Abstract
Aim:
The current study aimed to describe various types of myelitis associated with a novel coronavirus infection [coronavirus disease 2019 (COVID-19)] as well as to analyze cytokine profiles and cerebrospinal fluid (CSF) parameters in affected patients and to compare them to patients with other immune-mediated disorders—multiple sclerosis (MS), in order to identify possible common pathogenetic pathways and consequently treatment targets.
Methods:
Clinical, radiological, and laboratory characteristics were studied based on patients’ history. CSF from patients with myelitis associated with COVID-19 (11 patients) was compared with CSF of healthy controls (HC) (7 patients) and patients with MS (37 patients) from the non-COVID era. CSF cytological examination, protein levels and oligoclonal bands (OCBs) evaluation, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus detection and cytokine profiling using Bio-Plex Pro Human Inflammation Panel 1, 37-Plex were performed.
Results:
In total 11 patients with different types of myelitis developed up to 3 months after COVID-19 were enrolled in the study. Radiological findings were diverse: short transverse myelitis (lesion of fewer than 3 segments) (n = 6), longitudinal extensive transverse myelitis (LETM) (n = 2), multifocal spinal cord lesions (n = 1), and myelitis involving dorsal and lateral columns (n = 2). The most pronounced response to treatment was observed in patients with partial transverse myelitis and patients with anti-myelin oligodendrocyte glycoprotein (MOG) antibodies (MOG Abs). Multiple comparisons have demonstrated decreased levels of interleukin-10 (IL-10), interferon-α2 (IFN-α2), IFN-β, and thymic stromal lymphopoietin (TSLP), and increased IL-19 and B cell activating factor (BAFF) in patients with COVID-19 myelitis (CM) compared to the MS group. The highest BAFF and a proliferation-inducing ligand (APRIL) concentrations were found in patients with the most profound neurological disability.
Conclusions:
Myelitis associated with COVID-19 is clinically and radiologically heterogeneous. Evaluation of cytokine profiles in patients with myelitis associated with COVID-19 revealed their relative similarity with ones of MS patients, except for a few cytokines. BAFF/APRIL system as well as IL-10 is well-known for the role in the development and progression of autoimmune diseases, however, their links with COVID-19 and effects on the development of immune-mediated central nervous system (CNS) disorders after SARS-CoV-2 remain to be further studied.
Keywords
Novel coronavirus infection, cytokine profile, myelitis, immune-mediated diseases, multiple sclerosisIntroduction
Novel coronavirus infection [coronavirus disease 2019 (COVID-19)] caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus was first registered and described in December 2019 in the Chinese city of Wuhan [1]. Viruses of the coronavirus family, including SARS-CoV, SARS-CoV-2, and Middle East respiratory syndrome coronavirus (MERS-CoV), predominantly cause respiratory viral diseases that, if severe, can lead to the development of acute respiratory distress syndrome. As the number of COVID-19 cases increased, it became clear that the SARS-CoV-2 virus, in addition to the respiratory system damage, could lead to multi-organ pathology, in particular, to dysfunction of the cardiovascular system, gastrointestinal tract, coagulation system, and nervous system ranging from 22.5% to 36.4% among different studies [2–5]. The most common central nervous system (CNS) manifestations of COVID-19 are acute encephalopathy (53%), acute ischemic or hemorrhagic cerebrovascular accident (19%), and seizures (10%) [6]. The prevalence of another group of CNS disorders associated with COVID-19—immune-mediated and autoimmune diseases, remains not fully understood.
Virus SARS-CoV-2, along with SARS-CoV and MERS-CoV, has demonstrated its neuroinvasive potential in multiple studies, where various possible entry routes including axonal retrograde, transsynaptic, hematogenous, through angiotensin-converting enzyme (ACE) 2 receptors localized on the spinal roots have been identified [7]. However, generally, it is not the direct viral effects that are responsible for neurological complications as a minority of autopsies and cerebrospinal fluid (CSF) polymerase chain reaction (PCR) have detected the presence of virus in CNS, but indirect effects of SARS-CoV-2 such as systemic inflammation and hypoxia, blood-brain barrier damage, dysregulated immune response leading to the brain damage by proinflammatory molecules or autoantibodies.
The most frequently reported immune-mediated CNS diseases are transverse myelitis, acute disseminated encephalomyelitis, aquaporin-4 (AQP4), and myelin oligodendrocyte glycoprotein (MOG)-associated disorders. From a pathogenetic viewpoint, these complications could be linked with either direct virus effects (acute transverse myelitis, polio-like myelitis), para- or post-infectious immune-mediated mechanisms [transverse myelitis, longitudinal extensive transverse myelitis (LETM), myelitis as a presentation of MOG or AQP4-associated disorders] or effects stemming from systemic complications of COVID-19 (hypoxic myelopathy, subacute combined degeneration, and vascular pathology with the formation of spinal cord infarction). The rate of immune-mediated isolated transverse myelitis, according to a review by Garg et al. [8], accounts for the largest number of cases—18 patients out of 33 (54.5%).
