Abstract
Aim:
The immune system can experience temporary suppression following acute or prolonged strenuous exercise, potentially increasing susceptibility to infections. Young athletes, who must balance school, training, studies, and social life, may further disrupt their immune-inflammatory responses. This study aimed to investigate the effects of an 8-week pre-season training on circulating leukocyte responses in well-trained adolescent soccer-players at different stages of puberty.
Methods:
Thirty-six soccer players, divided into two groups based on their biological age-under sixteen (U16) and above sixteen (A16)-underwent two rounds of assessments. These included evaluations of anthropometrics, physical fitness, and immune parameters before and after an 8-week pre-season soccer training program.
Results:
No significant treatment or interaction effects were found for erythrocyte sedimentation rate (ESR), platelets, total leucocyte count, immune inflammation markers (IIM), weekly rating of perceived exertion, body composition, sit-and-reach flexibility, or VO2max (P > 0.05). However, post-intervention neutrophil counts were increased, while lymphocyte counts decreased, and IIM levels rose in both groups (P < 0.05). Neuromuscular explosiveness and strength endurance were significantly higher in the A16 group compared to the U16 group (P < 0.05). Body fat was reduced, and all physical fitness parameters improved in both groups over time (P < 0.05).
Conclusions:
The 8-week soccer-specific training program did not lead to different effects on immune-inflammatory parameters between youth soccer players at different stages of puberty. However, training adaptations, including improvements in physical fitness, were observed in both groups. This suggests that when the same training load (frequency, duration, intensity) is applied to adolescents at different stages of puberty, immune-inflammatory responses are not significantly influenced by age, but physical fitness improves regardless of puberty stage. A key limitation of this study is the lack analysis of lymphocyte subpopulations, which could provide a deeper understanding of the cellular effects of training on immune function.
Keywords
Soccer, pre-seasonal training, immune responses, puberty stages, training adaptationsIntroduction
There has been substantial focus on the influence of exercise-induced immunosuppression and disruptions to immunological health across the lifespan [1]. In adults, regular physical activity—whether moderate [2, 3] or vigorous [4, 5]—has been shown to improve immune-inflammatory parameters. This improvement may be linked to enhanced proliferation of CD8+ and B lymphocytes in animal models [5] or to increased anti-inflammatory and immunoprotective effects in humans, which could help mitigate immunosenescence and prevent age-related multimorbidity [6]. In contrast, high-performance athletes often experience changes in circulating hematological parameters, with various aspects of immunity being temporarily suppressed [7]. This can lead to respiratory inflammation and infections, particularly after acute [8, 9] or prolonged periods of intense physical activity [10]. The immune responses and distinct kinetic patterns in leukocyte subsets are influenced by different exercise intensities [8], and these changes may progressively limit training adaptations and exercise performance, especially in elite young athletes [11, 12].
A previous study examined the effects of acute exercise on immune responses in children across different age ranges, pubertal statuses, and genders and showed that when biological maturation was controlled for, cellular and soluble components of immune-inflammatory parameters responded similarly, regardless of pubertal stage [12]. Recently, Almeida-Neto et al. [13] investigated the impact of age and cardiorespiratory fitness on immune markers in healthy young adults (ages ~20–35 years) following a maximal cardiorespiratory capacity test. They found that immune responses to acute strenuous exercise were not influenced by fitness levels but were more closely related to chronological age, with older participants showing more pronounced immune responses. In a subsequent review, it was concluded that biological maturation stages, rather than chronological age, should be used to determine immune-inflammatory parameters in adolescent athletes [14]. Although it is well documented that acute high-intensity exercise can negatively impact training adaptations and performance in adults, it remains unclear whether chronic strenuous exercise influences immune-inflammatory parameters differently at various stages of puberty in youth athletes.
Puberty (ages ~13–18 years) is a period of significant physical development, including changes in body composition, hormonal fluctuations, and metabolic shifts—all of which can impact the immune system and influence future health [15]. Adolescent high-performance athletes, who must balance school, training, and social life, may be particularly vulnerable to further disruptions of their immune systems [11, 16, 17]. This makes them more prone to infections compared to adult athletes [18], and their training adaptations, performance, and overall health could be progressively impaired [10]. In a longitudinal study, Blume et al. [19] examined the effects of training load on stress and immunological markers in adolescent athletes (13.8 ± 1.5 years) over four years. They found that training load did not significantly alter immune parameters, but the susceptibility to infection increased as participants approached the end of puberty (ages 16–18 years) [19]. This suggests that further research is needed to examine how age and pubertal stages influence immune-inflammatory parameters during long-term intensified training. Supporting this, Liu et al. [20] found that chronic endurance training in young athletes induced transcriptional changes in white blood cell (WBC), upregulating genes associated with protein production and mitochondrial energetics, while downregulating genes involved in inflammatory responses.
