Abstract
Adaptor proteins are involved in various immune responses via the modulation of many signaling pathways. Signal-transducing adaptor protein-2 (STAP-2) is an adaptor protein that contains typical domains such as the pleckstrin homology (PH) domain, Src homology domain, and a proline-rich region from the N-terminal region. In T cells, STAP-2 positively regulates T cell receptor (TCR)-mediated signaling by associating with CD3ζ immunoreceptor tyrosine-based activation motifs (ITAMs) and lymphocyte-specific protein tyrosine kinase (LCK). Therefore, a peptide that inhibits the interaction between STAP-2 and CD3ζ ITAMs is likely to suppress TCR-mediated T cell activation, as well as T cell-mediated diseases. As expected, the peptide successfully inhibited the STAP-2/CD3ζ ITAM interaction and suppressed TCR-mediated signaling, cell proliferation, and interleukin (IL)-2 production in human/murine T cells. Furthermore, this inhibitor suppressed the pathogenesis of experimental autoimmune encephalomyelitis (EAE), which is widely recognized as a mouse model of multiple sclerosis, via the downregulation of T cell activation and infiltration of T helper (Th) 1/Th17 cells. These results suggest a new strategy for the treatment of multiple sclerosis and other immune diseases.
Keywords
Signal-transducing adaptor protein, adaptor protein, signal transduction, T cell receptor, immune response, autoimmunity, multiple sclerosis, peptidesIntroduction
T cells are key players in the adaptive immune system that eliminates pathogens from the body [1]. The T cell receptor (TCR) first recognizes antigenic peptides presented by major histocompatibility complex (MHC) components, followed by the transduction of various signaling pathways to initiate immune responses such as cell proliferation, cytokine production, differentiation, and selection [1]. As a result of various immune responses, invading pathogens are removed. Adaptor proteins are critical regulators of immune responses [2–4]. They interact with signaling molecules and positively or negatively modulate signal transduction [2–4]. Signal-transducing adaptor protein-2 (STAP-2) is a novel adaptor protein that has been isolated as a colony-stimulating factor-1 receptor (c-fms)-binding protein [5]. Previous studies showed that STAP-2 modulates various immune responses [6] such as the signal transducer and activator of transcription 3 (STAT3)/STAT5-related signaling [7–10], FS-7-associated surface antigen (Fas)-mediated apoptosis in T cells [11], high-affinity immunoglobulin E (IgE) receptor [Fc epsilon receptor I (FcεRI)]-mediated signaling in mast cell [12, 13], toll-like receptor (TLR)-mediated signaling in macrophages [14], integrin-mediated adhesion of T cells [15], stromal cell-derived factor-1α (SDF-1α)-mediated T cell migration [16], and TCR-mediated T cell activation (Figure 1) [17]. STAP-2 is a positive regulator of T cells via its interaction with CD3ζ immunoreceptor tyrosine-based activation motifs (ITAMs) and lymphocyte-specific protein tyrosine kinase (LCK) (Figure 2) [17]. Experimental autoimmune encephalomyelitis (EAE), which is widely known as a mouse model of multiple sclerosis, revealed that STAP-2 knockout (KO) mice had significantly lower clinical scores than wild-type mice and a higher clinical score in STAP-2 transgenic (Tg) mice [17]. Therefore, disrupting the interaction between STAP-2 and CD3ζ ITAMs is likely to help regulate TCR-mediated signaling and may be a new therapeutic target of T cell-mediated immune diseases. This perspective summarizes the function of an inhibitory peptide [STAP-2 inhibitor (iSP2)] which targets STAP-2/CD3ζ ITAM binding in vivo and in vitro.
