Affiliation:
1Department of Urology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan, China
ORCID: https://orcid.org/0009-0004-0733-3681
Affiliation:
1Department of Urology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan, China
ORCID: https://orcid.org/0009-0003-0550-6852
Affiliation:
2Medical Research Center, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan, China
Email: wangmingheda@163.com
ORCID: https://orcid.org/0000-0002-4913-5390
Explor Immunol. 2024;4:106–114 DOI: https://doi.org/10.37349/ei.2024.00131
Received: November 18, 2023 Accepted: January 17, 2024 Published: February 28, 2024
Academic Editor: Daishu Han, Chinese Academy of Medical Sciences & Peking Union Medical College, China
The article belongs to the special issue Immunobiology and Inflammation in the Male Reproductive System
Epididymitis or epididymo-orchitis is a common urological condition in males characterized by scrotal pain, swelling, and potential urinary symptoms. Although antibiotics can eliminate the causative pathogens, persistent inflammation may compromise spermatogenesis and steroidogenesis. The testis, an immune-privileged organ, possesses a specialized immune microenvironment that shields germ cells (GCs) from autoimmune attacks and orchestrates immune defenses against pathogens. This review focuses on the complex interplay between immune cells, including macrophages, dendritic cells (DCs), mast cells (MCs), and T cell subsets, in the testis. The roles of these immune cells in infection-induced orchitis were deliberated upon, emphasizing their involvement in inflammation and immune tolerance. Furthermore, the implications of testicular fibrosis and its effect on male infertility are discussed, emphasizing the role of MCs in tissue remodeling. The objective of this review is to expand comprehension of male reproductive health and foster the identification of potential therapeutic targets for epididymo-orchitis.
Epididymitis or combined epididymo-orchitis is a common disease in males and presents as scrotal pain and swelling that may be accompanied by urinary symptoms, such as dysuria and increased urinary frequency [1]. The causative pathogens include Chlamydia trachomatis, Ureaplasma urealyticum, Neisseria gonorrhoeae, Mycoplasma hominis, Mycoplasma hominis, Escherichia coli [2], and mumps virus [3]. These bacterial pathogens can be eliminated by antibiotics; however, inflammation may continue and impair spermatogenesis and steroidogenesis [4]. The testis serves as an immunologically privileged organ. It establishes a specialized microenvironment to safeguard germ cells (GCs) against autoimmune responses and orchestrates an immune defense mechanism to maintain the stability of the microenvironment in the face of pathogenic threats [5]. This immune privilege is facilitated by compartmentalization of the testis, with the seminiferous tubule consisting of peritubular, Sertoli, and spermatogenic cells, and interstitium-containing immune and Leydig cells [6]. In particular, adjacent Sertoli cells form the blood-testis barrier (BTB) through a complex network of specialized cell junctions, including tight junctions, basal ectoplasmic specialization (ES), and desmosomes, which play crucial roles in maintaining and regulating the immune privilege of the testis [7]. Recent research has shown that in a rat orchitis model, uropathogenic Escherichia coli (UPEC) infection can destroy the BTB by downregulating crucial cellular junctional proteins such as zonula occludens-1 (ZO-1), connexin-43 (CX-43), neural-cadherin (NCAD), and occludin in Sertoli cells, resulting in anti-sperm antibodies formation in the blood of infected animals [8].
The maintenance and regulation of testicular immune privileges are attributed to the immunosuppressive characteristics of immune cells and immunomodulatory factors originating from non-immune cells, including Sertoli cells, Leydig cells, leukocytes, fibrocytes, blood vessels, and lymph vessels within the interstitium [9]. Immune cells such as macrophages, mast cells (MCs), lymphocytes, natural killer (NK) cells, and dendritic cells (DCs) are predominantly located in the interstitial space of the testis and play a pivotal role in orchitis [10]. This interplay is intricately connected to the interstitial and immune cells. Notably, Leydig cells have been confirmed to stimulate the activity and proliferation of DCs, neutrophils, and NK cells, which are critical for resolving inflammation [11]. In addition to testosterone, corticosterone present in the interstitial fluid can induce macrophage polarization toward the M2 phenotype, contributing to the establishment of an immunosuppressive testicular microenvironment and ameliorating UPEC-induced orchitis [12]. This review discusses the roles of diverse immune cells in infection-induced orchitis, elucidates their functions, and introduces potential therapeutic strategies for epididymo-orchitis.