Recent studies have introduced new cellular inflammation markers—such as platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI), which could be useful in exercise physiology research [21–24]. Platelets, known for their role in primary hemostasis, were also observed to be released from the bone marrow into circulation during exercise, and their levels increase in response to both acute and chronic exercise [25]. Thrombocytosis therefore, increases significantly in response to exercise stimulus, similarly to exercise-induced neutrophilia [24, 25]. In competitive youth sports, these hematological indicators can help assess exercise strain, improve recovery, and identify periods of increased infection risk or overtraining, aiding in better exercise planning [17].
Building on these findings, the current study aims to evaluate blood leucocyte counts and cellular immune inflammation markers [neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), PLR, SIRI] in well-trained adolescent soccer players following 8 weeks of pre-seasonal preparation training, considering their puberty stage. To the authors’ knowledge, no studies have yet explored the impact of puberty on immune-inflammatory parameters in adolescent athletes after 8 weeks of soccer-specific pre-season training. Thus, it was hypothesized that the immune system’s response to the intensified pre-season training will vary according to the athletes’ pubertal stages.
Materials and methods
Participants
Forty-one well-trained youth soccer players (mean ± SD), all members of the same professional soccer academy, were initially recruited for the study. Participant suitability was examined with interviews. The participants were fully informed concerning the protocol and the procedures of the study. This study follows to the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1964) and received approval from the Institutional Research Ethics Committee (REC-010712). Written informed consent was gained from both the participants and their parents before any assessments were conducted. The participants completed a medical history questionnaire. None of the participants were taking medications or substances that could affect their immunological, haematological, or exercise performance. Additionally, none had a history of any illness or injury prior or during the period of the study. To ensure consistency, participants were not using creatine or other ergogenic supplements for at least 8 weeks before to the initiation of the experimental research.
Study design
Participants were divided into two groups based on their biological age: under sixteen years (U16, Tanner stage < 4.5) and above sixteen years (A16, Tanner stage 5). For detailed group characteristics, please refer to the results section. Randomization was not used to separate the groups. However, in order to minimize bias, all participants received equal encouragement during fitness tests, and test results were withheld until the end of the study. Both groups underwent the same 8-week soccer-specific pre-season training program. Participant retention was high, with only minimal dropouts. Two participants from the U16 group and one from the A16 group did not complete the blood analysis due to personal reasons. Additionally, one participant from each group withdrew from the fitness re-examination due to injuries. Ultimately, 36 participants completed both blood and fitness evaluations and were included in the statistical analysis. Before and after (within 48–72 h) the training intervention period, participants visited the laboratory on two occasions to complete the pre- and the post-intervention haematological, anthropometric, physiological, and physical fitness assessments [26].
Details of the dietary intake procedure, during the study period, have been previously published [27]. Briefly, dietary intake was not strictly monitored, but a caloric control regimen was provided. Participants and their parents attended a specific seminar explaining macronutrient and micronutrient categories and their effects on growth, performance, and health. Participants and parents were also taught practical methods to quantify food intake using cups, spoons, and digital scales. The participants were also counseled to follow their favored diet but having the regimen as a dietary guide. During the study period, participants aimed to maintain a daily diet of approximately 50% carbohydrates, 25% fats, and 25% proteins [27]. Three hours before each fitness assessment and friendly game, participants consumed a prescribed high-carbohydrate meal (~70% carbohydrates, 15% fats, and 15% proteins), followed by a 3-hour fasting period in which free water intake was allowed only [28]. Anthropometric, physiological (morning sessions), and most physical fitness tests (afternoon sessions) were conducted on the same day. The aerobic capacity test was conducted the following day. To reduce diurnal variation, all exercise performance evaluations were performed in the afternoon (5:30–8:30 pm), on both testing days. Participants rested the day before and avoided tea, caffeine, and alcohol for at least 48 hours before each fitness evaluation [29].
Physiological evaluations
Details of the test procedure have been published [27]. Briefly, when participants arrived at the laboratory, they were advised to seat comfortably. After 5 minutes, resting heart rate (HR) and blood pressure (BP) were measured using an OMRON M6 (Omron Healthcare, Milton Keynes, UK). Body weight and height were evaluated with a calibrated digital scale (Tanita Digital Scale, KD-400, UK). Skinfold thickness was measured at seven body sites using a Harpenden caliper (British Indicators Ltd., St. Albans, UK). Body density and body fat percentage (BF%) were calculated with the Jackson and Pollock [30] and Siri [31] equations, correspondingly. Pubertal status was evaluated with a self-administered pubertal development questionnaire [32], completed at home by each participant.
Aerobic capacity, neuromuscular explosiveness and strength endurance assessments
Leg explosiveness was assessed using the standing Broad Jump test (Gill Athletics Standing Long Jump Testing-Mat). Before each trial, participants were instructed to jump as far as possible, using an arm swing and countermovement for support. The take-off was performed with both feet simultaneously, without any preliminary steps or shuffling. Each participant completed three trials, with the best result recorded.