The leading role in neuroinflammation refers to the increased cytokine production, activation of microglia, and following tissue damage. Various viral infections could activate inflammatory endothelial and prothrombotic pathways leading to cytokines release. Numerous studies have investigated the profile of cytokines (pro-inflammatory and anti-inflammatory) in the CSF of patients with various neuropathologies following COVID-19, as they have also explored the correlation between cytokine levels and the severity of the disease, however, the acquired results have only outlined a diversity of cytokine changes.
Regarding cytokine changes in CSF, the results were highly variable. Thus, in the study of Espíndola et al. [9], patients with encephalopathy demonstrated an increased concentration of interleukin-2 (IL‐2) and C-X-C motif chemokine ligand 8 (CXCL8), while patients with inflammatory neurological diseases, including acute disseminated encephalomyelitis, encephalitis, meningitis, meningoencephalitis, acute myelitis, and neuromyelitis optica, also showed elevated levels of IL‐2, IL‐4, IL‐6, IL‐10, IL‐12, CXCL8, and CXCL10 compared to healthy controls (HC) [9]. In a multicenter study of Jarius et al. [10], 127 patients showed an increase in IL-6, IL-8, and in some cases tumor necrosis factor-alpha (TNF-α). In addition, during the CSF follow-up in 16 days, IL-6 and IL-8 were also still elevated after neurological COVID-19 onset [10]. At the same time, studies aimed to compare CSF cytokine profiles in COVID-19 patients with other acute infectious or autoimmune neuroinflammatory pathologies haven’t found meaningful neuroinflammatory changes. For example, the research of Garcia et al. [11] intended to compare patients after COVID-19 with headache, encephalopathy, and stroke with infectious, neuroinflammatory, and stroke controls and didn’t find any significant difference between corresponding groups [11]. And finally, according to the study of Bernard-Valnet et al. [12], cytokine profiles in patients with neurological complications after SARS-CoV-2 (encephalopathy, encephalitis, myelitis, optic neuritis, Guillain-Barré syndrome, etc.) were comparable to cytokine profiles seen in patients with multiple sclerosis (MS) [with an elevation of CXCL10/IP-10 (IFN-γ-inducible protein 10), CXCL12, CXCL13, and granulocyte colony-stimulating factor (G-CSF)], but not with CNS inflammatory diseases. Only in severe COVID-19 patients, a significant increase in CXCL8/IL-8 comparable with patients with inflammatory diseases was observed [12].
The clinical picture of post-infectious acute transverse myelitis associated with COVID-19 does not significantly differ from myelitis developing after other viral diseases (caused by varicella-zoster, herpes simplex, Epstein-Barr, etc.). Myelitis is generally characterized by acute or subacute development of motor, sensory, and/or autonomic dysfunction [13]. Motor signs are usually characterized by flaccid paraparesis turning into spastic if caused by white matter damage, sensory impairment by well-defined sensory level and could be accompanied by pain, dysesthesias/paresthesias. Spinal magnetic resonance imaging (MRI) shows a centrally located thoracic-two (T2) hyperintense lesion, which occupies about 2/3 of the diameter of the spinal cord [14]. Besides classical transverse myelitis, myelitis with tract-specific involvement of the lateral and/or dorsal columns was described in the literature [15]. Clinically this pathology is characterized by the subacute development of progressive muscle weakness, sensitive ataxia, and paresthesias, as well as the later onset of spasticity and pelvic organ dysfunction. Similar clinical and radiological pictures could be observed in subacute combined degeneration associated primarily with vitamin B12 deficiency, and also, in rare cases, with the deficiency of vitamin E, folic acid or copper. However, in these patients, the concentration of vitamins of groups B, E, and copper in the blood serum stayed within the reference values. The exact role of SARS-CoV-2 in the development of this type of myelitis remains unclear. It is assumed that SARS-CoV-2 can indirectly affect the methylation cycle, where the methyl group is transferred from methyltetrahydrofolate to various macromolecules, including myelin proteins, which ensures their stability [15].
Based on the MRI findings described in the literature, myelitis associated with COVID-19 typically extends more than four segments long and mainly localizes in the cervicothoracic region. Lesions from one to three segments long are described only in 30% of cases [8, 16]. Identified extended transverse myelitis requires differential diagnosis with AQP4 and MOG-associated diseases, which have a relapsing course in more than 50% of cases and consequently require an additional prolonged immunosuppressive therapy [17, 18].
The current study aimed to describe various types of myelitis associated with a SARS-CoV-2 infection as well as to analyze the cytokine profiles and CSF parameters in affected patients and to compare them with patients with other immune-mediated disorder—MS, to identify possible common pathogenic pathways and consequently treatment targets to prevent the exacerbation of neurological diseases triggered by viral infections.