There are usually two routes of entry of pathogens into the testis, one is through the blood circulation, and the other is retrograde entry through the urogenital tract [13]. After a pathogen invades the testis, local tissue initiates innate immunity against the invading pathogens [6]. After pathogen invasion, recruited immune cells such as macrophages, DCs, MCs, and T cells infiltrate the interstitium of the testis (Figure 1). They produce large amounts of proinflammatory cytokines, such as interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α) to facilitate pathogen elimination [14–16]. However, excessive inflammation can induce interstitial fibrosis and remodeling [17] and impair spermatogenesis and androgen production, eventually leading to male infertility [18]. The intricate characteristics of immune cell subtypes and their functions in the context of orchitis have been comprehensively outlined (Figure 1) and explored in the subsequent sections.
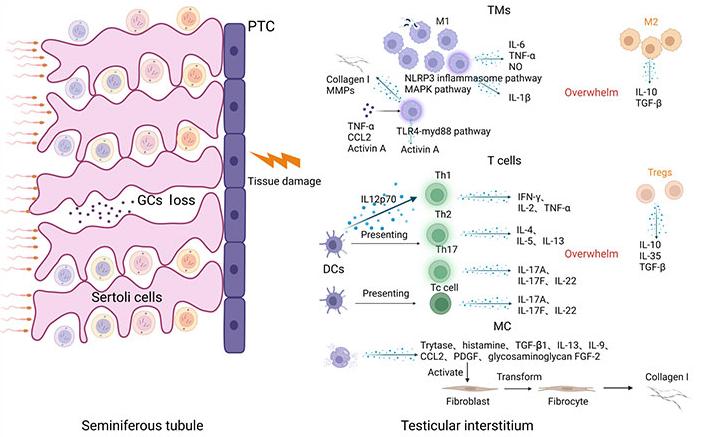
In the course of an infection, M1 macrophages, T helper (Th), and cytotoxic T (Tc) cells release diverse pro-inflammatory cytokines, overpowering the suppressive inflammatory role of M2 macrophages and regulatory T (Treg) cells. Simultaneously, macrophages have the ability to produce collagen I and matrix metalloproteinases (MMPs). Upon activation, MCs release a range of cytokines that foster the activation of fibroblasts, ultimately resulting in interstitial fibrosis. PTC: peritubular cell; NLRP3: pyrin domain (PYD) domain-containing protein 3; MAPK: mitogen-activated protein kinases; NO: nitric oxide; TGF-β: transforming growth factor-β; TLR4-myd88: toll-like receptors 4-myeloid differentiation factor 88; IFN-γ: interferon-γ; PDGF: platelet-derived growth factor; FGF-2: fibroblast growth factor 2; TMs: testicular macrophages; CCL2: chemokine (C-C motif) ligand 2. Created with BioRender.com
Testicular macrophages are the most abundant immune cells in the testes [19], and play an important role in maintaining the stability of the immune microenvironment and resisting pathogen infections [5]. Under physiological conditions, human testicular macrophages consist of two major subpopulations based on their functions and phenotypes: [macrophage colony-stimulating factor receptor (M-CSFR+) major histocompatibility complex class II (MHCII−)] interstitial macrophages (iTM) and (M-CSFRlo MHCII+) peritubular macrophages (pTM). Both cell subsets show the immunomodulatory and immunotolerant M2 phenotype [20]. The origin and function of testicular macrophages are controversial. iTM is closely associated with Leydig cells and provides cholesterol to them, which is necessary for steroid production. More importantly, iTM may play a significant role in the differentiation and maturation of Leydig cells [21]. pTM are present around seminiferous tubules and are generally considered important for the spermatogonial differentiation process of spermatogenesis [22, 23]. Because spermatogonia begin to mature and differentiate during puberty, they require an immunosuppressive environment to avoid being attacked by the immune system. Numerous experiments have shown that testicular macrophages can secrete large amounts of immunosuppressive factors such as IL-10 [19] and TGF-β [24] to suppress inflammatory responses to maintain a stable environment conducive to spermatogenesis.