Maximum oxygen consumption (VO2max) was measured through a multistage fitness (Beep) test to the point of exhaustion [33]. The examination was performed on a soccer field, with groups of 10–12 players wearing soccer cleats. Upper body muscular endurance was evaluated using the Push-up test [34], while abdominal and hip flexor endurance were measured with the Abdominal Curl Conditioning Test (Coachwise Ltd., Leeds, UK). Participants performed as many sit-ups as possible in sync with the beeps emitted from an audio recording, with the total number of correctly executed sit-ups recorded until participants could no longer maintain the pace.
All participants were familiar with these physical fitness tests, having completed them multiple times previously. For the standing Broad Jump and strength evaluations, the soccer-players completed at least 10 supervised practice trials. For the Beep test, participants had completed at least two familiarization trials (not to exhaustion). The test-retest reliability [35] correlation coefficients of were r = 0.97 for the Bleep test, r = 0.96 for BF%, r = 0.89 for the Broad Jump, r = 0.93 for push-ups, and r = 0.95 for sit-ups.
Haematological analyses
Participants visited the laboratory in the morning (7:00–8:30 am), following an overnight fast. Venous blood samples (20 mL) were collected from an antecubital vein and divided into EDTA, sodium heparin plasma, and serum separator tubes (Vacutainer; Becton Dickinson, Breda, The Netherlands). EDTA-treated whole blood was stored at 4°C, while sodium heparin plasma tubes were kept at room temperature (RT) until analysis. Serum separator tubes were left in the dark at RT for at least 30 minutes before centrifugation at 2000× g for 10 minutes. The resulting sera were aliquoted and stored at –80°C until further analysis.
Quantitative analysis of leukocyte subsets (lymphocytes, monocytes, neutrophils, eosinophils, basophils) was performed on EDTA whole blood using an ADVIA 1200 Chemistry Automated Haematology Analyzer (Siemens, Star-shl, Etten-Leur, The Netherlands). Total WBC count, leukocyte subsets, platelet count, and erythrocyte sedimentation rate (ESR) were determined according to standard procedures. Absolute values were calculated by multiplying the percentage values by the absolute WBC and dividing by 100. Inflammatory markers such as the NLR, PLR, MLR, and SIRI (calculated as SIRI = N × M/L) were determined [36]. Serum cortisol concentration was measured using the Cobas Mira Plus CC system (Roche), employing an Electro-Chemi-Luminescent Immunoassay (ECLIA, Rotkreuz, Switzerland). Intra-assay coefficients of variation for blood assays were within 5%.
Training intervention program
The training sessions were conducted on outdoor grass fields between mid-July and mid-September at 6:00 pm. When double training sessions were scheduled, the morning sessions started at 7:00 am for outdoor activities and at either 7:30 am or 9:00 am for gym-based activities. The 8-week pre-season training program was designed by the first author in collaboration with the coaching staff, following Jovanovic’s model [37]. The program included 48 training sessions and 8 friendly matches. Each session lasted between 60 and 90 minutes, including a 10–15-minute warm-up and approximately 5 minutes of post-session stretching. For further details on training quantification and specific components of the fitness program, see Table 1.
The 8-week of the soccer-specific training program: Fitness elements
| Week | Aerobic/AnaerobicEndurance (sessions) | Strength | Speed-Quickness-Agility | Flexibility(Dynamic/Static) |
|---|---|---|---|---|
| 1 | 2 Con (60–70% MHR);1 Fartlek (60–75% MHR) | 2 max Strength circuit sessions (60–70% 1RM) | 2 Agility sessions (low intensity: technique) | Warm-up: dynamicCool down: static + 3 specific sessions |
| 2 | 1 Con (75% MHR);2 Fartlek (60–80% MHR);1 MI interval (85% MHR);1 Friendly match | 2 max Strength circuit sessions (65–75% 1RM) | 2 Agility sessions (low intensity: technique) | Warm-up: dynamicCool down: static + 3 specific sessions |
| 3 | 1 Fartlek (60–85% MHR);2 MI intervals (85% MHR);1 HI interval (100% MHR);2 Friendly matches | 2 max Strength circuit sessions (70–90% 1RM) | 2 Quickness-Agility sessions (mid intensity: technique) | Warm-up: dynamicCool down: static + 3 specific sessions |
| 4 | 1 HI Fartlek (70-85% MHR);1 HI interval (95% MHR);1 Anaerobic endurance (85% MHR);1 Friendly match | 1 max Strength (~85% 1RM); 1 plyometric session | 1 Speed, 1 Quickness, 1 Agility sessions | Warm-up: dynamicCool down: static + 3 specific sessions |
| 5 | 1 HI interval (85% MHR);1 Anaerobic endurance (80% MHR);2 Friendly matches | 1 circuit Strength (~85% 1RM); 2 plyometric sessions | 1 Speed, 1 Quickness, 1 Agility sessions | Warm-up: dynamicCool down: static + 3 specific sessions |
| 6 | 1 HI interval (95% MHR);1 Anaerobic endurance (85% MHR);1 Friendly match | 1 circuit Strength (~80% 1RM); 2 plyometric sessions | 1 Speed, 1 Quickness, 1 Agility sessions | Warm-up: dynamicCool down: static + 2 specific sessions |
| 7 | 1 MI interval (80% MHR);1 Anaerobic endurance (80% MHR);1 Friendly match | 2 plyometric sessions | 2 Speed, 1 Quickness sessions | Warm-up: dynamicCool down: static + 1 specific session |
| 8 | 1 Short anaerobic endurance (80% MHR);1 Short HI interval (95% MHR) | 1 plyometric session | 2 Speed, 1 Agility sessions | Warm-up: dynamicCool down: static + 1 specific session |
MHR: maximum heart rate; Con: continuous with tempo; MI: moderate intensity; HI: high intensity; 1RM: one-rep max
Internal training load determination
The quantification of the intervention training program was controlled using internal training load (ITL) determination, which was estimated, in arbitrary units (AU), by multiplying the minutes of training duration with the session-Ratings of Perceived Exertion (RPE) using the CR10-scale modified by Foster et al. [38]. The soccer-players’ RPE was obtained around 15–30 min after each training session, for ensuring that the RPE was referred to the whole training session, rather than the last exercise effort of the session [39]. Before the beginning of the pre-seasonal training program, all soccer-players were fully familiarized with the Borg’s CR-10 scale.