Materials and methods
Study design
CSF from patients with myelitis associated with COVID-19 was compared to CSF of HC and patients with MS from the non-COVID era to find any similarities or differences in pathogenic pathways. A group of MS patients was chosen as a control because of its relatively similar pathogenesis with transverse myelitis and because of its more frequent occurrence in our hospital. The CSF control samples were collected from the before-COVID-19 pandemic era, and the samples were stored in the biorepository of the Research Center of Neurology in Moscow. CSF control samples included: 1) HC [patients who underwent diagnostic lumbar puncture for suspected ALS but were diagnosed with benign fasciculation syndrome or spondylogenic radiculopathy (n = 7)]; 2) patients with an established diagnosis of MS in accordance with the 2017 McDonald Criteria (n = 37).
Myelitis group
In total 11 patients with various immune-mediated spinal cord pathologies developed within 3 months after COVID-19 treated at the Research Center of Neurology in Moscow from September 2020 to December 2022 were enrolled in the study. The eligibility criteria included a confirmed diagnosis of myelitis based on the clinical and radiological data, and evidence of coronavirus infection confirmed by PCR testing no more than 3 months before the development of symptoms of myelitis. Exclusion criteria from the study were a history of chronic autoimmune or immune-mediated CNS disease (MS, neuromyelitis optica spectrum disorders, and autoimmune encephalitis), identification of an alternative non-inflammatory cause of spinal cord injury (compression myelopathy, ischemic spinal cord injury, and subacute combined degeneration of the spinal cord caused by B12 deficiency, etc.). All patients were screened for systemic antibodies (Abs) [antinuclear, beta2-glycoprotein I (beta2GPI), anti-DNA, anticardiolipin, anti-Smith, and antineutrophil cytoplasmic], antineuronal Abs (Hu, Yo-1, CV2, Ma2, Ri, amphiphysin), serum ACE, level of vitamins (B12, B1, B6, vitamin E), serum copper, iron and zinc levels, presence of MOG Abs (cell-based immunofluorescence assay), and AQP4 Ab (cell-based immunofluorescence assay). In CSF, specific Abs to herpes simplex, Varicella-Zoster, Epstein-Barr virus, and Abs to Borrelia burgdorferi were examined. Only after the subsequent exclusion of viral, bacterial, nutritional, or systemic genesis of spinal cord injury, the main group was formed.
The main group included 11 patients (6 females), and the mean age at diagnosis was 45.8 ± 2.24 years (age ranges from 25–64 years). The onset of myelitis symptoms was noted after 3.72 ± 0.19 weeks (time ranges from 2–5 weeks). The severity of the coronavirus infection varied from mild (n = 9) to moderate (n = 2). The severity of the infection was assessed depending on the duration of the COVID-19 infection and the need for hospitalization. One of the patients referred to the moderate form had subfebrile temperature (37.6°C) for a month after positive PCR SARS-CoV-2, and the second one required hospitalization due to the pneumonia development and saturation drop. The mean time of lumbar puncture after the symptoms of myelitis onset was 2.45 ± 0.32 weeks. The clinically and radiologically heterogeneous group was deliberately chosen for this study to provide a better overview of differences in cytokine profiles in the myelitis group in comparison with MS patients and HC.
Control groups
The MS group included 37 patients (17 females) with a mean age of 27.2 ± 2.7 years. Three patients (3/37) were for the first time diagnosed with MS onset, four patients (4/37) with secondary progressive, and 30 (30/37) with relapsing-remitting form. HC included 7 patients (2 females) with a mean age of 34.5 ± 4.9 years.
Laboratory studies
There are 3 types of laboratory studies.
1. Cytological examination, CSF total protein, and oligoclonal bands (OCBs): CSF pleocytosis was defined as > 5/mm3. The upper reference limit for CSF total protein was set at 0.45 mg/L. OCBs were assessed with isoelectrofocusing.
2. SARS-CoV2 virus detection in CSF: SARS-CoV2 RNA detection in CSF was performed by reverse transcription PCR (RT-PCR).
3. Cytokine profiling: In total 37 key biomarkers of inflammation from the TNF superfamily proteins, interferon (IFN) family proteins, regulatory T-cell (Treg) cytokines, and matrix metalloproteinases (MMPs) were assessed in CSF using a 37-plex Bio-Plex Pro Human Inflammation Kit (Bio-Rad Laboratories, USA) according to the manufacturer’s instructions. The panel included the following biomarkers: APRIL, B cell activating factor (BAFF), sCD30, sCD163, Chitinase-3-like 1, gp130, IFN-α2, IFN-β, IFN-γ, IL-2, sIL-6Rα, IL-8, IL-10, IL-11, IL-12 (p40), IL-12 (p70), IL-19, IL-20, IL-22, IL-26, IL-27 (p28), IL-28A, IL-29, IL-32, IL-34, IL-35, LIGHT (tumor necrosis factor superfamily member 14), MMP-1, MMP-2, MMP-3, Osteocalcin, Osteopontin, Pentraxin-3, sTNF-R1, sTNF-R2, thymic stromal lymphopoietin (TSLP), and tumor necrosis factor-like weak inducer of apoptosis (TWEAK).
