Abstract
Keratinocytes play an integral role in the human epidermis, serving as a barrier between the internal and external environment. They are immune-competent cells involved in both innate and adaptive cutaneous immune responses, crucial for maintaining skin integrity. Keratinocytes are essential for epidermal repair, facilitating proliferation and re-epithelialization following injury. They secrete pro-inflammatory markers such as cytokines and chemokines, which promote the recruitment of inflammatory cells like polymorphs and macrophages to the site of skin injury. The immune response mediated by keratinocytes involves signaling molecules like tumor necrosis factor (TNF), interleukin (IL)-1β, and IL-6. Langerhans cells respond to factors secreted by keratinocytes, migrating towards draining lymph nodes to activate T cells and initiate an adaptive immune response. Additionally, keratinocytes express Toll-like receptors (TLRs), enabling them to detect molecular patterns of pathogens. Recent studies have focused on understanding these interactions of keratinocytes to develop therapeutic strategies for managing various skin diseases. Genetic defects in keratinocytes underlie conditions like psoriasis. We also discuss the role of keratinocytes and the effect of neuro-endocrinal signaling and interventions, associated corticosteroidogenic pathways, and response to UV radiations to maintain a state of homeostasis. This article underlines and improves our understanding of the immune function of keratinocytes, which is crucial for developing more effective therapies against skin diseases.
Keywords
Keratinocyte, immunology, skin diseases, dermatologyIntroduction
The skin is the largest organ of the human body, serving as a protective barrier between the internal organs and the external environment. Comprising three primary layers—the epidermis, dermis, and hypodermis. It performs a range of crucial functions including protection against physical, chemical, and microbial insults, regulating body temperature, touch sensation, and synthesis of various protective agents such as sebum, anti-microbial peptides, vitamin D, and melanin [1]. As the main barrier between the internal and exterior environments in the complex environment of the human body, the skin is vulnerable to a wide range of assaults, from physical injury to microbial invasion. Thus, the skin’s immune system coordinates a complex defensive mechanism that is essential for maintaining skin integrity, homeostasis, and systemic immunity [2].
Cutaneous immune responses are important for more than just local defense; new research highlights their critical function in several physiological functions and diseases [3]. These research works indicate that the cutaneous immune system acts as a sentinel for systemic immunity, helping to shape distant immunological responses and contribute to the pathophysiology of systemic illnesses [4]. Understanding the molecular details behind skin-immune interactions is clinically relevant, as it explains the pathophysiology of a number of dermatological illnesses, autoimmune ailments, and even cancers. These disorders are all caused by dysregulation of cutaneous immune responses [5]. Given these diverse roles, it becomes imperative to clarify the vital part keratinocytes play in cutaneous immune responses [6]. Keratinocytes are the first line of defense against pathogens and environmental toxins because they are sentinel cells that live at the interface between the host and the external environment. They also actively modulate immune responses to maintain tissue integrity and homeostasis [7]. Comprehensive research on the interaction between keratinocytes and immune cells may provide new understandings of the etiology of skin conditions and lead to the development of focused therapy strategies that support skin health by re-establishing immunological homeostasis.
Keratinocytes make up over 90% of epidermal cells and are the main constituents of the epidermis, the skin’s outermost layer. Because they create a stratified epithelium that acts as a physical barrier against external assaults including infections, toxins, and UV radiation, keratinocytes are essential to the integrity and function of skin (Figure 1) [8]. Additionally, keratinocytes take an active role in cutaneous immune responses, serving as sentinels that recognize and react to tissue injury and microbial invasion [1]. They produce antimicrobial peptides (AMPs), cytokines, and chemokines in response to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which aid in the development of innate immunity. Furthermore, by acting as antigen-presenting cells (APCs) and promoting T cell activation and differentiation in the skin, keratinocytes are essential for adaptive immunity [9]. Determining the pathophysiology of different skin diseases and creating focused treatment strategies require an understanding of the complex roles that keratinocytes play in cutaneous immune responses. Some of these functions relevant to the scope of this review are discussed below.
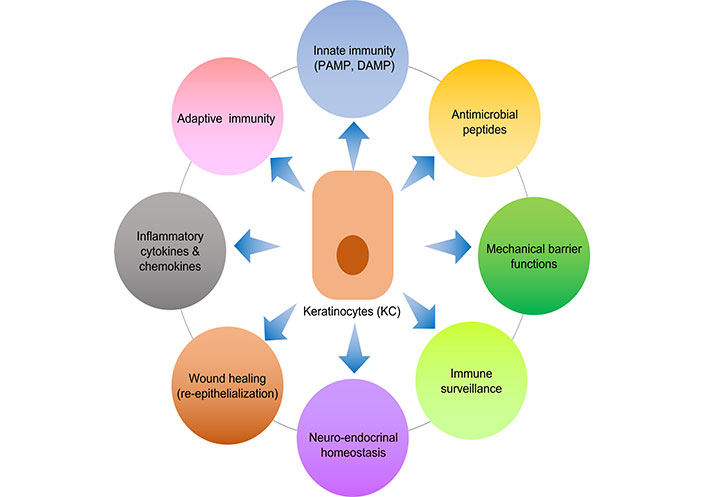
An illustration of various immune related functions of keratinocytes. PAMP: pathogen-associated molecular pattern; DAMP: damage-associated molecular pattern
Keratinocytes facilitate barrier and immune functions
The unique architectural arrangement of keratinocytes is essential to their function in preserving the integrity of the skin. These cuboidal-shaped cells, which have a strong proliferation capacity, are derived from the basal layer of the epidermis [10]. Keratinocytes travel upward through the epidermal layers during development, flattening and obtaining specific cytoskeletal proteins like keratins along the way [1, 6]. They go through final differentiation in the suprabasal layers, where they become densely packed, enucleated squamous cells that are abundant in cytokeratin filaments [11]. The creation of desmosomal connections between neighboring cells and the stratified arrangement of cells endow the epidermis with strength and resilience, which is essential for its barrier function against environmental assaults and microbial invasion [12]. Furthermore, keratinocytes collaborate with nearby cells like Langerhans cells and melanocytes to form intricate cellular networks that are necessary for immunological monitoring and cutaneous homeostasis, as discussed later in this review.