Statistical analysis
A G*Power (3.1.9.7) sample size calculation was conducted before testing, using a mixed repeated measures ANOVA. Parameters included an effect size of 0.7, an alpha error of < 0.05, a non-sphericity correction (ε) of 1, a correlation between repeated measures of 0.5, and a desired power (1 – β) of 0.95. This calculation indicated a required total sample size of 36 participants. All data are presented as means ± SD after confirming normal distribution with the Shapiro-Wilk test. Statistical analyses included an unpaired t-test for comparing chronological, biological, and training ages, as well as a mixed ANOVA for repeated measures (treatment: between groups; pre-post treatment: within groups) with a Bonferroni post-hoc adjustment for further comparisons. Partial Eta Squared (η2) was used to measure effect size, categorized as small if η2 < 0.06 and large if η2 > 0.14 [40]. Statistical significance was set at P < 0.05. Analyses were performed using SPSS 28 for Windows® (SPSS Inc., USA).
Results
Anthropometrics and body composition evaluations
The anthropometric characteristics and body composition evaluations for the groups are presented in Table 2. There were no significant differences in body weight, height, BMI, or BF% between the two groups (P > 0.05). However, a significant main time effect was observed for body weight [F(1,34) = 7.185, P = 0.011, η2 = 0.174], body height [F(1,34) = 9.239, P = 0.005, η2 = 0.214], BMI [F(1,34) = 10.50, P = 0.003, η2 = 0.236], and BF% [F(1,34) = 35.24, P < 0.001, η2 = 0.509]. Post-intervention, only the A16 group showed a significant reduction in body weight (P < 0.001) and BMI (P = 0.003). Additionally, body height increased significantly only in the U16 group (P = 0.016) compared to baseline values. BF% decreased significantly in both groups after the intervention, with a 13.8% reduction in the A16 group (P = 0.001) and an 8.3% reduction in the U16 group (P = 0.01).
Physiological and exercise performance assessments at pre- and post-training intervention
| Evaluations | A16 | U16 | ||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| Anthropometrics | ||||
| Age (yrs) | 17.3 ± 0.8# | 17.5 ± 0.8# | 15.5 ± 0.3 | 15.7 ± 0.3 |
| Training age | 10.5 ± 1.0# | 8.8 ± 0.7 | ||
| Tanner maturation | 5 ± 0# | 4.5 ± 0.5 | ||
| Height (cm) | 1.74 ± 0.06 | 1.74 ± 0.06 | 1.74 ± 0.06 | 1.75 ± 0.06* |
| Weight (kg) | 67.8 ± 7 | 66 ± 6.7* | 67 ± 6 | 67.3 ± 6 |
| BMI (kg·m-2) | 22.5 ± 1.3 | 21.8 ± 1.3* | 22.1 ± 1.9 | 22.2 ± 1.8 |
| Body fat (%) | 10.6 ± 2.9 | 9.1 ± 2.4* | 9.1 ± 1.9 | 8.3 ± 2.0* |
| Physiological and fitness evaluations | ||||
| Systolic BP | 122 ± 6 | 120 ± 8 | 120 ± 4 | 120 ± 5 |
| Diastolic BP | 73.2 ± 5 | 72.1 ± 6 | 71.2 ± 5 | 73 ± 5 |
| HR rest (beats/min) | 67 ± 9 | 62 ± 6* | 71 ± 6 | 65 ± 6* |
| Flexibility (cm) | 22.3 ± 8 | 24.4 ± 7* | 23.8 ± 5 | 26.7 ± 5* |
| VO2max (mL·kg-1·min-1) | 54.2 ± 3.9 | 56.9 ± 3.2* | 52.8 ± 3.8 | 54.3 ± 3.6* |
| Standing long-jump (cm) | 227 ± 13# | 238 ± 12*# | 216 ± 14 | 220 ± 16* |
| Sit-ups (rep/min) | 49 ± 5# | 55 ± 4*# | 43 ± 8 | 48 ± 7* |
| Push-ups (rep/min) | 43 ± 10# | 46 ± 1*# | 35 ± 9 | 41 ± 8* |
BP: blood pressure; MHR: Maximum heart rate; VO2max: maximum oxygen consumption. # Statistical differences between A16 and U16 (P < 0.05); * statistical difference over-time, between pre- and post-training (P < 0.05)
Physiological and physical fitness evaluations
Table 2 presents the results of the physiological and fitness evaluations. There were no significant main treatment, time, or interaction effects observed for resting BP (P > 0.05). Similarly, there were no significant main treatment or interaction effects for resting HR (P > 0.05). However, a significant main time effect was found for resting HR [F(1,34) = 19.24, P < 0.001, η2 = 0.361]. Post-intervention analysis with Bonferroni adjustment indicated that resting HR was significantly reduced in both the A16 group (P = 0.014) and the U16 group (P = 0.003) compared to baseline values.