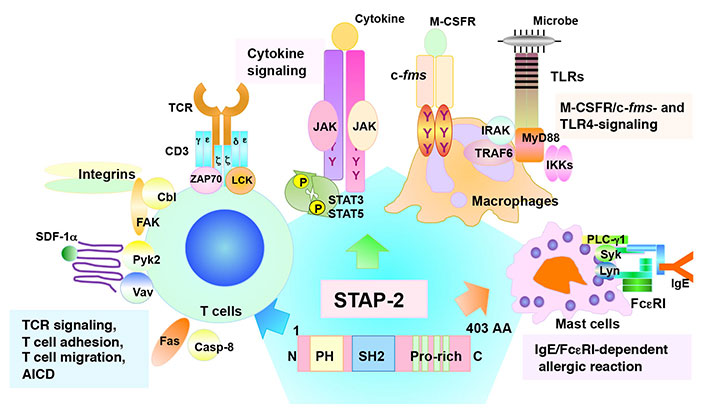
Structural features and functions of STAP-2. STAP-2 contains an amino (N)-terminal pleckstrin homology (PH) domain, a central Src homology 2 (SH2) domain, and a carboxy (C)-terminal proline-rich domain. The tyrosine-X-X-glutamine (YXXQ) motif is involved in the STAT3-mediated signaling. STAP-2 functions in various immune signaling pathways in several immune cell types. AA: amino acid; AICD: activation-induced cell death; PLC-γ1: phospholipase C-γ1; Pyk2: protein tyrosine kinase 2; FAK: focal adhesion kinase; Casp-8: caspase-8; ZAP70: zeta-chain-associated protein kinase 70; JAK: Janus kinase; IRAK: interleukin (IL)-1 receptor-associated kinase; TRAF6: tumor necrosis factor receptor-associated factor 6; MyD88: myeloid differentiation protein 88; IKKs: inhibitory kappa B kinases; Syk: spleen tyrosine kinase; Lyn: LCK/yes related novel protein linase; Cbl: Casitas B-lineage lymphoma; M-CSFR: macrophage-colony stimulating factor receptor; P: phosphoric acid; Y: tyrosine
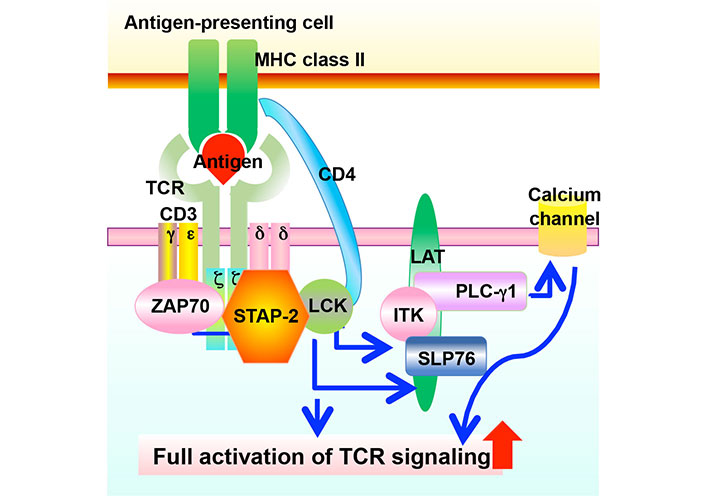
Involvement of STAP-2 expression in TCR-mediated signaling. TCR recognizes antigens presented by MHC class II, followed by the phosphorylation of various downstream molecules. These transmissions of phosphorylation induce cell proliferation, cytokine production, and other cellular activities in T cells. STAP-2 modulates the TCR-mediated signaling via interacting with CD3ζ ITAMs and LCK. ITK: IL-2 inducible T cell kinase; SLP76: SH2 domain-containing leukocyte protein of 76 kDa; LAT: linker for activation of T cells
Peptide as interfering tool of protein-protein interactions
Because the target is STAP-2/CD3ζ ITAM binding, which is a typical intracellular protein-protein interaction (PPI), it is very difficult to inhibit this PPI using small molecules or antibodies [18–20]. Recently, the discovery of peptide drugs has become remarkable. Peptides are now promising tools for targeting PPI as recent research has shown that they can be structured to have high stability, better binding affinity, and membrane permeability [21]. Therefore, peptides are suitable tools to inhibit PPI. Prior to constructing an iSP2, the important sequence for STAP-2/CD3ζ ITAM binding has been identified. A series of deletion mutants of STAP-2 were generated, and they were used to pull down the assay with Sepharose beads conjugated with the ITAM peptide. After the critical part and sequence for the interactions between STAP-2 and CD3ζ ITAMs were determined, the possible interfering peptide by adding cell-penetrating octa-arginine sequence to the STAP-2-CD3ζ ITAM binding site was constructed. This was further optimized by shortening the sequence without losing the capacity to inhibit T cell function [22]. The optimized and control peptides are shown as “iSP2” and “iCont”, respectively, in this perspective. iSP2 contains five AA [STAP-2-based 5 AA sequence (WPVIL)] corresponding to the C-terminal proline-lich region of STAP-2 [22].