Skin keratinocytes play essential functions in both innate immunity and adaptive immunity, supporting tissue integrity preservation and pathogen protection. Keratinocytes secrete ceramides, cholesterol, and fatty acids into the extracellular space, where they intercalate between keratinocyte layers to form lipid-rich lamellar bodies [13]. By acting as a hydrophobic barrier, this lipid matrix lowers water loss and stops toxic chemicals from penetrating. In addition, keratinocytes produce a range of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), which elicit inflammation and innate immune responses upon detection of conserved microbial structures [6]. These PRRs sense the PAMP in the early phase of the innate immune response. PAMPs are unique for different pathogens that are viral nucleic acid, lipopolysaccharide, lipoteichoic acid (LTA), peptidoglycan (PGN), lipoproteins, flagellin, etc. PRRs are either membrane or intracellular receptors which include TLRs, Dectin-1, nucleotide binding oligomerization domain (NOD)-like receptors (NLRs), and retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs). TLRs and NLRs show synergistic effects for the pathogen recognition process [5, 9]. Among all PRRs, TLRs are well studied and have a very important role against pathogens (Figure 2). This receptor activation leads to the secretion of cytokines, chemokines, and the production of AMPs [14]. As a result of these reactions, the epidermal barrier is strengthened by enhanced keratinocyte differentiation and proliferation as well as the synthesis of cytokines and AMPs [9, 14].
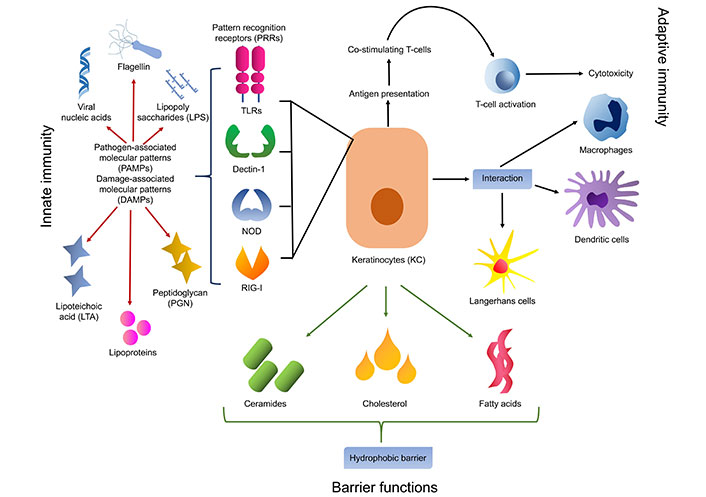
Keratinocyte involved in the innate and adaptive immune functions along with barrier functions of the epithelium. TLRs: Toll-like receptors; NOD: nucleotide binding oligomerization domain; RIG-I: retinoic acid-inducible gene-I
TLRs: sensor for microbial invasion
Both innate and adaptive immunity depend heavily on TLRs, which are specific PRRs that identify PAMPs exhibited by various microbes and pathogens. TLRs 1 to 6 and TLR9 are exhibited by keratinocytes, along with TLR7 and TLR8, which are found localized in intracellular organelles such as endosomes and lysosomes [14]. TLR 1, 2, 4, 5, and 6 are positioned on the cell membrane whereas TLR 3, 7, 8, and 9 are placed in the intracellular compartment of keratinocytes on the endosomal and lysosomal membrane [14, 15]. These TLRs except TLR3 recognize their cognitive ligand to activate the myeloid differentiation primary response 88 (MYD88) complex which is a downstream signaling cascade to commence immune response involving the production of cytokine, chemokines, and AMPs [16]. TLRs have the capacity to recognize bacterial products and set off pro-inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-8, and IL-6. TLR4 along with CD14 recognizes lipopolysaccharides of gram-negative bacteria [16, 17]. TLR5 is specifically activated by bacterial flagellin, a structural protein of bacterial flagella which helps to incite nuclear factor of κ-light chain-enhancer of activated B cells (NF-κB) translocation and IL-8 secretion [14, 18]. Intracellular TLRs like TLR3 recognize viral or bacterial broken nucleic acid (dsRNA) which has been taken into the cells and leads to activation of cytokines like interferon-B (IFN-β), TNF-α, and various ILs such as IL-8, IL-18, and IL-36γ [14, 16]. CpG-methylated DNA selectively activates TLR9 on keratinocytes and induces CXCL9, CXCL10, and type 1 IFNs [19]. TLRs also act as a negative regulator to maintain the balance of inflammation and defense process in keratinocytes, e.g., Staphylococcus epidermidis found as a normal bacterial flora, activates TLR2 to express miR-143 which in turn inhibits TLR2 expression and decreases Propionibacterium acnes induced pro-inflammatory cytokines like IL-6 and TNF-α in keratinocytes (Figure 3) [20, 21].