Hamstring and lower back flexibility did not differ significantly between the groups (P > 0.05). However, a significant main time effect on flexibility was observed within both groups [F(1,34) = 27.40, P < 0.001, η2 = 0.466], with notable improvements post-intervention in both the A16 (P = 0.009) and U16 (P = 0.0002) groups compared to baseline. There was no significant main treatment effect for VO2max (P > 0.05). However, significant main time [F(1,34) = 131.67, P < 0.001, η2 = 0.795] and interaction [F(1,34) = 36.46, P < 0.001, η2 = 0.517] effects were found for VO2max. Post-intervention, VO2max showed significant increases in both the A16 group (+5.1%, P < 0.001) and the U16 group (+2.8%, P < 0.001) relative to baseline. For the standing long-jump, there were significant main treatment [F(1,34) = 11.35, P = 0.002, η2 = 0.250], time [F(1,34) = 36.46, P < 0.001, η2 = 0.517], and interaction [F(1,34) = 8.99, P = 0.005, η2 = 0.209] effects. Post-hoc analysis indicated that the standing long-jump score was higher at baseline (P = 0.016) and post-intervention (P = 0.0004) in the A16 group compared to the U16 group. Both groups showed improvement post-intervention: A16 (P < 0.001; 5% improvement) and U16 (P = 0.039; 1.8% improvement) relative to baseline. In the sit-up test, there was a significant main treatment [F(1,34) = 4.84, P = 0.035, η2 = 0.125] and time [F(1,34) = 39.88, P < 0.001, η2 = 0.540] effects. Post-hoc analysis revealed that the sit-up performance was significantly higher at baseline (P = 0.013) and post-intervention (P = 0.001) in the A16 group compared to the U16 group. Post-intervention, sit-up scores improved in both A16 (P < 0.001; 14.4% improvement) and U16 (P < 0.001; 13.9% improvement) compared to baseline. The push-up test showed significant main treatment [F(1,34) = 10.22, P = 0.003, η2 = 0.231] and time [F(1,34) = 94.75, P < 0.001, η2 = 0.736] effects. Post-hoc analysis indicated that the A16 group had higher scores at baseline (P = 0.022) compared to the U16 group. Post-intervention, both groups showed significant improvements: A16 (P < 0.001; ~10% improvement) and U16 (P < 0.001; ~18% improvement) relative to baseline.
Internal training load
The mean weekly ITL is depicted in Figure 1. Mean weekly session RPE was not different between the groups (P > 0.05).
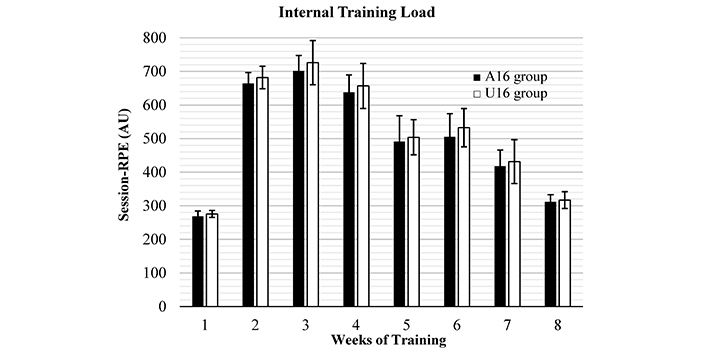
Weekly periodization determined using (CR10-scale) mean weekly RPE-based training load (session-RPE) during the 8 weeks of training intervention in both groups. AU: arbitrary unit; RPE: Ratings of Perceived Exertion
Blood and immunological markers evaluation
Table 3 presents the circulating blood and immunological parameters. No significant treatment, time, or interaction effects were observed for total WBC, ESR, platelets, or counts of monocytes, eosinophils, and basophils (P > 0.05). However, there was a significant main time effect for neutrophil [F(1,34) = 36.55, P < 0.001, η2 = 0.518] and lymphocyte [F(1,34) = 37.59, P < 0.001, η2 = 0.525] counts. Post-intervention, neutrophil counts increased in both the A16 (P < 0.001) and U16 (P < 0.0002) groups, while lymphocyte counts decreased in both A16 (P = 0.0004) and U16 (P = 0.0003) compared to baseline. Significant main time effects were also observed for PLR [F(1,34) = 14.63, P < 0.001, η2 = 0.301], NLR [F(1,34) = 20.00, P < 0.001, η2 = 0.370], SIRI [F(1,34) = 14.84, P = 0.001, η2 = 0.304], MLR [F(1,34) = 11.2, P = 0.002, η2 = 0.250], and serum cortisol concentration [F(1,34) = 10.51, P = 0.003, η2 = 0.236]. Post-intervention, PLR, NLR, and SIRI levels were significantly higher in both the A16 (P = 0.032, P = 0.002, P = 0.004, respectively) and U16 (P = 0.004, P = 0.013, P = 0.04, respectively) groups compared to baseline. MLR levels increased only in the A16 group (P = 0.005) relative to baseline. Serum cortisol levels post-intervention were lower in both A16 (P = 0.004) and U16 (P = 0.03) groups compared to baseline values.