Functional roles of iSP2 in vitro
Since STAP-2 is important for TCR-mediated T cell activation, including TCR-mediated signaling, cell proliferation, and IL-2 production [17], the inhibition of STAP-2 functions by iSP2 was investigated. In the human leukemia-derived cell line Jurkat and human peripheral blood mononuclear cells, iSP2 shows an inhibitory effect on TCR-mediated signaling, cell proliferation, and IL-2 production [22]. iSP2 effects on murine T lymphoma EL-4 cells and murine CD4+ T cells were tested. Consistent with its suppressive effect on human-derived cells, iSP2 has the capacity to attenuate TCR-mediated signaling, cell proliferation, and IL-2 production [22]. Collectively, these results indicate that iSP2 functions as an iSP2 for T cells by blocking the interaction between STAP-2 and CD3ζ ITAMs. In drug discovery research, the off-target responses or cell-specific functions of a drug are important in terms of cytotoxicity or adverse events [23]. Regarding its possible effects on other signaling pathways in T cells, iSP2 did not affect cell proliferation upon stimulation with phorbol myristate acetate (PMA)/ionomycin, indicating that it has a TCR signaling-specific function [22]. When the function of iSP2 was analyzed using non-T cells, iSP2 failed to affect spontaneous proliferation in the human prostate cancer cell line DU145 and murine melanoma cell line B16F10, as well as FcεRI-mediated IL-6 production in bone marrow-derived basophils [22]. Thus, iSP2 is likely to exert T cell-specific suppressive functions.
Effect of iSP2 in EAE
As iSP2 negatively regulates not only human but also murine T cells [22], the therapeutic potential of iSP2 in T cell-mediated immune diseases was analyzed. In the previous study using an EAE model, STAP-2 KO mice exhibited significantly lower clinical scores while STAP-2 Tg mice exhibited higher scores than wild-type mice [17]. During EAE development, STAP-2 messenger RNA (mRNA) expression is enhanced and STAP-2 in CD4+ T cells contributes to the activation and expansion of autoreactive myelin oligodendrocyte glycoprotein (MOG)-specific T cells [17]. Thus, STAP-2 is likely involved in worsening the severity of EAE. EAE is widely recognized as a mouse model of multiple sclerosis and is induced by immunization with MOG peptide emulsified with complete Freund’s adjuvant, followed by an intravenous injection of pertussis toxin. Approximately 12–14 days after immunization, autoreactive immune cells infiltrate the central nervous system (CNS) and induce demyelination; thus, multiple sclerosis-like symptoms are detected [24–27]. Using this model, the role of iSP2 in EAE development was examined. After treatment with iSP2 following MOG immunization, the peak disease severity was significantly lower than that in iCont-treated mice [22]. Histologically, hematoxylin and eosin staining and Luxol fast blue staining revealed that immune cell infiltration and spinal cord demyelination during EAE development were significantly lower in iSP2-treated mice than in iCont-treated mice [22]. To test whether iSP2 modulates the T cell response, CNS-infiltrating T cells was analyzed. T helper (Th) 1 and Th17 cells are critical for the pathogenesis of EAE. They are first activated in peripheral tissues, after which they migrate across the blood-brain barrier to enter the CNS. Infiltrated Th1 and Th17 produce their specific cytokines, namely interferon (IFN)-γ and IL-17, respectively [28–30]. These cytokines not only directly damage myelin and cause neuroinflammation, but also induce the migration and activation of glial cells, followed by additional neuroinflammation and axonal damage. Thus, Th1 and Th17 cells play direct and indirect critical roles in pathogenesis. Therefore, the CNS-infiltrating Th1 and Th17 in iSP2- and iCont-treated mice were analyzed. In iSP2-treated mice, the absolute number and proportion of both Th1 and Th17 cells were significantly attenuated compared to iCont-treated mice [22], indicating that iSP2 suppressed the activation of autoreactive T cells, contributing to lower disease severity. To investigate the mechanism by which iSP2 regulates EAE pathogenesis, single-cell RNA-sequencing of CNS-infiltrating cells from iSP2- and iCont-treated mice was performed. Based on the expression of cell markers, pathogenic T cell-related gene expression decreased in iSP2-treated mice [22]. Flow cytometric analysis also confirmed that iSP2 treatment suppressed the migration of immune cells to the CNS [22]. Collectively, these results indicate that iSP2 suppresses the pathogenesis of EAE by reducing Th1 and Th17 cell activation and immune cell infiltration (Figure 3).