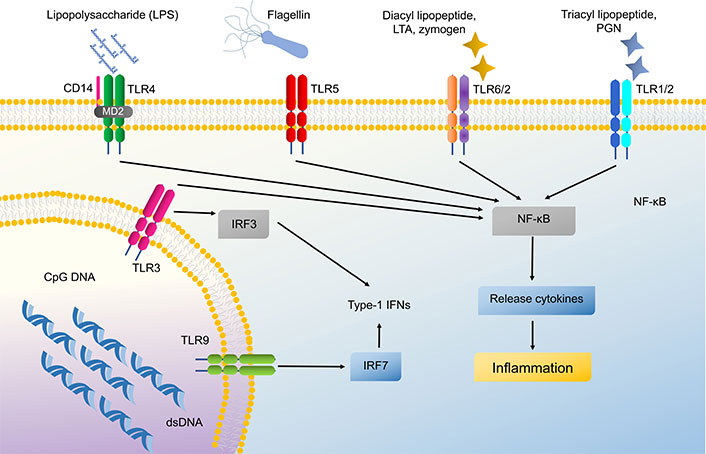
Signaling pathway of Toll-like receptors (TLRs) in keratinocytes. These TLRs can activate nuclear factor of κ-light chain-enhancer of activated B cells (NF-κB) and cause cytokine production. LTA: lipoteichoic acid; PGN: peptidoglycan; IFNs: interferons; MD2: myeloid differentiation factor 2; IRF3: IFN regulatory factor 3
Dectin-1
Dectin-1 is another PRR found in keratinocytes, though it is mainly expressed by dendritic cells, monocytes, and macrophages [22]. It is found on membranes as well as intracellularly in keratinocytes [23]. Dectin-1 acts through the β-glucan-induced MAPK pathway and IL-8 cytokine production in normal human epidermal keratinocytes, which helps in the proliferation and migration of keratinocytes thus enhancing wound healing [23, 24]. Dectin-1 is also capable of recognizing Mycobacterium ulcerans and causes the release of intracellular reactive oxygen species (ROS) like CXCL8, CCL2, and LL-37 in keratinocytes [25–27].
Nucleotide binding oligomerization domain
NLRs are divided into 3 main subfamilies NOD-containing, the NLRP, and the NLRC, which are intracellular sensors that recognize cellular damage and bacterial infection [28]. NOD-containing subfamilies are functional in human keratinocytes and have two main characteristic members which are NOD-containing proteins 1 and 2 (NOD-1 and NOD-2) [28, 29]. NOD-1 recognizes bacterial PGN while NOD-2 recognizes muramyl dipeptide, γ-D-glutamyl-meso-di-amino-pimelic acid, and PGN from gram positive bacteria [28, 30]. Literature review shows that NOD-1 recognizes Pseudomonas aeruginosa and enhances the secretion of IL-8 (CXCL8) in keratinocytes [31]. Moreover, NOD-2 also acts in defense against Staphylococcus aureus via the secretion of cytokine IL-17C, helping in decreasing the survival of S. aureus in keratinocytes. TLR2 ligand shows a synergistic effect with NOD-2 ligand to hasten the immune response [28, 31–33]. Recently NLRP3 inflammasome has been discovered which forms as a result of a complex of NLRs with caspase-1 and an adaptor protein apoptosis-associated speck-like protein containing a CARD domain (ASC) in the cytosol. NLRP3 functions as a PRR. NLRP3 inflammasome activated via dsRNA in keratinocytes with the help of dsRNA-induced protein kinase [33, 34]. Type 1 IFN is responsible for the enhancement of NLRP3 activation. NLRP3 anti-pathogen response is mediated via cleavage of caspase-1 along with the formation of IL-1β and IL-18 [28, 31–33].
RIG-I-like receptors
Keratinocytes also own cytosolic nucleic acid sensors named RLRs, which activate type 1 IFN production after binding with dsDNA or dsRNA [35, 36]. Type 1 IFN has a critical role in regulating host defense against viral infection by promoting myeloid dendritic cell maturation, T-cell proliferation, and priming of CD8+ T-cells [35, 37]. They also stimulate B-cell differentiation into antibody-secreting plasma cells. Impairment in the production of type 1 IFN can lead to psoriasis. Apart from RLRs, cGMP synthase and absent in melanoma 2 (AIM2) are other cytosolic nucleic acid sensors of keratinocytes [38]. Cyclic GMP-AMP synthase (cGAS) and cGMP activate the stimulator of IFN genes (STING) and lead to type 1 IFN production. cGMP is therapeutically used for topical treatment of wound healing which works through the building up of IFN-β in the wound sites (Figure 2) [39, 40].