Blood components and immune responses considering the time factor (pre- vs. post-intervention) and the puberty stage
| Blood variables | A16: Puberty 5-Tanner Scale | U16: Puberty < 4.5-Tanner Scale | ||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| WBC (× 103/μL) | 7.1 ± 1.4 | 6.7 ± 1.6 | 6.4 ± 1.5 | 6.6 ± 1.2 |
| ESR (mm/h) | 3.6 ± 3.0 | 3.3 ± 2.4 | 3.1 ± 1.8 | 3.7 ± 3.2 |
| Platelets (× 103/μL) | 212 ± 50 | 205 ± 37 | 195 ± 54 | 208 ± 50 |
| Neutrophils (%) | 47 ± 5 | 55 ± 7* | 44 ± 8 | 51 ± 8* |
| Neutrophils (× 103/μL) | 3.3 ± 0.7 | 3.9 ± 1* | 2.8 ± 0.9 | 3.2 ± 0.8* |
| Lymphocytes (%) | 41 ± 5 | 32 ± 7* | 42 ± 8 | 35 ± 8* |
| Lymphocytes (× 103/μL) | 2.9 ± 0.6 | 2.3 ± 0.6 | 2.7 ± 0.8 | 2.3 ± 0.8 |
| Monocytes (%) | 8.7 ± 1.8 | 9.2 ± 2 | 9.4 ± 2 | 9.2 ± 2 |
| Monocytes (× 103/μL) | 0.61 ± 0.17 | 0.64 ± 0.18 | 0.59 ± 0.16 | 0.57 ± 0.14 |
| Eosinophils (%) | 2.6 ± 1.4 | 2.9 ± 2.3 | 3.9 ± 2.4 | 3.8 ± 2.6 |
| Eosinophils (× 103/μL) | 0.19 ± 0.12 | 0.19 ± 0.16 | 0.25 ± 0.17 | 0.26 ± 0.19 |
| Basophils (%) | 0.5 ± 0.3 | 0.6 ± 0.3 | 0.4 ± 0.2 | 0.4 ± 0.2 |
| Basophils (× 103/μL) | 0.033 ± 0.02 | 0.044 ± 0.02 | 0.027 ± 0.01 | 0.026 ± 0.02 |
| PLR | 76.8 ± 24 | 100 ± 50* | 78.3 ± 30 | 103 ± 39* |
| NLR | 1.2 ± 0.3 | 1.8 ± 0.7* | 1.1 ± 0.4 | 1.6 ± 0.9* |
| MLR | 0.2 ± 0.05 | 0.3 ± 0.01* | 0.2 ± 0.08 | 0.3 ± 0.01 |
| SIRI | 10.3 ± 3.2 | 17 ± 7.7* | 10.5 ± 4.8 | 15.5 ± 10* |
| Serum cortisol (nmol/L) | 421 ± 110 | 343 ± 108* | 446 ± 137 | 371 ± 141* |
WBC: white blood cell; ESR: erythrocyte sedimentation rate; PLR: platelets to lymphocytes ratio; NLR: neutrophils to lymphocytes ratio; MLR: monocytes to lymphocytes ratio; SIRI: systemic inflammation response index. * Statistical difference over-time, between pre- and post-training (P < 0.05)
Discussion
The main purpose of the current research was to evaluate the impact of puberty stage on blood leukocyte counts and cellular immune-inflammatory biomarkers in well-trained adolescent soccer players after an 8-week soccer-specific pre-season training program. To the authors’ knowledge, this is the first study to investigate how such a training program affects training adaptations and immune-inflammatory responses in adolescents at different stages of puberty.
