, Japan
Explor Immunol. 2023;3:286–299 DOI: https://doi.org/10.37349/ei.2023.00103
Received: March 08, 2023 Accepted: June 12, 2023 Published: August 30, 2023
Academic Editor: Jean Amiral, Hyphen BioMed, France
The article belongs to the special issue Autoantibodies Associated to Thrombosis and Hemostasis
In patients with autoimmune coagulation factor deficiency (AiCFD), the production of autoantibodies that inhibit coagulation factors in the blood reduces the activity of those relevant coagulation factors, resulting in severe bleeding symptoms. Recently, reports of patients with AiCFD have noted the concomitant detection of lupus anticoagulant (LA), a risk factor for thrombosis. LA-positive patients may show bleeding symptoms due to decreased activity of coagulation factor II (FII) caused by autoantibodies against FII, in addition to thrombotic symptoms, a condition termed LA-hypoprothrombinemia syndrome (LAHPS). Anti-FII antibodies in LAHPS cases are frequently cleared antibodies that can be detected using immunological techniques, such as enzyme-linked immunosorbent assay (ELISA). Recently, several cases of coagulation FV inhibitors, known as autoimmune FV deficiency, have been reported. Some of these cases may be complicated by LA, which can cause thrombosis. False-positive results for anticoagulant inhibitors are known to occur in LA cases; therefore, immunological confirmation of antibodies against coagulation factors is recommended. Additionally, acquired hemophilia A (AHA), caused by autoantibodies against FVIII, is a typical acquired hemorrhagic diathesis, although affected patients may present with thrombosis associated with LA. Thus, it is important to remember that hemorrhagic diathesis due to autoantibodies against clotting factors can also result in thrombosis, as demonstrated by the co-detection of LA. When clotting factor inhibitors are detected in LA-positive individuals, it is important to confirm the presence of autoantibodies against coagulation factors using immunological methods, such as ELISA, to avoid false-positive results.
Autoimmune coagulation factor deficiency (AiCFD) is an acquired hemorrhagic diathesis that causes severe bleeding symptoms by decreasing the activity of coagulation factors, which is due to the production of autoantibodies (inhibitors) against such coagulation factors in the blood [1]. A representative disease observed in such cases is acquired hemophilia A (AHA), caused by autoantibodies against coagulation factor VIII (FVIII) [2]. Recently, several cases with autoantibodies against coagulation FV, known as autoimmune FV deficiency (AiFVD), have been reported [3]. However, it has recently been demonstrated that AiCFD is related to lupus anticoagulant (LA) detected in association with autoimmune diseases with the same pathology [4–6]. Cases of LA-hypoprothrombinemia syndrome (LAHPS), where autoantibodies against prothrombin (FII) are detected, are well-known AiCFD cases associated with LA [4]. LA is a significant thrombotic risk factor, and findings indicating AiCFD, a typical acquired hemorrhagic diathesis associated with LA, frequently cause confusion in clinical practice. This study discusses cases of AiCFD, particularly autoantibodies to prothrombin, FV, and FVIII, along with factors regarding the potential for complications related to LA and its influence on clinical symptoms and laboratory findings.
LA is defined as a condition that inhibits phospholipid-dependent coagulation reactions without inhibiting the activity of individual coagulation factors. It is a diagnostic antiphospholipid antibody (aPL) available for detecting antiphospholipid syndrome (APS) and is known to be an independent risk factor for thrombosis and recurrent pregnancy loss [7]. APS is diagnosed when aPL is persistently detected (detected more than twice, 12 weeks apart) and presents with clinical manifestations, such as arteriovenous thrombosis and pregnancy complications [7]. LA is primarily detected using dilute Russell’s viper venom time (dRVVT) and activated partial thromboplastin time (APTT) examinations [8]. Furthermore, it has been shown that LA may be overlooked if both of those are not measured [9]. When prolonged clotting time is observed in APTT and dRVVT, a confirmation test using an excess phospholipid addition (neutralization test) and a cross-mixing test is performed [8]. Therefore, it is recommended that the screening test be followed by a mixing and confirmation test in all samples showing a prolonged clotting time in the screening test. For example, suppose the screening and confirmatory steps are positive with a negative mixing step and the presence of anticoagulants excluded. In that case, measuring coagulation factor levels can help identify the reason for prolonged screening times [8, 10, 11]. It has been recently reported that omitting the mixing test step may yield false positive and negative results [12–14]. Particularly, the APTT mixing test is recommended to exclude the effects of anticoagulants such as warfarin and identify LA [15, 16].