When the PRR of keratinocytes gets activated in response to the pathogen, it secretes cytokines, chemokines, and thymic stromal lymphopoietin (TSLP) [41]. Pro-inflammatory cytokines are IL-1, IL-6, IL-18, TNF, and IFN, and anti-inflammatory cytokines include IL-10 [42]. These pro-inflammatory cytokines and chemokines recruit immune cells and induce AMP production [43]. IL-1β and IL-18 belong to the IL-1 family and their secretion is regulated by inflammasome and dependent on caspase-1. IL-1α is also a member of the IL-1 family, which induces calprotectin (antimicrobial protein complex), also known as S100A8/A9 over oral keratinocytes and epithelial cells [44]. IL-1α also enhances the expression of chemokines for neutrophil recruitment like CXCL1, CXCL2, and IL-8 in keratinocytes. A new cytokine IL-36 is also secreted by keratinocytes which involves in inflammation [45].
Antimicrobial peptides
AMPs play an important role in wound healing as an anti-pathogen and immunomodulatory agent. Cathelicidins and human β-defensins (HBD) are the families of AMP expressed in keratinocytes [46]. AMPs prevent bacterial infection by alteration of their structure. It may also inhibit the pathogenicity of viruses and fungi [46, 47]. HBD were found to inhibit cell surface bacteria S. aureus. Staphylococcus epidermidis, fungi like Candida albicans and Malassezia furfur induce HBD expression in keratinocytes via TLR2 signaling [48]. Cathelicidin [human cationic antimicrobial protein 18 (hCAP18)] has a synergistic function with HBD against microbes. LL-37 is the mature form of hCAP18, which is generated after cleavage of hCAP18 by proteinase 3 [49]. LL-37 shows antiviral response in keratinocytes by enhancing dsRNA-induced IFN-β expression [50]. It also works by up-regulating TLRs in keratinocytes, cytokines of the IL-1 family, and chemokines. Moreover, LL-37 also helps in eliminating S. aureus and C. albicans [49, 50]. In a recent study, LL-37 is found to be effective in polymicrobial wound treatment [51].
RNAase
Keratinocytes also express RNAase 5 and RNAase 7, which catalyze the degradation of microbial RNA. RNAase 7 can be induced by IL-1β in human keratinocytes and ocular epithelial cells to act against S. aureus [52]. Other AMPs expressed by keratinocytes are S100A7 (psoriasin), S100A8 (calgranulin A), and S100A9 (calgranulin B) belonging to the S100 protein family [53]. Flagellin of Escherichia coli infection is recognized by S100A7 (psoriasin) which initiates bacteria killing properties [52, 54]. Psoriasin also helps in wound healing along with calprotectin (S100A8/A9) which is another antimicrobial complex formed by S100A8 (calgranulin A) and S100A9 (calgranulin B), and induced by bacterial flagellin via TLR5 [52, 53]. Calprotectin also helps in keratinocyte proliferation and chronic inflammation. The expression of such AMPs is mostly regulated by cytokines, especially pro-inflammatory cytokines like IL-1β, TNF-α, IL-17, IL-22, etc. (Figure 4) [52]. The receptor for IL-22 is located on epidermal keratinocytes which acts through signal transducer and activator of transcription 3 (STAT3) and up-regulate the expression of HBD, S100A7, S100A8, and S100A9 [55]. IL-22 is helpful in increasing the thickness of the epidermis by inducing AMP expression. Hidradenitis suppurativa, a chronic inflammatory disease can occur due to a deficiency of IL-22 in keratinocytes [56]. The nuclear translocation of Bcl-3 in keratinocytes (IL-22-STAT3-Bcl-3 pathway) is probably responsible for STAT3 mediated gene expression [57]. In psoriasis and atopic dermatitis (AD), IL-22 is up-regulated indicating that IL-22 and its downstream Bcl-3 may be involved in the pathogenesis of these diseases [56, 57]. Similarly, platelet-released growth factors enhance AMP production and up-regulate the expression of keratinocytes psoriasin using EGF receptor and IL-6 receptor to boost wound healing [58]. Expression of filaggrin (FLG; a protein required to maintain skin barrier integrity) and HBD can be upregulated by S100A11 (a S100 calcium-binding protein) [59, 60].