Statistical analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences Statistics software (version 26.0). Scale variables were described as median, 25 and 75 percentiles. After normality testing with the Shapiro-Wilk method, scale variables were compared between three independent groups. Variables with normal distribution were compared using a one-way analysis of variance (ANOVA) with Tukey’s post hoc. Variables with non-normal distribution were compared using the Kruskal–Wallis H-test with Dunn’s post hoc. The level of significance was set at P < 0.05. In the case of post hoc analysis, the level of adjusted significance was set at Padj < 0.05.
Note: Results of IFN-γ, IL-2, IL-12(p40), IL-12(p70), IL-20, IL-22, IL-27(p28), IL-28A IFN-lambda2, IL-29 IFN-lambda1, IL-34, IL-35, LIGHT, and MMP-1 should be interpreted with caution, because the level of cytokine was out of definition range in many participants. These cytokines wouldn’t be included in the results and discussion sections.
Results
Patients’ clinical characteristics
The clinical picture of myelitis varies depending on the lesion localization and size. Patients with short transverse myelitis (lesion of less than 3 segments) mostly had mild or moderate motor and sensory impairment. Patients with an extended lesion, as well as patients with tract-specific lesions, had pronounced motor, sensory, and pelvic dysfunction. The age and sex distribution, date of SARS-CoV-2 positive swab, date of first symptoms onset, and MRI and CSF features for all myelitis patients were described in Table 1.
Clinical, radiological, and laboratory characteristics of 11 patients with myelitis following SARS-CoV-2 infection
| Case | Diagnosis | Symptoms | Time from infection to symptoms onset | MRI | Lumbar puncture | Treatment | Response | 1-year follow-up |
|---|---|---|---|---|---|---|---|---|
Case 1 (F, 25) | Myelitis involving posterior and lateral columns (cervical and thoracic) | Legs weakness (2/5 MRC), urinary urgency, voiding difficulty, and bowel constipation | 3 weeks | Posterior and lateral column damage at the cervical and thoracic levels | Cytosis 10 cells/mm3 Protein 0.213 g/L OCB type 1 | PLEX, IVMP Vitamin B12 | None (sent for rehabilitation) | Legs weakness (3/5 MRC), bladder and bowel symptoms persist |
Case 2 (F, 55) | Myelitis (С1) | Hands weakness (3/5 MRC) and neck numbness | 5 weeks | Lesion at the С1 | Cytosis 1 cell/mm3 Protein 0.287 g/L OCB type 2 | IVMP | Residual sensory disturbances | Total recovery |
Case 3 (M, 49) | Myelitis С2-С4 | Arms weakness (4/5 MRC), neck weakness, and Lhermitte sign | 4 weeks | Lesion at the С2-С4 | Cytosis 7 cells/mm3 Protein 0.662 g/L OCB type 1 | IVMP | Residual sensory disturbances | Residual sensory disturbances Radiologically stable |
Case 4 (M, 34) | Myelitis С3-С4 | Hands clumsiness, Lhermitte sign, and bilateral numbness up to shoulder | 4 weeks | Lesion at the С3-С4 | Cytosis 4 cells/mm3 Protein 0.282 g/L OCB type 1 | IVMP | Residual sensory disturbances Lhermitte sign persists | NA |
Case 5 (F, 42) | Myelitis С6 | Left arm weakness (3/5 MRC), legs weakness (4/5 MRC), left-sided numbness, Lhermitte sign | 3 weeks | Lesion at the С6 | Cytosis 2 cells/mm3 Protein 0.251 g/L OCB type 3 | IVMP | Residual sensory disturbances Lhermitte sign persists | Total recovery |
Case 6 (F, 52) | Myelitis С6-С7 | Right arm numbness, right arm burning, and Lhermitte sign | 5 weeks | Lesion at the С6-С7 | Cytosis 7 cells/mm3 Protein 0.513 g/L OCB type 1 | IVMP | Residual sensory disturbances | Total recovery |
Case 7 (M, 48) | LETM | Legs weakness (1/5 MRC), urinary urgency, voiding difficulty, and bilateral numbness up to abdomen | 3 weeks | Longitudinal lesion at the T4-T11 | Cytosis 1 cell/mm3 Protein 0.391 g/L OCB type 2 | PLEX IVMP | Residual sensory disturbances and legs weakness (3/5 MRC) | Lower limbs weakness (4/5 MRC) Radiologically stable |