As the LA sensitivity of commercial APTT reagents also depends on the combination of activator and phospholipid composition, laboratories should evaluate the sensitivity of their APTT reagents using well-characterized LA-positive samples [10]. Notably, LA measurements were highly sensitive. Therefore, referring to the guidelines published in 2020 for LA measurement is recommended.
Although the antibodies responsible for LA have not been fully elucidated, anti-prothrombin [anti-phosphatidylserine/prothrombin antibodies (aPS/PT), and others] and anti-β2-glycoprotein I antibodies (aβ2GPI) are thought to be involved. aPS/PT is frequently detected in patients with APS and thrombosis, particularly in those who are LA-positive [17]. Additionally, aPS/PT detection rates have been shown to correlate with LA positivity rates and the incidence of thrombosis [18]. Thus, some reports exist that aPS/PT is the antibody responsible for LA; however, there are currently various theories regarding this issue, and it is within the realm of possibility.
LA has also been reported to inhibit negatively charged phospholipids involved in coagulation reactions [19]. By inhibiting intrinsic coagulation reactions that involve phospholipids, LA may cause an apparent decrease in intrinsic coagulation factor activities and false-positive results for inhibitors of intrinsic coagulation factors in LA-positive individuals [20], especially when using APTT reagents with high sensitivity for LA. It is also well known that the administration of direct oral anticoagulants (DOACs), such as rivaroxaban, can affect LA measurements and lead to false-positive LA findings [21–23]. Since the introduction of DOACs, several studies have demonstrated their effects on LA testing [10, 24]. However, the effects of autoantibodies against coagulation factors on LA measurement systems have not been clearly determined. Autoantibodies that function as inhibitors of coagulation factors bind to them in a time-dependent manner and reduce their activities, although they do not directly inhibit phospholipids that contribute to coagulation reactions. The LA test generally confirms the differences or ratios in clotting times with and without the addition of phospholipids. Antibodies against coagulation factors do not appear to have a strong effect on these differences or ratios, and the presence of these autoantibodies is unlikely to directly affect the LA measurement system [5]. Nevertheless, LA measurements in patients with autoantibodies against coagulation factors should be performed cautiously and with multiple related reagents [25] and the mixing test [10]. Because LA-containing aPL is frequently transiently detected, retesting for aPL after 12 weeks is recommended.
Although extremely rare, AiFIID is a pathological condition where autoantibodies against FII (prothrombin) are detected, along with bleeding symptoms known to occur with decreased FII activity in affected patients. Since the report by Scully et al. [26] in 1982 on a case of autoantibodies that showed binding to both thrombin and FII, there have been very few other related studies [27]. Recently, most of these cases have been reported to be associated with APS or LA, with many of them diagnosed as LAHPS [28].
LAHPS is defined as an occasional bleeding disorder due to decreased FII activity caused by autoantibodies against FII in LA-positive individuals [4]. However, bleeding symptoms are not necessarily essential for diagnosing LAHPS because sometimes these symptoms are not observed [4]. In the previous study, only 20% of the 20 patients examined presented with bleeding symptoms during diagnosis [4].
In a report by Mazodier et al. [28], which summarized the findings obtained in 74 patients with LAHPS, the average age was 22.7 years (2–76 years), with 42% of them aged 15 years or older, and a male (M)-to-female (F) ratio of 42:58. Although LAHPS is considered to be a group of diseases that commonly occurs in children, it is also found relatively frequently in elderly individuals. The underlying diseases are diverse, with autoimmune diseases being the most common, followed by infectious diseases.