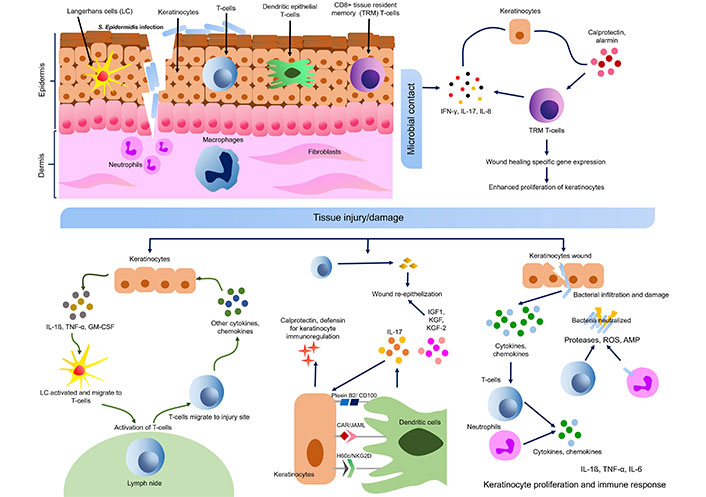
Cross talk of keratinocyte-immune cells in skin wound healing and microbial contact. S. epidermidis: Staphylococcus epidermidis; IL-17: interleukin 17; GM-CSF: granulocyte-macrophage colony-stimulating factor; TNF-α: tumor necrosis factor α; ROS: reactive oxygen species; AMP: antimicrobial peptide; IFN: interferon; IGF1: insulin-like growth factor 1; KGF: keratinocyte growth factor; JAML: junctional adhesion molecule-like protein
DNA is a potent stimulator of the innate immune response. However, there is a lack of information about the response of keratinocytes to cytosolic DNA. Cinat et al. [61] found that human keratinocytes are sympathetic to cytoplasmic DNA, and activation of DNA in the immune response is tightly regulated by keratinocytes. However, these keratinocytes require collateral stimulation with inflammatory cytokines to facilitate immunological reactions [61]. They also found that IL-37 antagonizes DNA driven immune activation. Further, they noticed that IFN-inducible protein 16 (IFI16) is essential for DNA directed innate immune response in keratinocytes [62]. They reported that in psoriatic patients, the pattern of IFI16 and DNA location in specific perinuclear foci in the keratinocyte cytoplasm is similar to in vitro study after DNA stimulation [62, 63]. Cytokine treatment induces an important factor in the DNA sensing pathway which plays an upstream of STING. Inflammatory environment in the skin lesion leads to malfunction of DNA in keratinocytes and results in skin inflammatory diseases [64, 65].
Role of keratinocytes in hypersensitivity reactions
The role of keratinocytes is also described in hypersensitivity reaction which is more related to cytokine secretion in response to antigenic proteins. Keratinocytes modulate the immune responses through B-responsive genes and indirectly regulate immune function by activating IL-8, RANTES, and macrophage inflammatory protein 1 α (MIP1α) [66]. Imiquimod is found very useful for the treatment of various skin diseases as it directly stimulates keratinocytes for cytokine and IFN-α production [67, 68]. A recent study described that CD44 is a unique target for the treatment of group A Streptococcus (GAS)-induced pharyngeal infection. CD44 is a hyaluronic acid-binding protein present in keratinocytes that mediates cell to cell or cell to extracellular matrix interaction [69, 70]. It works as a receptor for GAS colonization in the pharynx. Cywes et al. [70] found that the binding of GAS to murine epithelial keratinocytes is mediated via CD44. The colonization of GAS could be blocked by pre-treatment of wild mice with exogenous hyaluronic acid or by simultaneous administration of monoclonal antibodies to CD44. These findings support the potential application of interfering GAS to CD44 adherence as a crucial approach to prevent pharyngeal infection by GAS [71, 72]. Schistosoma mansoni, a parasite is identified to produce prostaglandin 2 from keratinocytes and is capable of down regulating IL-10 dependent host immune response [73]. It was also observed that PGE2 production by parasites failed to be blocked by COX2 inhibitors [74–76]. Gamma irradiation can significantly reduce the capacity of parasites to produce PGE2 and IL-10 from skin cells [76].
Among cytokines, IL-17 augments keratinocyte activation via the release of various factors including IL-6, growth-related oncogene α (GRO-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [77]. IL-18 secreted by keratinocytes, modulates the migration of Langerhans cell and T helper (Th) cell responses. IL-20, a novel homolog of IL-10 is identified in keratinocytes [77, 78]. IFN-α also modulates cytoskeletal responses of keratinocytes which makes it more contractile [77, 79]. Tacrolimus (FK506), an immunomodulatory drug can directly regulate the productions of cytokines and chemokines by keratinocytes [80]. Wakugawa et al. [81] reported the role of RANTES in skin inflammation. IFN-γ or IL-1β strongly induces keratinocytes to produce RANTES. RANTES production can be regulated or balanced by immunosuppressive drugs like dexamethasone and tacrolimus (FK-506) [81, 82]. RANTES production by keratinocytes can be significantly inhibited when treated with dexamethasone even in the presence of IFN-γ and IL-4 and partially inhibited by tacrolimus [80]. Studies have noted the production of macrophage derived chemokine (MDC) by human keratinocytes cell line (HaCaT cells) which is regulated by Th1/Th2 cytokines [83]. They also found significant upregulation of MDC production with TNF-α and IFN-γ stimulation, however, decreased MDC production by IL-4 and IL-13. They concluded that IL-4 and IL-13 partly contribute to maintaining Th1/Th2 balance in inflammatory skin diseases, especially in AD [84–86].
Chemokines
Chemokines and their receptors have an important role in the modulation of keratinocyte functions in inflammation [87]. Keratinocytes express liver- and activation-regulated chemokine (LARC)/CCL20, which strongly builds up the healing response in AD lesions. Besides endothelial cells, keratinocytes also produce CC chemokine, thymus and activation-regulated chemokine (TARC) via CCR4 receptor in the lesional area of AD [83, 84]. For the chemokine receptors like CXCR3 and CCR4, keratinocytes produce ligands like IP-10, MIG, and MDC which are helpful in chemokine trafficking and may help in healing psoriatic lesions [88].