A key finding of this study is that long-term exercise training, conducted at the same frequency, duration, and intensity, does not cause significant differences in immune-inflammatory responses between adolescents at different puberty stages. In terms of training adaptations, both groups showed similar positive changes in body composition and comparable improvements in aerobic capacity, strength endurance, and neuromuscular explosiveness. However, older adolescents demonstrated higher performance in anaerobic and strength parameters. These results indicate that while pre-season soccer-specific training enhances physical fitness, it does not substantially disrupt immune-inflammatory parameters in adolescents, regardless of puberty stage. Therefore, a soccer-specific pre-season training program (mean weekly ITL ~450 AU; daily session RPE ranging from ~300 to 700 AU) can be safely implemented in adolescents (approximately 15 years and older) to achieve positive training adaptations without significantly affecting hematological and immune-inflammatory markers. Notably, older adolescents exhibit greater performance in neuromuscular explosiveness and strength endurance compared to younger ones following the same 8-week training program.
The current study found no main treatment or interaction effects for blood parameters such as ESR and platelets, or for immune-inflammatory markers including NLR, PLR, MLR, and SIRI. Additionally, there were no time effects (pre- vs. post-training) for total WBC, ESR, platelets, and counts of monocytes, eosinophils, and basophils. These findings align with previous research involving acute exercise protocols [12, 13], chronic training interventions [20], and longitudinal studies [19]. The absence of significant differences between groups may be attributed to cellular and molecular adaptations in WBCs resulting from chronic exercise, irrespective of age or puberty stage. Blum et al. [19] examined the effect of training load on stress perception and various immunological markers in adolescent athletes (mean age 13.8 ± 1.5 years) over four years and found that training load did not significantly alter immunological parameters compared to an inactive control group. Similarly, Liu et al. [20] reported that chronic endurance exercise gradually induces transcriptional changes in WBCs, upregulating genes related to protein synthesis and mitochondrial function while downregulating those involved in inflammatory responses. Horn et al. [22] also found that endurance athletes had significantly lower total WBC and neutrophil counts compared to athletes in team or skill-based sports, suggesting that lower WBC counts in endurance sports may result from the immune system adapting to chronic training. Therefore, the training age of the groups (A16: 10.5 years, U16: 8.8 years) likely contributed to the development of leukocyte, ESR, and platelet adaptability to chronic training, potentially obscuring any significant differences in immune-inflammatory responses due to the training intervention.
Previous studies have shown that exercise can lead to increased NLR values, which are associated with fatigue and immune stress [23]. If recovery periods are insufficient, this can result in persistent inflammatory processes [9, 24]. Platelets, beyond their role in primary hemostasis, exhibit several pro-inflammatory properties, establishing them as significant markers of inflammation [41]. Indeed, similar to exercise-induced neutrophilia, increases in NLR, PLR, and SIRI, platelet counts can acutely rise (thrombocytosis) in response to exercise [24]. In the present study, post-intervention levels of neutrophils, NLR, PLR, and SIRI were elevated, while lymphocyte counts were reduced in both groups compared to baseline. These findings align with previous research, indicating a temporary immunosuppressive response that is a common adaptation of the immune system following periods of intense short- [17] or long-term exercise [22, 42, 43]. When these immune biomarker levels return to baseline after exercise stress or adapt due to chronic training, it is viewed as a restoration of homeostasis. This suggests a successful recovery process after intensified exercise [9, 17, 22, 24, 43]. In the current study, blood samples were collected 48 to 72 hours after the training program concluded, showing similar immune-inflammatory results for both groups. This may indicate that adolescents’ immune system is well-adapted to long-term exercise stimuli from approximately 15 years of age, without adverse effects on physiological training adaptations.
To further explore the potential inflammatory response to the 8-week training program, ESR and serum cortisol levels were also analyzed. ESR is a blood test that indicates the level of inflammation in the body, potentially triggered by injury, infection, or various clinical conditions, including immune system and blood disorders, as well as cancer [44]. The results of this study showed that the training intervention did not affect ESR, suggesting that both groups managed the exercise stressors similarly. These findings, consistent with studies involving healthy young soccer players [45] and certain disease states [46], imply that, as with adults, adolescents’ immune systems are not significantly impacted by chronic exercise stress when training and recovery are balanced [17, 41, 43]. Although RPE values increased significantly over the 8 weeks in both groups compared to baseline, the recovery periods provided during this timeframe appeared sufficient to prevent or mitigate inflammation in both younger and older adolescents. However, it is noteworthy that no blood data were collected during the 8-week training period itself. Future research should investigate weekly ESR and immune-inflammatory responses throughout a soccer-specific pre-season training program in adolescents to gain deeper insights.
Cortisol, a key indicator of catabolic metabolism, plays a significant role in daily physiological functions, particularly under exercise-induced stress [47, 48]. This study found no treatment or interaction effects on serum cortisol levels, but a significant time effect was observed. Cortisol levels decreased notably from pre- to post-training, with both groups showing a similar reduction. These findings align with previous research [49, 50], suggesting that the long-term, intensive training employed in this study primarily induced an anabolic response. The lower cortisol levels could reflect a reduction in overall stress, potentially due to adaptive responses during the 8-week training period and effective recovery strategies, indicating that the appropriate selection of training loads, combined with adequate recovery periods, helps mitigate catabolic stress in adolescent players [51].