UPEC is the most common pathogen causing genitourinary tract infections [25, 26]. Studies have shown that UPEC induces an inflammatory response by promoting the activation of the NLRP3 inflammasome in testicular macrophages and that chemical mediators such as prokineticin 2 (PK2) in testicular macrophages and testicular interstitial fluid can enhance this process [27, 28]. At the same time, UPEC infection can activate the MAPK signaling pathway in testicular macrophages, promote the secretion of IL-1β by testicular macrophages [29], and inhibit the production of testosterone by Leydig cells [27]. Consequently, immunosuppression is further weakened and, with the development of inflammation, autoantigens are attacked by the immune system. During orchitis, the number of macrophages in the interstitium increases significantly [29–31], and testicular macrophages can produce a large number of NO and proinflammatory chemokines such as IL-6 and TNF-α [31, 32]. Spermatogenic disorders can be caused by excessive immune responses. Our previous study found that corticosterone can maintain the immunosuppressive phenotype of testicular macrophages during infection by strengthening the adenosine monophosphate-activated protein (AMP)-activated protein kinase pathway, thereby reducing damage by the inflammatory response in the testis [12]. UPEC infection can activate the TLR4-myd88 pathway, promote activin A expression, and induce fibrosis of the male reproductive system [33]. Markers of fibrosis such as α-smooth muscle actin and fibronectin are significantly increased during orchitis. Cytokines such as activin A, TNF-α, and CCL2 induce macrophages to secrete large amounts of collagen I and MMPs, which eventually lead to interstitial fibrosis [17]. Recent studies have shown that macrophages may play an important role in testicular viral infections and may be crucial for viral transmission, sperm damage, and the establishment of viral reservoirs [34].
Testicular macrophages have a variety of cell subsets that play key roles in spermatogenesis, removal of foreign particles, and resistance to pathogen invasion. However, their specific functions remain unclear, and the mechanism by which pro-inflammatory M1 macrophages replace anti-inflammatory M2 macrophages during inflammation remains controversial. Clarifying these mechanisms will help in the exploration of testicular damage caused by immune responses and may provide new treatment options for male infertility.
Physiologically, lymphocytes in the interstitium are mainly T cells, T cell subsets in the testis mainly include Treg, Th, Tc, NK T, and γδT cells [35]. T cells play an important role in immune responses and are important immunomodulators. Tregs are mainly composed of clusters of differentiation 4 (CD4) cell subsets [36, 37], they secrete various inhibitory cytokines, such as IL-10, IL-35, and TGF-β, interfere with the metabolism of effector T cells, and regulate the maturation of DCs, classical antigen-presenting cells, so as to achieve the effect of immunosuppression [38]. Tregs reside in the interstitial space and draining lymph nodes (TLN) of the testes and interact with tissue-specific antigens to maintain testicular immune privilege [39, 40]. Studies have shown that Treg plays a crucial role in preventing spontaneous organ-specific autoimmunity induced by persistent endogenous danger signals [41]. Furthermore, the removal of Treg results in the onset of autoimmune orchitis and epididymitis, marked by increased infiltration of immune cells, including CD4 T cells, monocytes, and mononuclear phagocytes, along with the emergence of anti-sperm antibodies [13, 42].
Th cells are a subset of CD4+ T cells, which are mainly composed of Th1, Th2, and Th17 subsets in human and mouse testes, and help the testis clear a variety of invading pathogens [35]. In the context of an infection, Th1 cells play a crucial role in pathogen clearance, specifically viruses and bacteria, through the production of cytokines such as IFN-γ, IL-2, and TNF-α. Th2 cells eliminate extracellular pathogens by releasing IL-4, IL-5, and IL-13. Th17 cells, on the other hand, aid in the body’s defense against bacterial and fungal infections through the secretion of IL-17A, IL-17F, and IL-22 [43]. Research findings indicate that, in cases of chronic testicular inflammation, the Th17 subset takes precedence, releasing a substantial quantity of proinflammatory cytokines [35]. This surge in proinflammatory cytokines overwhelms the suppressive influence of Tregs, perpetuating an ongoing inflammatory state within the testes. Tc lymphocytes are a subset of CD8+ T cells that help clear pathogen-infected, damaged, and tumor cells [35]. Tc17 cells release significant quantities of pro-inflammatory cytokines, including IL-17A, IL-17F, and IL-22. These cytokines play pivotal roles in the onset and progression of experimental autoimmunity orchitis (EAO) [44, 45]. NK T cells regulate the immune response, and their number is significantly increased in the testes during infection [46], however, their role in testicular immunity is unclear. γδT cells are a subset of CD4− and CD8− T cells, whose T cell receptor expression chain is γδ, which may act as a signal amplifier to induce adaptive immunity [35, 47]. However, the function of γδT cells in human testes remains unclear.
T cell subsets play an important role in immune response. Studies have found that T cell infiltration is closely related to the pathological process of orchitis [35]. By exploring the complex relationship between T cell subsets, it can be clarified that the role of T cells in testicular immune tolerance and inflammation will help in identifying new drug targets.