LAHPS is screened using prothrombin time prolongation (APTT prolongation is also common in affected individuals) and is diagnosed when LA is positive, and decreased FII activity is acquired. A procedure for detecting autoantibodies against FII using an immunological technique, including enzyme-linked immunosorbent assay (ELISA), was subsequently used to confirm the diagnosis of LAHPS [4]. The following two types of antibodies affect coagulation factor activity: neutralizing antibodies that inhibit the activities of coagulation factors and clearance antibodies that are removed from the blood by forming complexes with coagulation factors [29]. In addition to neutralizing and clearing antibodies, those detected using ELISA (nonfunctional antibodies) do not directly affect coagulation factor activity; however, the detection of these antibodies suggests the possibility of AiCFD onset and relapse (Figure 1). Decreased FII activity in LAHPS is believed to be primarily caused by the clearance of antibodies against FII [30]. However, anti-FII antibodies in LAHPS are frequently undetected using the Bethesda method, which is used to detect neutralizing antibodies [4]. When bleeding symptoms can be confirmed in association with the present condition or past history, it can serve as a diagnostic aid.
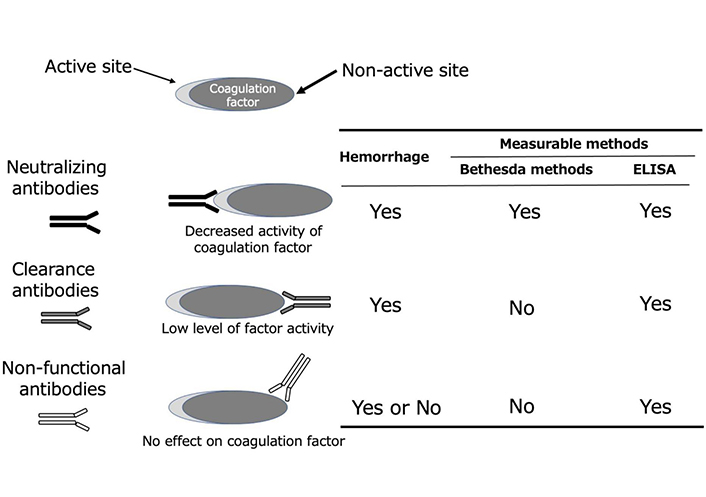
Types of autoantibodies against coagulation factors and detection methods. The following are the three types of autoantibodies against coagulation factors: neutralizing antibodies that bind to the active portion of coagulation factors and inhibit their activity, clearance antibodies that bind to a part other than the active part and are excreted as a complex, and nonfunctional antibodies that do not affect coagulation factor activity. The Bethesda method, which is used to detect coagulation factor inhibitors, can only detect neutralizing antibodies. In contrast, ELISA for detecting antibodies to coagulation factors can detect all autoantibodies, including nonfunctional antibodies
LAHPS presents as hemorrhagic manifestations ranging from mild to severe in 20–89% of affected patients, although it can also be absent [4, 28]. Mild bleeding symptoms, such as epistaxis, have been found in many reported cases, although severe symptoms, including irregular vaginal bleeding and macrohematuria, are also common. In contrast, a previous study reported that 13% of LAHPS cases were accompanied by thrombosis [30]. Therefore, it is important to remember that LAHPS originates from the LA, which increases the risk of thrombosis. Additionally, thrombocytopenia, possibly caused by LA, was observed in 24% of cases in that study. Among the patients with LAHPS (shown in Table S1), those seven of the 10 cases where both LA and anti-FII antibodies were detected had bleeding symptoms, while there were no thrombotic symptoms (Table 1) [4].