Case 8 (F, 49) | MOG-Abs associated LETM | Legs weakness (4/5 MRC) and legs numbness | 5 weeks | Longitudinal lesion at the T5-T8 | Cytosis 1 cell/mm3 Protein 0.391 g/L OCB type 2 | IVMP | Residual sensory disturbances | Placed on Rituximab therapy Clinically and radiologically stable |
Case 9 (M, 49) | Multifocal myelitis (cervical) | Left arm numbness, left arm pain, and Lhermitte sign | 5 weeks | Multiple lesions at the С2-3, С4, and С5-6 | Cytosis 7 cells/mm3 Protein 0.347 g/L OCB type 2 | IVMP | Residual sensory disturbances | NA |
Case 10 (M, 37) | Myelitis involving posterior and lateral columns | Legs weakness (1/5 MRC) and legs paresthesia | 2 weeks | Posterior and lateral column damage throughout the spinal cord | Cytosis 10 cells/mm3 Protein 0.360 g/L OCB type 1 | PLEX IVMP Vitamin B12 | None (sent for rehabilitation) | Legs weakness (3/5 MRC) and less bladder and bowel symptoms |
Case 11 (F, 64) | Myelitis T4-T5 | Right leg weakness (3/5 MRC), right-sided numbness and pain, urinary urgency and voiding difficulty | 2 weeks | Lesion at the T4-T5 | Cytosis 2 cells/mm3 Protein 0.277 g/L OCB type 1 | IVMP | Residual sensory disturbances, right leg weakness (4/5 MRC), and persistent urinary urgency | NA |
F: female; M: male; С1: level of the 1st cervical vertebra; MRC: Medical Research Council; PLEX: plasma exchange; IVMP: intravenous methylprednisolone; NA: not available
Patients’ and controls’ laboratory characteristics
All patients underwent screening for serum systemic, paraneoplastic Abs, MOG, and AQP-4 Abs. In one patient with myelitis, MOG Abs were detected. CSF pleocytosis was observed in 5/11 patients (up to 10 cells/mm3), and increased protein in 2/11 patients (up to 0.662 g/L). OCBs type 2 were found in 4/11 cases, type 3 in 1/11 case, and polyclonal synthesis in 6/11 cases. SARS-CoV-2 CSF PCR was negative in all patients with myelitis. In MS patients, pleocytosis was seen in 10/37 patients (up to 32 cells/mm3), and OCBs type 2 were found in 31/37 patients.
Patients’ radiological characteristics
Radiological findings were diverse: short transverse myelitis (lesion of less than 3 segments) (n = 6), LETM (n = 2), multifocal spinal cord lesions (n = 1), and myelitis involving dorsal and lateral columns (n = 2). The spinal cord MRI of Patients 5 (Figure 1) and 10 (Figure 2) are presented below.
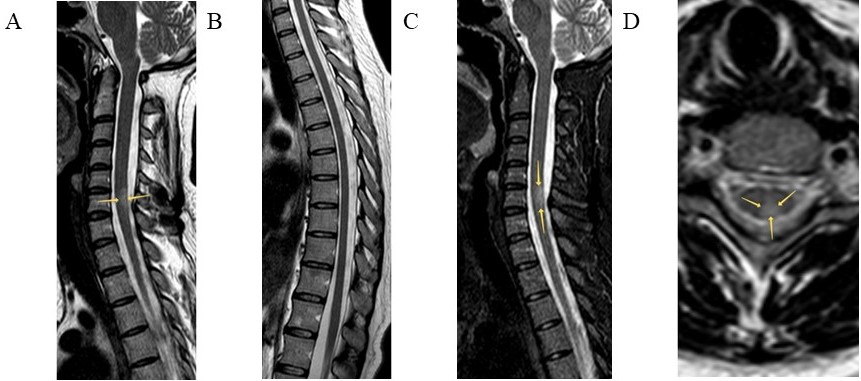
Spinal cord MRI of Patient 5. Sagittal T2 (A, B) and T2 weighted short tau inversion recovery (T2-STIR) (C) modes and axial T2 mode (D) are presented. Intramedullary, in the central and posterior regions of the spinal cord at the C6 level, a hyperintense lesion is visualized (arrows). Throughout the rest length, including the thoracic level, the spinal cord is intact, with homogeneous intensity of the magnetic resonance signal.
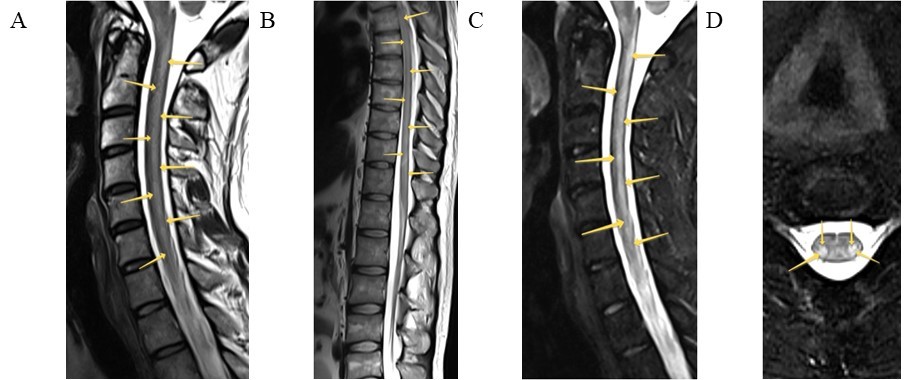
Spinal cord MRI of Patient 10. Sagittal T2 (A, B) and T2-STIR (C), axial T2 with fat suppression (D) modes are presented. Intramedullary, in the lateral and posterior columns of the spinal cord at the cervical and thoracic levels, symmetrical extended zones are detected (arrows)
Treatment and response
IVMP with an average daily dose of 1,000 mg from 3 days to 5 days (up to a maximal total dosage of 7,000 mg) was administered to all patients. Three patients (Patients 1, 7, and 10) also underwent high-volume PLEX due to the severity of neurological symptoms. Two patients (Patients 1 and 10) with myelitis involving lateral and posterior columns additionally received vitamin B12 injections despite its normal values during lab tests.