Both groups demonstrated the ability to tolerate the 8-week pre-season training program, regardless of puberty stage. This suggests that youth soccer players can handle soccer-specific, high-intensity training without significant disruptions to their immune-inflammatory markers. However, younger adolescents (under 16 years; puberty stage < 4.5 on the Tanner Scale) may not yet possess the biological maturity to achieve the same training adaptations in neuromuscular explosiveness and strength endurance as their older counterparts. While both groups showed significant improvements in these areas, the older adolescents (over 16 years; Tanner stage 5) exhibited greater gains. Armstrong et al. [52] noted that muscle metabolism evolves with maturation, allowing older adolescents to develop adaptations relevant to anaerobic performance due to enhanced muscle phosphocreatine kinetics and uptake. Indeed, in this study, older adolescents outperformed younger ones in all anaerobic fitness measures, including neuromuscular explosiveness and strength. Nevertheless, both groups improved their overall physical fitness over the training period. Post-intervention results showed an increase in VO2max (A16: 5.1%, U16: 2.8%), standing long-jump performance (A16: 5%, U16: 1.8%), sit-up repetitions (A16: 14.4%, U16: 13.9%), and push-ups (A16: 10%, U16: 18%). These results indicate that the pre-season training program was effective for both groups, achieving similar adaptation rates irrespective of puberty stage, although older adolescents were stronger and more explosive.
Training intensity for each participant was monitored using session RPE, recorded 15–30 minutes after each training session. This method effectively tracks training load/intensity across various age groups and expertise levels [53]. Unlike HR monitoring, session RPE evaluates total body exertion, including resistance training sessions, which HR alone cannot accurately measure [54, 55]. Thus, the ITL evaluation used in this study is validated as a reliable method for assessing exercise load, especially in the age group studied [27, 38, 56]. Notably, weekly RPE did not differ between groups, which was anticipated.
The current findings highlight specific immune-metabolic responses in well-trained male adolescent soccer players and should not be generalized to other populations. Female adolescents and adults should interpret these results cautiously, as hormonal fluctuations during the menstrual cycle can affect immune cell activity and inflammatory responses. Future studies should include diverse populations, such as female athletes and individuals with inflammatory conditions, to better understand the effects of exercise training on various groups. Additionally, further research should investigate weekly immune-inflammatory responses during soccer-specific pre-season training in youth.
The study limitations are: 1. A broader evaluation of lymphocyte subpopulations and their kinetic responses could provide deeper insights into the cellular effects of the training intervention on well-trained youth athletes. 2. Randomization was not conducted as the groups were separated based on biological age. 3. To better understand the immune-inflammatory responses throughout the training period, weekly assessments of ESR, C-reactive protein, and related immune-inflammatory parameters should be conducted. 4. Including inactive control groups at the same puberty stage as the experimental groups would enhance the methodological design, especially for blood analyses; however, this was not feasible due to financial limitations.
In conclusion, soccer-specific pre-season training appears to have a similar impact on immune-inflammatory parameters in well-trained youth soccer players, regardless of their stage of puberty. While physical fitness improvements were evident in both groups, the training did not affect immune-inflammatory markers differently across puberty stages. This suggests that when the same training load (in terms of frequency, duration, and intensity) is applied, adolescents at different stages of puberty experience similar effects on immune-inflammatory parameters. However, physical fitness improves significantly in all players, regardless of their puberty stage. From a practical perspective, coaches should note that soccer-specific pre-season training is well-tolerated by adolescents (ages 15–18 years) across different stages of puberty. This type of training significantly enhances physical fitness without causing significant disruptions to immune-inflammatory parameters.
Abbreviations
| AU: | arbitrary units |
| BF%: | body fat percentage |
| BP: | blood pressure |
| ESR: | erythrocyte sedimentation rate |
| HR: | heart rate |
| ITL: | internal training load |
| MLR: | monocyte-to-lymphocyte ratio |
| NLR: | neutrophil-to-lymphocyte ratio |
| PLR: | platelet-to-lymphocyte ratio |
| RPE: | Ratings of Perceived Exertion |
| RT: | room temperature |
| SIRI: | systemic inflammation response index |
| VO2max: | maximum oxygen consumption |
| WBC: | white blood cell |
Declarations
Acknowledgments
The authors would like to thank the coaching teams’ members for their excellent collaboration and support throughout the study period. The excellent cooperation of the participants and their parents is strongly appreciated.
Author contributions
MH: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing—original draft, Writing—review & editing, Project administration. EC and NZ: Data curation, Validation, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study conforms with the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1964) and it was approved by the Institutional Research Ethics Committee (REC-010712).
Consent to participate
Written informed consent to participate in the study was obtained prior to any assessment from all participants and their parents.
Consent to publication
Not applicable.
Availability of data and materials
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Funding
Not applicable.
Copyright
© The Author(s) 2024.