Cases of coexistent LA and anti-FII antibodies [4]
| No. | Age (years) | Gender | Underlying conditions | FII:C (%) | Anti-FII antibodies | LA | Clinical presentation | Remarks | |
|---|---|---|---|---|---|---|---|---|---|
| IgG (AU/mL) | IgM (AU/mL) | ||||||||
| II-1 | 34 | F | RA | 4.6 | 344 | 6.8 | dRVVT, Staclot LA, SCT, and CMT positive | Hematoma | Positive for aPS/PT |
| II-2 | 48 | M | SLE/SjS | 10 | 271.1 | 33.1 | dRVVT, Staclot LA, and CMT positive | Ecchymosis and hematuria | Positive for aCL, aβ2GPI, and aPS/PT |
| II-3 | 13 | M | SLE | 12.1 | 430.7 | 5.5 | dRVVT, Staclot LA, SCT, and CMT positive | Ecchymosis | positive for aCL, aβ2GPI, and aPS/PT |
| II-4 | 2 | M | Infection | 18 | 37.1 | 5.6 | dRVVT, SCT, and CMT positive | Ecchymosis | Positive for aCL |
| II-5 | 4 | F | Infection | 35 | 15.2 | 140.8 | dRVVT, SCT, and CMT positive | Ecchymosis | Positive for aCL and aβ2GPI |
| II-6 | 22 | M | Non-hodgikin lymphoma | 39 | 33.8 | 3.7 | dRVVT, SCT, and CMT positive | Not applicable | None |
| II-7 | 85 | M | Colon cancer/infection | 48 | 33.1 | 3.9 | dRVVT, SCT, and CMT positive | Not applicable | Positive for aβ2GPI |
| II-8 | 6 | M | Infection | 53 | 107.5 | 14.3 | dRVVT, Staclot LA, and CMT positive | Ecchymosis and ematoma | Positive for aPS/PT |
| II-9 | 57 | F | Multiple myeloma | 55 | 27.1 | 62.9 | dRVVT, SCT, and CMT positive | Hemorage | Positive for aCL and aβ2GPI |
| II-10 | 2 | F | Infection | 57 | 370.6 | 12.7 | dRVVT, Staclot LA, and CMT positive | Not applicable | Positive for aβ2GPI |
Normal range: FII:C < 60%, anti-FII Ab-IgG < 24 AU/mL, and anti-FII Ab-IgM < 24 AU/mL. No.: patient number; FII:C: FII activity; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; SjS: Sjogren syndrome; SCT: silica clotting time; CMT: APTT cross mixing test; aCL: anti-cardiolipin antibody; IgG: immunoglobulin G
Laboratory findings of LAHPS include prolonged prothrombin time and decreased activity of plasma FII, while autoantibodies against FII have been reported in 55–88% of diagnosed patients [4, 28]. First, an acquired reduction in the plasma FII activity, which causes bleeding, is essential for diagnosing LAHPS. The patients shown in Table 1, verified through their attending physicians that no decrease in FII activity had been confirmed in the past. In contrast, detecting anti-FII antibodies is not a diagnostic requirement, although it is certainly a strong indication of such a diagnosis [4]. In individuals with LAHPS, anti-FII antibodies, which are clearance antibodies, are considered the cause of decreased plasma FII activity. Recently, a case was reported where aPS/PTs, which are known as aPLs, were detected instead of autoantibodies to FII [31]. However, no known reports have shown a clear inhibition of FII activity by aPS/PT. Naturally, LA is detected in all LAHPS cases, and prolongation of APTT is common. Previous studies have also reported the detection of aCLs and aβ2GPI in 40–70% and 40–73% of the diagnosed cases, respectively [4, 28].
AiFVD is a group of diseases where autoantibodies, sometimes known as inhibitors, are produced against coagulation FV in the blood, resulting in decreased FV activity, primarily causing bleeding symptoms. AiFVD cases are extremely rare, with an annual incidence of 0.023–0.09 per one million individuals in the general population reported [32]. However, many of these cases go unnoticed, with the actual number of cases expected to be more than 10-fold higher than those reported [33, 34]. Regarding the underlying disease, it was previously considered that antibody production against bovine FV present in the bovine-derived fibrin glue used in surgical procedures was a common factor [35, 36], although more recent studies have shown associations between AiFVD and infectious diseases, drugs, malignant tumors, surgery, and other factors [37–40]. AiFVD was diagnosed by detecting decreased FV activity, and inhibitors of FV were measured using the Bethesda method. Most clinical manifestations observed in AiFVD cases are associated with bleeding, although reports have noted that some have been related to thrombotic manifestations [41, 42]. Notably, FV not only plays a coenzyme-like role in promoting coagulation but also participates in anticoagulation by protein C [43]. Bleeding symptoms in individuals with AiFVD have been reported to be relatively mild compared with the symptoms in those with AiCFD, although some patients with AiFVD may show spontaneous remission [3]. Nevertheless, the early initiation of hemostatic and immunosuppressive therapies results in high recovery rates.