The most pronounced therapy response was observed in patients with partial transverse myelitis and with anti-MOG Ab. Patient 7 with LETM showed incomplete recovery reaching MRC 3/5 in lower limbs. Patients with tract-specific myelitis did not have any notable symptom improvement after PLEX and IVMP. They were recommended for rehabilitation treatment and after 3–4 months, marked amelioration was noted. One year follow-up was available for 8 patients. Detailed information is presented in Table 1.
Cytokine profiles
Compared to HC, patients with COVID-19 myelitis (CM) and MS had significantly higher levels of gp130 sIL-6Rb and osteopontin, significantly lower levels of sCD30, IFN-β, IL-8, IL-10, IL-11, IL-26, IL-32, MMP-3, osteocalcin, pentraxin-3, sTNF-R2, TSLP, and TWEAK. Besides that, patients with CM had significantly higher levels of APRIL than healthy participants, whereas the same trend for the MS group was not statistically significant. Patients with MS had significantly higher levels of sCD163, chitinase 3-like 1, and IL-6Ra; and significantly lower levels of IL-19 and MMP-2 than healthy participants, whereas the same trend for the CM group was not statistically significant.
Compared to participants with MS, patients with CM had significantly lower levels of IL-10 (P = 0.037). A trend towards significance was shown for the cytokines IFN-α2, IFN-β, TSLP (decreased in the myelitis group), and IL-19 (increased in the myelitis group). For the cytokine BAFF P-value for the three groups comparison was close to the threshold, so an additional comparison between CM and MS was carried out using a Student’s t-test. It showed a significantly higher level of BAFF in the myelitis group (P = 0.013).
Descriptive statistics are presented in Table S1, and P-values for all comparisons are presented in Table S2.
Discussion
Myelitis after coronavirus infection is clinically and radiologically heterogeneous. This study aimed both to describe the different phenotypes of immune-mediated spinal cord injury in patients after the infection and to analyze cytokine profiles and compare them to MS patients to determine common or different pathogenic mechanisms. Similar studies for other pathologies have already been performed, for example, the comparison of patients with headache, encephalopathy, and stroke after coronavirus infection, with corresponding controls, but no significant differences were found [11]. However, current study has demonstrated that patients with myelitis after COVID-19 have their distinct cytokines characteristics.
The mean age of the patients was 45.8 ± 2.24 years, which is higher than reported in the literature (30–39 years) [19], and no sex-related differences were found. According to radiological data, short transverse myelitis (n = 6) localized in the cervical region (n = 5) was more common in this group, though based on published studies, post-covid myelitis in 70% of cases is characterized by an extended longitudinal lesion [16]. Patients with myelitis involving lateral and posterior cords represent distinct and most interesting cases. Huang et al. [15] described five cases of similar spinal cord injury after COVID-19. Clinically, patients predominantly presented with a decreased deep sensitivity (3/5), motor (3/5), and pelvic dysfunction (2/5) [15]. Patients were treated with PLEX in combination with immunoglobulins or IVMP, or IVMP alone, and yet in two cases out of five, therapy did not lead to clinical improvement, which is generally consistent with current clinical observations.
CSF analysis didn’t reveal any signs of meaningful neuroinflammatory changes such as increased cytosis or protein level. OCBs were detected in 4/11 cases, which is consistent with previous findings, where OCBs are detected in an average of 38% of myelitis patients [20]. None of the patients with myelitis had SARS-CoV-2 RNA detected in the CSF. Thus, the most likely mechanism for the development of spinal cord injury is an immune-mediated pathway.
Analysis of CNS cytokine profile of patients with myelitis after SARS-CoV-2 and its following comparison with control groups is complicated, since the cytokine profiles are affected by both SARS-CoV-2 infection and CNS neuroinflammation. In this regard, clinically and radiologically heterogeneous groups of myelitis patients have been deliberately chosen for this study in order to visualize only clear differences. So, multiple comparisons have demonstrated the relative similarity of CSF cytokine profiles of myelitis and patients with MS, and the two groups differed only in IL-10 level. Compared to HC in myelitis and MS groups, increased levels of gp130 sIL-6Rb and osteopontin, and decreased levels of sCD30, IFN-β, IL-8, IL-10, IL-11, IL-26, IL-32, MMP-3, Pentraxin-3, osteocalcin, sTNF-R2, TSLP, and TWEAK were found.