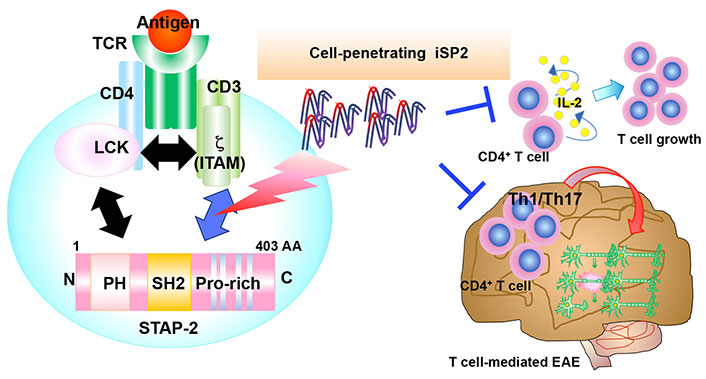
Highlight of T cell suppression by iSP2. iSP2 inhibits STAP-2/CD3ζ ITAMs interactions and suppresses T cell activation in vitro. In EAE, iSP2 also negatively regulates the pathogenesis by suppressing T cell activation and infiltration of immune cells
Discussions
Possibility of iSP2
As described in this perspective, STAP-2 is involved in various T cell functions, such as TCR-mediated T cell activation [17], SDF-1α-related migration [16], and AICD [11] independently of each other. To initiate various T cell responses, STAP-2 has the capacity to interact with many other signaling molecules through each domain. Its PH domain binds to breast tumor kinase (Brk), c-fms, and Pyk2 [8, 31, 32], and its SH domain binds to MyD88, IKKβ, FAK, Pyk2, Cbl, Vav, and PLC-γ1 [12, 14–16, 32, 33]. The STAP-2 C-terminal proline-lich region binds to Vav and PLC-γ1 [12, 16]. Therefore, STAP-2 is a key regulator of T cell functions, and understanding the mechanisms underlying STAP-2-related T cell functions can provide new clinical applications. In this perspective, focusing on T cell activation via TCR ligation, it was sought to apply the previous finding that STAP-2 is involved in TCR-mediated T cell activation by interacting with LCK via its PH domain and CD3ζ ITAMs via its C-terminal proline-lich regions [17]. The previous study [22] suggested that inhibiting the interaction between STAP-2 and CD3ζ ITAMs can suppress the activation of T cells and T cell-mediated immune diseases. STAP-2-derived peptides that penetrated the cell membrane and interfered with their interaction were successfully generated. An optimized peptide, iSP2, could successfully inhibit STAP-2/CD3ζ ITAM binding, as well as TCR-mediated signaling, cell proliferation, and IL-2 production in human/murine T cells [22]. To test the potential of iSP2 in a new clinical application, its functions in vivo using EAE were analyzed. EAE is widely considered a model of multiple sclerosis characterized by motor dysfunction and paralysis of the four limbs, initiated by immune cell infiltration and demyelination. In the EAE model, peptides were administered from day 8 after MOG immunization every other day until day 20 as a therapeutic treatment. Mice injected with iSP2 exhibited significantly lower clinical scores than iCont-injected mice. The function of iSP2 as a prophylactic agent for EAE was also evaluated. During prophylactic treatment, peptides were injected shortly after MOG immunization (day 1) to day 9. iSP2 suppresses EAE pathogenesis both therapeutically and prophylactically. Regarding this mechanism, iSP2 modulates the activation of T cells (Th1 and Th17), which is critical for initiating pathogenesis and reduces the migration of immune cells to the CNS region [22]. Indeed, EAE-induced IFN-γ and IL-17 mRNA expression levels in the CNS were reduced, although no alteration was observed in anti-inflammatory cytokines IL-10 and transforming growth factor (TGF)-β mRNA levels (data not shown). Because the development of many autoimmune diseases is dependent on the excess of T cell activation, iSP2 may have the potentiality to be applicable to various T cell-dependent immune diseases. However, the mechanisms underlying the regulation of cell migration remain unclear. In the previous research [16], the STAP-2 proline-rich region was found to be involved in SDF-1α-mediated chemotaxis via interaction with Vav. Therefore, iSP2 likely modulates SDF-1α-mediated signaling. Although it is not clear whether this acts positively or negatively, the inhibition of chemokine signals may be off-target effects of iSP2.