Laboratory findings for these cases included decreased FV activity and FV inhibitors, measured using the Bethesda method. However, because phospholipids are partially involved in the coagulation reaction system including FV, the measurement of FV activity may be affected by LA. Consequently, LA cases sometimes show an apparent decrease in FV activity and false-positive findings for FV inhibitors [44]. In such cases, it is recommended that FV activity and the inhibitor of FV be measured first using the APTT reagent, which is less sensitive to LA [10]. If this is not possible, or if FV activity declines or FV inhibitors are still detected, anti-FV antibodies can be detected using an immunological method such as ELISA, which can lead to an accurate diagnosis of AiFVD [5, 44].
Several cases of AiFVD co-detected with LA have been reported [5, 43, 45–51]. The confirmed cases of AiFVD co-detected with LA presented in the literature are shown in Table 2.
Cases of coexistent LA and anti-FV antibodies [5]
| No. | Age (years) | Gender | Underlying conditions | FV:C (%) | FV-inhibitor (BU/mL) | LA | Symptoms | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|
| V-1 | 71 | M | Temporo-parietal astrocytoma | < 3 | 61 | dRVVT positive | Left popliteal vein thrombosis | Positive for aCL-IgG and-IgM | Kamphuisen et al. [45] |
| V-2 | 29 | M | Appendicular infiltrate and antibiotics | 23 | 1 | dRVVT positive | No symptoms (abnormal coagulation tests) | None | Van den Berg et al. [46] |
| V-3 | 71 | F | Hemangiopericytoma, UTI, and antibiotics | 1 | 1.4 | dRVVT and Staclot LA positive | Left femoral DVT | None | Donohoe and Levine [47] |
| V-4 | 58 | M | Unknown | 8 | 2.65 | dRVVT positive | DVT | None | Rief et al. [48] |
| V-5 | 71 | F | Proctoscipyand antibiotics | < 2 | 6 | PNP positive | Ecchymosis and hematoma | None | Sridharan et al. [49] |
| V-6 | 63 | M | Lithotripsy and antibiotics | 2 | 16 | PNP positive | Hematuria | None | Sridharan et al. [49] |
| V-7 | 76 | F | Pneumonia and antibiotics | 2 | 70 | dRVVT positive | Subdural hematoma | None | Olson et al. [50] |
| V-8 | 71 | F | Pancreatic cancer | < 3 | 1.7 | dRVVT positive | Multiple purpura | None | Yokota et al. [51] |
| V-9 | 71 | F | HT, HL, and stroke | < 3 | 8 | dRVVT positive | Petechiae, ecchymoses, and melena | Positive for aCL-IgG | Mima et al. [43] |
| V-10 | 56 | M | HT and DM | 2.3 | 1.9 | Staclot LA positive | Purpura and hematuria | None | Ieko et al. [44] |
| V-11 | 52 | F | SLE and DM | 2.5 | 3 | Staclot LA positive | Multiple purpura | None | Ieko et al. [44] |
Normal range: FV:C < 60% and anti-FV inhibitor < 1 BU/mL. FV:C: factor V activity; UTI: urinary tract infection; HT: hypertension; HL: hyperlipidemia; DM: diabetes mellitus; DVT: deep vein thrombosis; PNP: platelet neutrization procedure
Among the eleven confirmed patients with LA-positive AiFVD (Table 2), the median age of the affected individuals was 63 years (range 29–76 years), and the proportion of Ms was 54.5%. These patients were also affected by various underlying diseases, such as autoimmune, infectious, and malignant diseases, although there were also cases where the underlying disease was unclear. A reliable LA test, or even multiple LA tests, if possible, along with accurate detection of FV inhibitors, is important for the proper diagnosis of LA-positive AiFVD. Autoantibodies against FV were confirmed using ELISA in some cases. LA was confirmed by phospholipid neutralization studies using dRVVT and APTT. However, the possibility of false LA positives cannot be ruled out in cases where LA was measured using only one LA test reagent.