These findings are in line with a previous report where decreased levels of IL-8 during the disease onset and decreased levels of anti-inflammatory cytokine (IL-10, IL-19, IL-26) during an exacerbation for MS patients were observed [21, 22]. Increased concentrations of gp130 sIL-6Rb and osteopontin are typical for MS and are considered markers of disease severity and progression [23, 24]. Similar cytokine profiles for the myelitis group only support the immune-mediated mechanisms underlying the development of the disease.
APRIL and BAFF upregulation, as well as IL-10 downregulation in myelitis patients compared to MS and HC, seems interesting for a closer look (Figure 3).
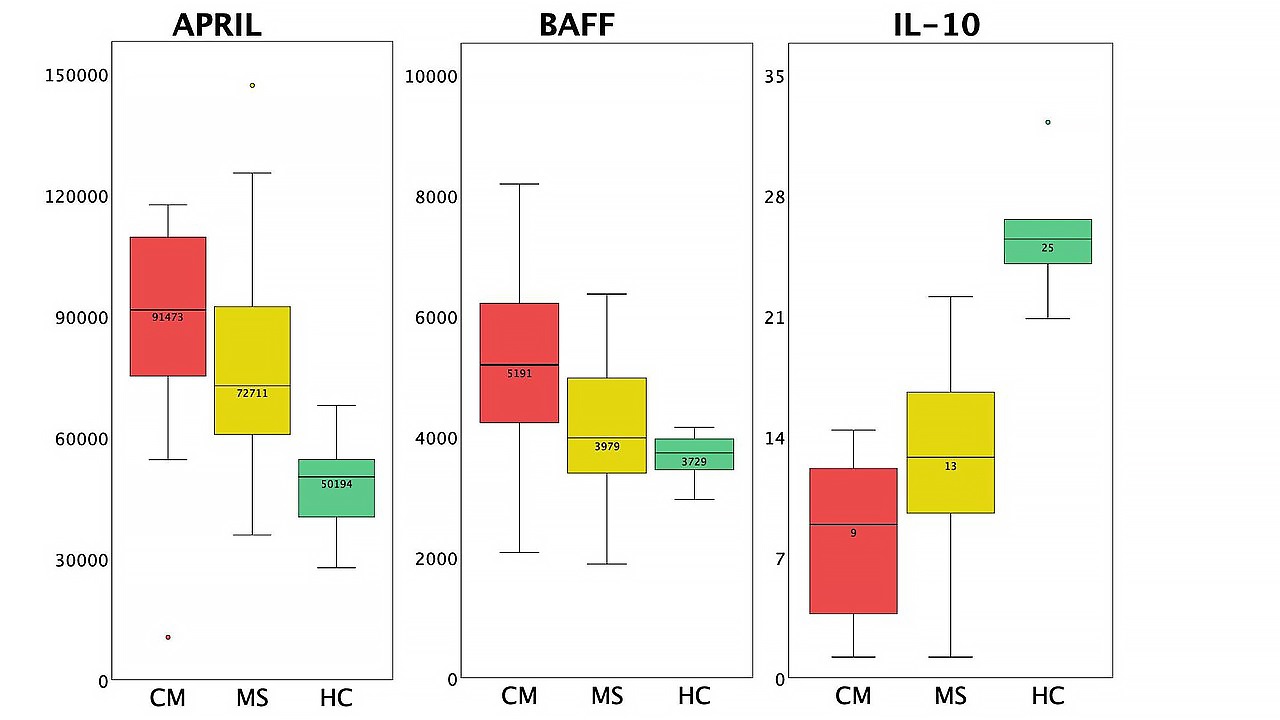
Differences in cytokine profiles between patients with CM, patients with MS, and HC (box plots). The circles standard for outliers.
BAFF (B cell activating factor) and its homologue APRIL are known to play a key role in autoimmunity development, as they are responsible for B cell survival and selection as well as dendritic cell maturation and IL-6 release [25, 26]. Increased CSF BAFF and CSF APRIL were associated with increased disease disability in both neuromyelitis optica spectrum disorders and MS as well as high EDSS scores in neuromyelitis optica [27]. High serum APRIL activity has been described in ICU COVID-19 patients, and BAFF in non-ICU patients [28], however, its increase in CSF has not been widely reported. The increase of APRIL and BAFF in the CSF of patients with myelitis may be due to both previous coronavirus infection and the presence of an autoimmune disease. It remains unclear whether an increase in BAFF and APRIL during COVID-19 has an impact on the subsequent development of an autoimmune lesion.