Further, although Fas-induced cell death might also be not recommended in autoimmune disorders, iSP2 targets the ITAM motifs through the STAP-2’s C-terminal but not SH2 domain which interacts with Casp-8 activated Fas [11].
Further studies
The WPVIL is a lead candidate for peptide drugs. Further research is needed for additional optimizations, such as the capacity for cell penetration, metabolic stability, and intracerebral migration. A 29 AA branched peptide behaving as a programmed cell death protein 1 decoy showed greater inhibition of mouse colorectal carcinoma cells (CT26) subcutaneous tumor growth when combined with tumor vaccines and/or cyclophosphamide in a xenograft model [34]. Similarly, the iSP2 peptide may function better when combined with other immunosuppressive or anti-inflammatory drugs.
Conclusion
The STAP-2 genomic sequence has potential binding sites for c-Rel, activator protein-1 (AP-1), p65/nuclear factor-κB (NF-κB), and STAT proteins [5]. These transcription factors are activated by bacterial pathogens and inflammatory cytokines. Indeed, STAP-2 expression is highly induced by several inflammatory cytokines and reagents such as IL-6 and lipopolysaccharide [5], although STAP-2 mRNA is ubiquitously expressed in a variety of tissues and cells under steady-state conditions. Several mouse models of immune/inflammatory diseases, such as inflammatory bowel disease, hematopoietic stem cell transplantation, and graft-versus-host disease, clearly show that STAP-2 expression influences the onset and development of these diseases [35–37].
Therefore, a better understanding of the molecular interactions related to these models will provide valuable information on the application of iSP2 in other diseases in the near future.
Abbreviations
| AA: |
amino acid |
| c-fms: |
colony-stimulating factor-1 receptor |
| CNS: |
central nervous system |
| EAE: |
experimental autoimmune encephalomyelitis |
| Fas: |
FS-7-associated surface antigen |
| FcεRI: |
Fc epsilon receptor I |
| IgE: |
immunoglobulin E |
| IL: |
interleukin |
| iSP2: |
signal-transducing adaptor protein-2 inhibitor |
| ITAMs: |
immunoreceptor tyrosine-based activation motifs |
| LCK: |
lymphocyte-specific protein tyrosine kinase |
| MHC: |
major histocompatibility complex |
| MOG: |
myelin oligodendrocyte glycoprotein |
| mRNA: |
messenger RNA |
| PH: |
pleckstrin homology |
| PLC-γ1: |
phospholipase C-γ1 |
| PPI: |
protein-protein interaction |
| Pyk2: |
protein tyrosine kinase 2 |
| SDF-1α: |
stromal cell-derived factor-1α |
| SH2: |
Src homology 2 |
| STAP-2: |
signal-transducing adaptor protein-2 |
| STAT3: |
signal transducer and activator of transcription 3 |
| TCR: |
T cell receptor |
| Th: |
T helper |
| TLR: |
toll-like receptor |
Declarations
Acknowledgments
We thank Editage (www.editage.com) for English language editing.
Author contributions
Y Sasaki and TM: Writing—original draft. SK, Y Sekine, JIK, and KO: Conceptualization, Methodology. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This study was supported in part by
Copyright
© The Author(s) 2023.