Bleeding symptoms, such as subcutaneous hemorrhage, were observed in seven (63.6%) with AiFVD with LA, while three (27.3%) also had thrombotic symptoms, such as DVT. One patient was discovered incidentally during a routine clinical examination. APL other than the LA, such as aCL, may also be detected in patients with AiFVD [52]. Some patients with LA-associated AiFVD develop thrombosis. However, it is unclear whether the onset of thrombosis is due to coexisting LA or inhibition of the FV protein C anticoagulant system by anti-FV antibodies.
The patients with AiFVD suspected of being LA-positive are shown in Table 3 [5, 53–55]. In these patients, the results of an LA test were reported to be inconclusive because the samples did not clot when dRVVT was performed as part of the LA test. LA is an autoantibody that inhibits phospholipids involved in coagulation reactions. Since the coagulation reaction involving FV is also a phospholipid-dependent process, LA may affect not only the measurement of FV activity but also the detection of FV inhibitors with the Bethesda method, especially using LA-sensitive reagents.
Suspicious cases of coexistent LA and ant-FV antibodies [5]
| No. | Age (years) | Gender | Underlying conditions | FV:C (%) | FV-inhibitor (BU/mL) | Symptoms | LA | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|
| VS-1 | 92 | F | HT, HF, Af, PE, erysipelas, leg infection, and antibiotics | 1 | 496 | Extensive hematomas | dRVVT: IM | None | Hoffmann et al. [53] |
| VS-2 | 87 | M | DM | < 1 | 415 | Recurrent bleeding from the hemodyalysis puncture site | dRVVT: IM | None | Ogawa et al. [54] |
| VS-3 | 70 | M | Unknown | 12.4 | NR | Subcutaneous hemorrhage and right thigh hematoma | dRVVT: IM | Positive for aCL | Kato et al. [55] |
Normal range: FV:C < 60% and anti-FV inhibitor < 1 BU/mL. FV:C: FV activity; HF: heart failure; Af: atrial fibrillation; IM: immeasurable (not clotting); NR: not reported; PE: pulmonary embolism
In these cases, both anti-FV antibodies and LA may result in abnormally prolonged clotting times in LA tests, such as dRVVT. However, such an abnormal prolongation of clotting time was not observed in all cases of AiFVD. Therefore, the abnormal clotting times observed in the cases shown in Table 3 suggest the presence of LA.
AHA, which is an AiFVIIID, is a rare autoimmune disease that causes clinically significant bleeding diathesis due to inhibiting FVIII activity by circulating autoantibodies against FVIII. The annual incidence of AHA in the United Kingdom has been reported to be 1.48 per million in the general population [56]. AHA occurs most frequently in older individuals and is associated with underlying medical conditions, such as autoimmune diseases, cancer, or the use of certain drugs; it is also observed during pregnancy or the postpartum period [57]. In some cases, no underlying diseases are found, although cancer and autoimmune diseases frequently develop later [58]. The overall mortality rate of AHA ranges from 7.9% to 33% [59], whereas the recurrence rate is approximately 20% in all cases [56, 60]. AHA is diagnosed by detecting decreased FVIII activity and FVIII inhibitors, with the initial indication frequently being bleeding symptoms in patients with no family or personal history of bleeding. Bleeding manifestations are usually found in the skin, mucous membranes, or soft tissues, although bleeding findings are occasionally present in vital organs, such as intracranial hemorrhage [56]. Recently, measurement of anti-FVIII autoantibodies using ELISA has been recommended as a diagnostic aid for AHA because this method can reliably detect all autoantibodies that bind to FVIII, including antibodies that neutralize coagulation factor activity, as well as clearance antibodies that form complexes with coagulation factors and are removed from the body [44]. Immunosuppressive therapy is recommended for treating AHA and should be administered immediately after diagnosis. Additionally, hemostatic therapy, such as the use of bypassing agents, should be considered for treating cases of bleeding in vital organs or marked anemia.