Keratinocyte also expresses another potent chemokine, MIP3α which captivates Langerhans cell precursors at inflammatory lesions. Stimulation with TNF-α and IL-1β results in the production of another chemokine CCL27 by keratinocyte [89]. This grouping is also beneficial in psoriatic lesions through up-regulation of MIP3α and CCR6 [4]. A chemokine, MIP3α (CCL20) and its receptor CCR6 are significantly up-regulated in psoriasis. It was proved in a study conducted by Homey et al. [90]. They showed that cultured primary keratinocytes, dermal microvascular endothelial and dermal fibroblast, and dendritic cells when induced with pro-inflammatory mediators like TNF-α/IL-1β, CD40 ligand, IFN-γ, or IL-17 are major sources of CCL20 production by RT-PCR and ELISA [91, 92]. LARC/MIP3α/CCL20 may play a significant role in the migration of Langerhans cells in lesional skin of AD [90, 91, 93].
Role of keratinocytes in eczematous and allergic skin disorders
Keratinocyte is the major immunologically effective cell interacting with different types of T-cells to produce immune responses in an eczematous skin lesion [94]. Keratinocytes display CCL27/CTACK, which binds to CCR10 expressed on most lymphocytes infiltrated in the skin. IL-1 and TNF-α have an important role in the chemokine induction by keratinocytes. These keratinocyte-derived chemokines include CCL5/RANTES, CCL27/CTACK, CCL17/TARC, CCL22/MDC, and CCL18/PARC which are highly expressed in AD [95].
IFN-γ has a great role in the induction of apoptosis in eczematous skin lesion of AD which occur through TNF-α induced expression of Fas on keratinocytes [96]. IFN-γ also induces CCL22/MDC production in human keratinocytes. CD54, CCL2/MCP-1, CCL5/RANTES, and CXCL10/IP-10 are other IFN-γ inducible molecules which are activated through IL-4 or IL-13 in human keratinocytes [95].
Recently it has been demonstrated that high expression of IL-31 occurs in atopic inflammatory lesions and epidermal keratinocytes express IL-31 receptor α (IL-31RA) in AD [97]. IL 31, a T-cell cytokine may result in skin dermatitis like AD and pruritus [98]. Bilsborough et al. [99] have studied skin biopsy specimens and peripheral blood cells of healthy individuals and of patients of AD using immunohistochemistry and RT-PCR to assess the expression of IL-31 and IL-31RA. They also assessed IL-31 protein production by skin-homing cutaneous lymphocyte antigen (CLA)-positive T cells. They found that IL-31 expression is associated with CLA+ T cells, thus found to contribute to the causation of AD like lesion and pruritus [100, 101]. Sonkoly et al. [102] found that IL-31 is a novel target for the treatment of pruritus. IL-31 is also involved in non-pruritic psoriatic inflammatory skin lesions [103]. Staphylococcal superantigen is capable of inducing IL-31 expression in patients of AD. Thus IL-31 is crucial for targeted therapy. Thus, IL-31 is an important cytokine that supervises keratinocyte-T cell interaction [102, 103].
In the early stages of allergic contact dermatitis (ACD), there is direct activation of keratinocytes by contact allergens. In the elicitation phase of ACD, IFN-γ attacks keratinocytes and liberates much of ICAM-1, MHC class II, MHC class I, and Fas [104]. In the amplification phase, keratinocyte also releases chemotactic factors and express chemokine receptors, thus attracting distinct cells to modulate immune responses [102, 103, 105]. CXCL9, CXCL10, and CXCL11 are the most commonly produced chemokines by activated keratinocytes [106]. Among the infiltrating T cells, more than 70% of cells express CXCR3. Other chemokines include CCL27, CCL5, CCL22, and CCL1 [104].
Keratinocytes in AD skin also exhibit an abundance of GM-CSF and TNF-α which may be due to uncontrolled and poorly regulated signal transduction [107]. Keratinocyte of AD lesion also releases a high amount of TSLP, which has an impact on dendritic cells and stimulates dendritic cells to secrete Th2-recruiting chemokines and also upregulate differentiation of T-cells into inflammatory Th2 cells [107, 108]. Increased Th2 cytokine expression in AD is mainly responsible for AMP deficiency including HBD2, HBD3, and LL-37 [109, 110]. Keratinocytes also express an epidermal differentiation complex protein known as FLG, which is involved in barrier function [60].
Role of keratinocytes in psoriasis
Keratinocyte is identified as auto-antigen generating cells in the pathogenesis of psoriasis. The interaction of skin resident keratinocytes and auto-reactive T-cells is the mainstay for the commencement and progression of the disease [111]. Various keratinocyte-derived auto-antigens have been identified which include LL-37 cathelicidin/nucleic acid complexes and newly generated lipid antigens [110]. In addition, due to some genetic reshaping that occurs within keratinocytes are responsible for a cross-talk with T-cell derived cytokine and initiation of signal transduction pathway [112].
Blockage of T-lymphocytes co-stimulation has a beneficial role in reversing psoriatic pathology which is possible by using a soluble chimeric protein (CTLA4 Ig) and minimizes cellular activation of keratinocytes, dendritic cells, and endothelial cells [113]. In biopsies from the psoriatic lesion, it is observed that keratinocytes show lowered expression of CD40, CD54, and also MHC class II HLA-DR antigens [114]. The efficacy of recombinant IL-10 inhibits the IL-8 pathway by suppressing its receptor CXCR2 and IL-17, thus preventing the maturation of abnormal keratinocytes of the psoriatic lesion [114, 115].