Interestingly, the highest BAFF and APRIL values were observed in patients with tract-specific myelitis. Considering the similarity of the clinical and radiological picture with subacute combined degeneration, the influence of the SARS-CoV-2 virus on the methylation cycle was first assumed to be the cause [15], however, given the pronounced increase in pro-inflammatory B lymphocyte-dependent cytokines, the autoimmune genesis of the lesion may predominate in these patients.
In turn, IL-10 has shown both pro-inflammatory properties, such as inducing B cell differentiation, enhancing B cell proliferation and antibody production, and anti-inflammatory properties such as down-regulating the expression of T helper 1 (Th1) cytokines. The effect of IL-10 on B-lymphocytes, as well as its influence on the balance of Th1 versus Th2 cytokines, is apparently of great importance to the development and progression of autoimmune diseases [29–31]. In MS patients, a high level of IL-10 in the CNS is one of the factors required for disease remission and its decrease could serve as a predictor of an early exacerbation [21, 32]. Considering the results of multiple studies showing an increased IL-10 level in the CSF during COVID-19, its pronounced decrease in this case may be a consequence of infection, however, the possible influence of this drop on the development of immune-mediated spinal cord injury remains to be studied.
Regarding the prognosis of patients with myelitis after COVID-19, based on such cohort it could be seen that the outcome differs within patients depending on the lesion location and its size as well as on the presence of specific Abs (anti-MOG in this group). The best recovery rate was noted for patients with short transverse myelitis, while the worst was seen in patients with tract-specific myelitis where higher values of BAFF and APRIL in CSF were found.
Further studies probably on more homogenous groups are needed to confirm the role of BAFF/APRIL and IL-10 in the development of myelitis after SARS-CoV-2 infection and subsequently justify the possible use of target therapy in these patients such as BAFF-neutralizing therapies.
In conclusion, novel coronavirus infection is a complex multisystemic disorder that could cause various types of neurological dysfunction acting through different mechanisms. The ability of SARS-CoV-2 to cause various immune-mediated nervous system lesions is supported by multiple studies, including data obtained on the neurotropic, neuroinvasive properties of SARS-CoV-2 as well as on mechanisms of initiation/development of the immune response in COVID-19. The current study has demonstrated the clinical and radiological diversity of immune-mediated spinal cord lesions in patients after SARS-CoV-2. However, even for this highly heterogeneous group, similar patterns of cytokine changes were found. In particular, evaluation of CSF cytokine profiles in patients with myelitis associated with COVID-19 revealed their relative similarity with the ones in MS patients, except for a few cytokines including BAFF, proinflammatory B lymphocyte dependent molecules, and IL-10—cytokine known for its potent anti-inflammatory effect, all of which are well-known for their role in development and progression of autoimmune diseases. The effect of BAFF and APRIL upregulation and IL-10 downregulation after COVID-19 on the development of immune-mediated CNS disorders remains to be further studied.
Abbreviations
| Abs: |
antibodies |
| APRIL: |
a proliferation-inducing ligand |
| AQP4: |
aquaporin-4 |
| BAFF: |
B cell activating factor |
| С1: |
level of the 1st cervical vertebra |
| CM: |
coronavirus disease 2019 myelitis |
| CNS: |
central nervous system |
| COVID-19: |
coronavirus disease 2019 |
| CSF: |
cerebrospinal fluid |
| CXCL8: |
C-X-C motif chemokine ligand 8 |
| HC: |
healthy controls |
| IFN: |
interferon |
| IL-10: |
interleukin-10 |
| IVMP: |
intravenous methylprednisolone |
| LETM: |
longitudinal extensive transverse myelitis |
| MMPs: |
matrix metalloproteinases |
| MOG: |
myelin oligodendrocyte glycoprotein |
| MRI: |
magnetic resonance imaging |
| MS: |
multiple sclerosis |
| OCBs: |
oligoclonal bands |
| PCR: |
polymerase chain reaction |
| PLEX: |
plasma exchange |
| SARS-CoV-2: |
severe acute respiratory syndrome coronavirus 2 |
| T2: |
thoracic-two |
| Th1: |
T helper 1 |
| TNF-α: |
tumor necrosis factor-alpha |
| TSLP: |
thymic stromal lymphopoietin |
| TWEAK: |
tumor necrosis factor-like weak inducer of apoptosis |
Supplementary materials
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1003132_sup.pdf.
Declarations
Author contributions
AK: Conceptualization, Investigation, Formal analysis, Writing—original draft. AD: Resources, Conceptualization, Investigation. AT: Resources, Supervision, Writing—review & editing. IZ, TS, LA, DE, NS, and IK: Resources. EB: Supervision. MZ: Validation, Supervision, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The current study was approved by the ethical committee of the Research Center of Neurology in Russia (1-3/23). The study complies with the Declaration of Helsinki.
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The dataset for this study can be found in the Open Science Framework at https://osf.io/4r29k/?view_only=59ab004eb92143bca0c990b96b40686b
Funding
Not applicable.
Copyright
© The Author(s) 2024.