Although rare, patients with AHA have been reported to have LA complications, with 16 affected individuals reported in the literature to date (Table 4) [6]. In these sixteen patients, the median age was 61 years (range 30–92 years), and 25% were M. The underlying conditions are diverse and include connective tissue diseases and hematologic malignancies. It is well known that LA affects the Bethesda assay used for examining FVIII inhibitors, resulting in false-positive results [20]. Therefore, accurate detection of LA and measurement of coagulation FVIII activity and inhibitors were required for the diagnosis in this case. To determine the clotting factor activity and its inhibitors, it is recommended to first retest LA-insensitive APTT reagents. If decreased clotting factor activity or inhibitors are still detected, it is important to confirm the presence of anti-FVIII antibodies using immunological methods, such as ELISA. To diagnose LA, it is recommended to perform screening, mixing, and confirmatory tests using multiple reagents, such as dRVVT and APTT (e.g., silica clotting time), according to the 2020 guidelines [10].
Cases of coexistent LA and autoantibodies to FVIII [6]
| No. | Age (years) | Gender | Underlying conditions | FVIII:C (%) | FVIII-inhibitor (BU/mL) | LA | Clinical presentation | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|
| VIII-1 | 70 | M | RA | < 1 | 500 | dRVVT | Bleed postliver biopsy | None | Ballard and Nyamuswa [61] |
| VIII-2 | 92 | F | Pemphygoid | 2 | 32 | Staclot LA/PNP | Ecchymosis | None | Biron et al. [62] |
| VIII-3 | 63 | F | EV virus reactivation | 6 | 123 | KCT | Bleeding mouth | None | Grossmann et al. [63] |
| VIII-4 | 62 | F | CLL | 0 | 48 | dRVVT | DVT and PE | positive for aCL | Ghirarduzzi et al. [64] |
| VIII-5 | 38 | F | Postpartum | < 1 | 16 | KCT | DVT → ecchymoses* | None | Saxena et al. [65] |
| VIII-6 | 68 | M | None | < 1 | 320 | KCT | Hematoma | None | Saxena et al. [65] |
| VIII-7 | 60 | F | None | < 1 | 80 | KCT | Hematomas | None | Saxena et al. [65] |
| VIII-8 | 64 | F | Non-Hodgikin lymphoma | 12 | 9 | Staclot LA/PNP | DVT and PE | Positive for aCL | Liozon et al. [66] |
| VIII-9 | 64 | F | None | < 1 | 64 | dRVVT | DVT → hematoma* | None | Brings et al. [67] |
| VIII-10 | 30 | F | Postabortion | 56 | Staclot LA/PNP | Hematoma | None | Wullen et al. [68] | |
| VIII-11 | 30 | M | Monoclonal gammapathy | 20 | 52 | dRVVT | Hematoma | None | Taher et al. [69] |
| VIII-12 | 59 | M | None | < 1 | 70.4 | dRVVT | Hematoma | None | Dreisbach et al. [70] |
| VIII-13 | 37 | F | Postpartum | 2 | 35 | dRVVT | DVT → hematoma* | None | Spencer et al. [71] |
| VIII-14 | 60 | F | SLE/RA | < 1 | 1 | dRVVT | Ecchymosis | None | Seethala et al. [72] |
| VIII-15 | 69 | F | HT | 4 | 8 | dRVVT | Ecchymosis and gingival bleeding | None | Gupta et al. [73] |
| VIII-16 | 50 | F | None | 5 | 2.1 | dRVVT/SCT | Incidental (asymptomatic) | None | Jacobs et al. [6] |
Normal range: FVIII:C < 60% and anti-FVIII inhibitor < 1 BU/mL. *: the clinical presentation was initially thrombosis (DVT), but later changed to hemorrhagic manifestations. FVIII:C: FVIII activity; KCT: kaolin clotting time; CLL: chronic lymphocytic leukemia; EV: enterovirus
As for clinical symptoms, bleeding symptoms, such as ecchymosis and hematoma, were finally recognized in those thirteen patients, whereas DVT and pulmonary embolism were found in five. Interestingly, three patients showed hemorrhagic symptoms after thrombosis. Unsurprisingly, all patients had decreased levels of FVIII activity (median 1.0%, range 0–20.5%) and FVIII-inhibitor (median 41.5 BU/mL, range 1–500 BU/mL). LA was frequently detected using dRVVT, and aCLs were detected simultaneously in two cases. However, when LA was measured only using the dRVVT, the possibility of LA false-positive results could not be ruled out.