Neuro-endocrine role of keratinocytes and responses to environmental stressors
Throughout the skin, neuroendocrine signaling coordinates a wide range of physiological functions essential for maintaining homeostasis and reacting to outside stimuli [116]. Neuropeptides produced and secreted by cutaneous nerve terminals, sensory neurons, and immune cells in the skin include substance P, calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP) [117]. These neuropeptides attach to specific receptors on keratinocytes upon release, starting a series of biological reactions. In order to react to neurogenic stimuli, keratinocytes express a variety of neuropeptide receptors, such as VIP receptors, CGRP receptors, and neurokinin receptors for substance P [117, 118]. Through these connections, keratinocyte physiological functions such as proliferation, differentiation, inflammation, and wound healing are modulated by epidermal neuropeptides [119]. Additionally, it is possible for keratinocytes to synthesize neuropeptides and express enzymes related to neuropeptide metabolism, indicating that keratinocytes and neurons can communicate in both directions [119]. The complex interaction between keratinocytes and epidermal neuropeptides highlights the critical function of neuroendocrine signaling in preserving skin homeostasis and coordinating reactions to external cues and pathological states [120, 121]. Vasodilation, inflammation, and pain perception are regulated by epidermal neuropeptides such as substance P and CGRP through complex interactions with surrounding keratinocytes, immune cells, and sensory nerve terminals [122, 123]. Pro-opiomelanocortin (POMC) and corticotropin-releasing hormone (CRH) are two examples of molecules that are important regulators [124]. A number of physiologically active peptides, such as α-melanocyte-stimulating hormone (α-MSH), which regulates skin pigmentation, inflammation, and wound healing, are precursors of POMC [125, 126]. Additionally, POMC-derived peptides exhibit antimicrobial properties, further highlighting their role in skin defense mechanisms [125]. Moreover, the local production of CRH in the skin is involved in modulating many effects such as acting as a barrier, immunomodulatory effect, and most importantly in stress response against stressors such as UV and pathogens [127, 128].
The neuroendocrine role of the skin was unraveled when cutaneous production of melanotropins was reported. This highlights that the skin expresses important hormones involved in the hypothalamus-pituitary-adrenal axis, such as CRH, urocortin, and POMC, along with its byproducts, beta-endorphin, adrenocorticotropic hormone (ACTH), and α-MSH. Local production of other hormones like vitamin D3, acetylcholine, catecholamines, and PTH-related protein (PTHrP) are also involved in responding to and mitigating harmful effects of environmental stressors such as UV radiation [116]. UV radiations (more importantly UVB radiations) by influencing local cutaneous production of chemical and neuronal signals can impact some regulatory functions of the brain via the skin, which ultimately help maintain homeostasis [126]. This may influence opioidogenic effects or immunosuppression that was earlier believed to be mediated by vitamin D or beta-endorphins in the skin [126, 129]. Recent research has illuminated the presence of corticosteroidogenic pathways [130] within the epidermis, highlighting the skin’s capacity for autonomous regulation of local glucocorticoid synthesis [131–133]. Key enzymes involved in corticosteroidogenesis, including 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) and steroidogenic enzymes such as CYP11A1 and CYP11B1, are expressed in keratinocytes [134, 135]. These enzymes catalyze the conversion of precursor molecules, such as cholesterol, into active glucocorticoids, including cortisol. Additionally, the epidermis harbors receptors for ACTH and CRH, which stimulate corticosteroid production in response to stress and inflammation [136]. Local synthesis of glucocorticoids within the epidermis plays a crucial role in regulating cutaneous immune responses, inflammation, and barrier function, highlighting the intricate interplay between the skin and the neuroendocrine system [136, 137].
Another interesting facet of cutaneous defense and keratinocytes is their role in ultraviolet radiation (UVR) induced damage to the skin. UVR exerts a multitude of homeostatic actions on both cutaneous and systemic physiology, while also presenting keratinocytic stress [138]. On the one hand, controlled exposure to UVR is essential for the synthesis of vitamin D in the skin, thereby regulating calcium metabolism and skeletal health systemically [138, 139]. Furthermore, UVR-induced production of β-endorphins contributes to mood elevation and stress reduction. However, excessive or chronic UVR exposure can disrupt cutaneous homeostasis, leading to keratinocytic stress characterized by DNA damage, oxidative stress, and inflammation [139]. In response, keratinocytes activate intricate cellular defense mechanisms, including DNA repair pathways, antioxidant systems, and the secretion of cytokines and growth factors to mitigate UVR-induced damage [139]. One such example is the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. Upon UV exposure, ROS are generated, leading to oxidative stress and cellular damage. In response, keratinocytes upregulate the expression of antioxidant response element (ARE)-dependent genes through the activation of Nrf2. Nrf2 translocates to the nucleus and binds to ARE sequences in the promoter regions of target genes, including heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), and glutathione S-transferase (GST) [140, 141]. These genes encode antioxidant enzymes and phase II detoxification enzymes, which scavenge ROS, repair oxidative damage, and promote the detoxification of harmful compounds. By enhancing the cellular antioxidant defense system, keratinocytes mitigate oxidative stress induced by UVR, thereby reducing DNA damage, inflammation, and the risk of skin disorders such as photoaging and skin cancer. Moreover, UVR-induced stress triggers adaptive immune responses and melanogenesis, serving to protect against further UVR insult [140–142].