MCs are mononuclear granule cells that play an indispensable role in immune regulation, mediating and regulating allergic reactions, and tissue remodeling after chronic inflammation [48]. Within the testicular environment, MCs are typically observed within the interstitial compartment in humans, a pattern not commonly found in traditional experimental models such as rats and mice [48–50]. MCs appear to play a pivotal role in the development of testicular fibrosis in various instances of infertility. MCs hold numerous granules containing histamine, chymase, tryptase, carboxypeptidase A, and TNF-α [51]. Following MC activation, these granules are discharged into the adjacent tissues, leading to the activation of various substances such as TNF-α, IL-6, and IL-1β, as well as the synthesis of prostaglandins and the production of leukotrienes [52].
In response to an infection, there is a notable rise in the population of MCs within the testis, and these activated MCs discharge cytokines, including TNF-α, from their granules, facilitating the advancement of inflammation. Concurrently, stromal fibroblasts are stimulated to transform into fibroblasts through the release of tryptase, histamine, TGF-β1, IL-13, IL-9, CCL2, PDGF, and the glycosaminoglycan FGF-2. Subsequently, these fibroblasts generate substantial quantities of collagen [53]. Testicular fibrosis is a significant and severe complication of orchitis and is potentially linked to the enduring pro-inflammatory effect of MCs and fibrotic remodeling of the tissue during chronic inflammation. However, the precise mechanisms governing MC-mediated immune regulation in the testes under pathological conditions remain unclear. This knowledge gap presents an opportunity to identify potential therapeutic targets to mitigate testicular fibrosis in the context of chronic inflammation.
DCs are antigen-presenting cells that play an important role in adaptive immunity and stimulate T cell proliferation and differentiation [54]. Cytokines such as IL-10 and IL-12 released during DC maturation can affect the immune responses of Th1, Th2, and Th17 cells [55]. Under physiological conditions, DCs isolated from the testis fail to stimulate the proliferation and differentiation of naive T cells, and the chemokine receptor C-C chemokine receptor type 2 (CCR2), a marker of immature DCs, can be detected [9]. These observations indicate that, under normal circumstances, DCs in the testis maintain an immune-tolerant state, rendering them incapable of presenting antigens to immune cells. This promotes the establishment of an immunosuppressive environment within the testicular milieu.
During orchitis, the number of mature DCs in the interstitium increases significantly [10], which can activate T cells and promote the occurrence and development of inflammation. At the same time, the DCs secrete IL12p70, which promotes the activation of T cells to produce the Th1 type inflammatory response [56]. As inflammation progresses, DCs phagocytose self-antigens and present them to T cells, inducing the immune system to attack their own tissues and cause tissue damage [57]. After the spermatogenic tubules are damaged, semen enters the stroma of the testis, and semen antigens are phagocytosed by DCs and returned to the lymph nodes, inducing an autoimmune attack that affects semen quality [9]. Although the number of DCs in the testes is small, their immune functions cannot be ignored. Clarifying the mechanism of interaction between DCs and lymphocytes will help with the identification of new therapeutic targets for patients with infertility.
The testis relies on the active involvement of immune cells to maintain normal physiological functions. Through the collaborative efforts of various immune cells, the testis maintains an immunosuppressive state, shielding its tissue and GC antigens from potential immune system attack. Immune cell activity is triggered in response to pathogenic invasion, leading to the secretion of diverse cytokines that promote inflammation. An excessive inflammatory response can result in damage to the testicular tissue and differentiation of fibroblasts, ultimately leading to testicular fibrosis. The application of advanced technologies such as single-cell sequencing and transgenic mouse models in future investigations holds the promise for offering more insights into immune cell subtypes and their distinct roles in orchitis. These developments will enhance the comprehension of male infertility, a complex condition, and present potential new therapeutic targets and treatment alternatives.
CD4: clusters of differentiation 4
DCs: dendritic cells
IL-6: interleukin-6
iTM: interstitial macrophages
MCs: mast cells
NK: natural killer
Tc: cytotoxic T
TGF-β: transforming growth factor-β
Th: T helper
TNF-α: tumor necrosis factor-α
Treg: regulatory T
UPEC: uropathogenic Escherichia coli
YZ and JZ: Writing—original draft. MW: Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was financially supported by the National Natural Science Foundation of China [81901466]; Ming Wang was financially supported by Henan Province Scientific and Technological Research [232102520026]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Xiaoyu Wu ... Xinzong Zhang
Chuxiong Wang ... Donghui Huang
Prity Yadav, Pratap Chand Mali
Marina Izvolskaya
Xue Zhang ... Donghui Huang