The presence of LA can result in the decreased activity of intrinsic coagulation factors and false-positive results for inhibitors of these factors [20]. Therefore, the laboratory findings caused by LA are similar to those observed in AHA. In patients with decreased FVIII activity or detectable FVIII inhibitors, confirming whether these abnormal values are “false results” caused by LA is necessary. To avoid those false detections, FVIII activity, and FVIII inhibitors should be first measured using an LA-insensitive APTT reagent [10]. If low coagulation factor activity or inhibitors are still detected, or if the measurement is impossible, it is recommended to measure FVIII activity using a chromogenic assay and directly determine the amounts of autoantibodies against FVIII using ELISA [44].
Patients with AiCFDs affected by LA can be difficult to treat. Immunosuppressive therapy should be administered immediately after the diagnosis. For the treatment of thrombosis and hemorrhage, the therapeutic plan must be selected according to the clinical symptoms noted. Anticoagulant and antiplatelet drugs are administered for thrombosis, whereas bypass agents, such as recombinant activated FVII, should be chosen for significant bleeding episodes. As clinical symptoms may change from thrombosis to hemorrhage, frequent monitoring, and timely treatment are recommended for these cases.
Cases with autoantibodies against coagulation factors are typical of acquired hemorrhagic diathesis, and LA may be detected in such conditions in a minority of these cases. The presence of LA may lead to thrombotic complications, and changes in clinical symptoms in such patients should be monitored. In contrast, coagulation factor inhibitors are sometimes detected using the Bethesda method in individuals positive for LA. The inhibitor result may be false positive; therefore, it may be useful to measure coagulation factor inhibitors using APTT reagents, which are less sensitive to LA [10]. Additionally, confirming the presence of autoantibodies against coagulation factors by immunological methods, such as ELISA, would provide a definitive diagnosis of AiCFD, even in LA-positive individuals. Conversely, in clinical practice, if there are signs of bleeding in LA-positive patients, it is recommended to actively investigate the activity of FII, FV, and FVIII and the inhibitor of those coagulation factors using LA-insensitive APTT reagents [10]. In conclusion, hemorrhagic diathesis due to autoantibodies to coagulation factors can also cause thrombosis when LA is co-detected. Also, when coagulation factor inhibitors are detected in LA-positive individuals, it is important to confirm the presence of autoantibodies to the coagulation factors using immunological methods such as ELISA to avoid false positive results.
aCL: anti-cardiolipin antibody
AHA: acquired hemophilia A
AiCFD: autoimmune coagulation factor deficiency
AiFVD: autoimmune factor V deficiency
aPL: antiphospholipid antibody
aPS/PT: anti-phosphatidylserine/prothrombin antibodies
APS: antiphospholipid syndrome
APTT: activated partial thromboplastin time
aβ2GPI: anti-β2-glycoprotein I antibodies
dRVVT: dilute Russell’s viper venom time
DVT: deep vein thrombosis
ELISA: enzyme-linked immunosorbent assay
F: female
FII: factor II
LA: lupus anticoagulant
LAHPS: lupus anticoagulant-hypoprothrombinemia syndrome
M: male
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1003103_sup_1.xlsx.
Thanks are due to Yayoi Ishida of the Health Sciences University of Hokkaido for assistance with this study and Editage (www.editage.com) for the English language editing.
MI: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Funding acquisition, Project administration, Supervision. KO: Data curation, Software. SN: Investigation, Data curation. MY and HS: Investigation. TS and NS: Writing—original draft, Validation. NT: Writing—review & editing, Validation. AI: Writing—review & editing, Funding acquisition, Project administration. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study was funded in part by a research grant (to Prof. Akitada Ichinose) for the Japanese Nationwide Survey on Autoimmune Coagulation Factor Deficiencies from the Japanese Ministry of Health, Labor and Welfare [21FC1008]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The funder managed this study and helped write and revise this paper.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gary W. Moore
Osamu Kumano ... Jean Amiral
Fariza A. Cheldieva ... Tatiana M. Reshetnyak
Tiffany Pascreau ... Marc Vasse