Interplay between keratinocytes and other immune cells
A crucial component of the cutaneous immune response is the interaction between keratinocytes and other immune cells, which creates a dynamic network necessary for skin homeostasis and pathogen protection [7]. Key sentinels, keratinocytes use PRRs like TLRs to identify microbial invasion and danger signals, which trigger innate immune responses [14, 16, 18, 19]. Keratinocytes recognize pathogens and inflammatory stimuli and release a variety of cytokines, chemokines, and AMPs that not only fight the invaders but also draw in and influence the activity of other skin cells that are part of the immune system [7, 12, 14].
The relationship between keratinocytes and Langerhans cells—specialized dendritic cells found in the epidermis—is one of the most important ones. Dendrites from Langerhans cells are extended between keratinocytes, creating a complex network that is used for antigen presentation and sampling [115]. When antigens are presented to T cells in draining lymph nodes by Langerhans cells in response to infections or antigenic stimuli, the T cells then begin to mount an adaptive immune response [105]. The exchange of information between Langerhans cells and keratinocytes is essential for the skin’s innate and adaptive immune responses to work together, clearing infections and forming immunological memory [105, 143].
Additionally, keratinocytes regulate immunological activation, inflammation, and tissue healing processes through their interactions with T cells, mast cells, and natural killer (NK) cells, among other immune cells [7, 15]. In the skin microenvironment, keratinocytes control the recruitment, activation, and activity of these immune cells by expressing adhesion molecules, cytokines, and co-stimulatory molecules [56, 104]. This complex interaction between keratinocytes and other immune cells emphasizes how diverse cutaneous immunity is and how crucial keratinocytes are as the primary organizers of immune responses in the skin [14].
Future prospects
In recent years, keratinocyte physiology received high attention due to its powerful first line of defense in our immune system. They not only recognize harmful microbes but are also involved in the production of AMPs, several pro-inflammatory cytokines, and chemokines and regulate the function of other immune cells. Such chemokines have a crucial function in triggering signal transduction pathways to incite the expression of essential genes [144]. In viral infection, the invasion and replication of viruses is mostly reliant on keratinocytes. Hence any functional abnormality in keratinocytes may lead to dermatosis [95]. Many physical and chemical factors influence the function of keratinocytes which are targeted in clinical practice [144]. For example, calcium has a very useful role in modulating calcium concentration in the signal transduction pathway. High calcium can stimulate dsRNA sensor expression including TLR3, melanoma differentiation-associated protein 5 (MDA5), and RIG-I of keratinocytes [35, 36]. It also enhances the expression of S100/A11 and HBD which show effective immunity against the pathogen. In the early stages of injury CCL20, IL-15, and IL-23A are strongly over-expressed in keratinocytes and ease the adaptive immune response [144].
Recently keratinocyte role has been emphasized and studied for targeted therapy in various skin diseases. New mediators and receptors have been identified and studied which interact with keratinocytes to facilitate molecular mechanisms and immune responses for healing. More analysis of autologous cell-cell interaction is required for detailed study of various mediators interacting with keratinocytes in skin diseases to understand the pathogenetic immune mechanism of keratinocytes for identifying and targeting therapeutic intervention [145, 146]. Recently, photodynamic therapy has been introduced for the treatment of various skin diseases, which activates IL-10 gene promoter via AP-1, thus modulating the immune response of keratinocytes [147].
Conclusions
Keratinocytes play a crucial role in cutaneous immune response which serves as an important barrier between the internal and external environment. Keratinocytes are pivotal in skin injuries, various inflammatory dermatological diseases, and skin infections by their ability to secret different cytokines, cross-talk with other immune cells like Langerhans cells, and sense molecular patterns of pathogens. A better understanding of keratinocyte immunology will pave the way for the development of newer targeted therapeutic options in different skin diseases including malignancy.
Abbreviations
| AD: | atopic dermatitis |
| AMPs: | antimicrobial peptides |
| CGRP: | calcitonin gene-related peptide |
| CRH: | corticotropin-releasing hormone |
| GAS: | group A Streptococcus |
| HBD: | human β-defensins |
| hCAP18: | human cationic antimicrobial protein 18 |
| IFN-β: | interferon-B |
| IL: | interleukin |
| MDC: | macrophage derived chemokine |
| MIP1α: | macrophage inflammatory protein 1 α |
| NLRs: | nucleotide binding oligomerization domain-like receptors |
| NOD: | nucleotide binding oligomerization domain |
| NOD-1: | nucleotide binding oligomerization domain-containing protein 1 |
| Nrf2: | nuclear factor erythroid 2-related factor 2 |
| PAMPs: | pathogen-associated molecular patterns |
| PGN: | peptidoglycan |
| POMC: | pro-opiomelanocortin |
| PRRs: | pattern recognition receptors |
| RIG-I: | retinoic acid-inducible gene-I |
| RLRs: | retinoic acid-inducible gene-I-like receptors |
| ROS: | reactive oxygen species |
| STAT3: | signal transducer and activator of transcription 3 |
| Th: | T helper |
| TLRs: | Toll-like receptors |
| TNF: | tumor necrosis factor |
| UVR: | ultraviolet radiation |
Declarations
Author contributions
RKG: Conceptualization, Writing—review & editing, Supervision. PW: Writing—original draft. DS: Conceptualization, Supervision. DM: Writing—original draft, Visualization, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.