Affiliation:
1Department of Gynecologic Oncology, The First Affiliated Hospital of Henan Polytechnic University, Jiaozuo 454000, Henan Province, China
Affiliation:
1Department of Gynecologic Oncology, The First Affiliated Hospital of Henan Polytechnic University, Jiaozuo 454000, Henan Province, China
Affiliation:
1Department of Gynecologic Oncology, The First Affiliated Hospital of Henan Polytechnic University, Jiaozuo 454000, Henan Province, China
2Academy of Medical Engineering and Translational Medicine, Medical College, Tianjin University, Tianjin 300072, China
Email: niugaoli@hpu.edu.cn
ORCID: https://orcid.org/0000-0003-0167-8406
Explor Immunol. 2024;4:853–870 DOI: https://doi.org/10.37349/ei.2024.00177
Received: September 11, 2024 Accepted: December 11, 2024 Published: December 18, 2024
Academic Editor: Calogero Caruso, University of Palermo, Italy
The article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies
Ovarian cancer is the deadliest malignant tumor in the female reproductive system. Despite advancements in standard treatments such as tumor debulking surgery and platinum-based chemotherapy, the overall survival rate remains low. The emergence of targeted therapies, including Poly(ADP-ribose) polymerase (PARP) inhibitors and anti-angiogenic agents, has provided new avenues for treatment. However, drug resistance and disease heterogeneity continue to pose significant challenges. Immune checkpoint inhibitors (ICIs), as an emerging therapeutic approach, primarily target the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) pathways to restore anti-tumor immune responses. Although ICIs have shown significant efficacy in other malignancies, their effectiveness in ovarian cancer is limited, with a response rate of only 10–15% for monotherapy. Recent studies have focused on combining ICIs with chemotherapy, anti-angiogenic agents, or PARP inhibitors to enhance therapeutic outcomes. This article reviews the progress of ICIs in ovarian cancer, including monotherapy and combination treatment strategies, and explores emerging therapeutic targets and strategies aimed at improving patient prognosis and achieving personalized treatment. By gaining a deeper understanding of the tumor microenvironment and its immune evasion mechanisms, there is hope for developing more effective treatment options in the future, ultimately improving the survival rates and quality of life for ovarian cancer patients.
Ovarian cancer is one of the three most common malignant tumors in the female reproductive system and has the highest mortality rate among gynecological malignant tumors [1, 2]. Currently, the recommended treatment strategy is tumor debulking surgery, supplemented by chemotherapy primarily with platinum and paclitaxel [3]. In recent years, anti-angiogenic drugs such as bevacizumab, Poly(ADP-ribose) polymerase (PARP) inhibitors, and antibody-drug conjugates (ADCs) have also been widely applied in the treatment of ovarian cancer, achieving significant research progress [4–6]. Notably, multiple phase III clinical trials have demonstrated the efficacy of PARP inhibitors as first-line maintenance therapy [7]. However, the efficacy of these drugs is closely related to the patient’s homologous recombination (HR) repair status or BRCA gene mutation status. Some patients may develop resistance to PARP inhibitors, which limits their broader clinical application [8]. Ovarian cancer originates from different types of cells, resulting in various tumor subtypes with distinct biological behaviors and treatment responses. This heterogeneity further complicates treatment [9, 10]. Many patients experience multiple recurrences after receiving standard initial treatment and gradually develop resistance to chemotherapeutic agents. Due to these complex factors, the overall survival (OS) rate of ovarian cancer remains low, and the prognosis is poor.
To address these challenges, researchers are exploring novel therapeutic approaches, including ICIs. ICIs primarily target immune checkpoints such as PD-1, PD-L1, and CTLA-4, aiming to restore anti-tumor immune responses [11]. Over the past two decades, studies have shown that ovarian cancer exhibits a certain degree of immunogenicity, capable of triggering anti-tumor immune responses in the host. Tumor-infiltrating lymphocytes (TILs) can be detected in approximately half of ovarian cancer cases [12]. However, ovarian cancer is a highly heterogeneous malignant tumor, capable of evading immune surveillance during its spread and proliferation [13–15]. Due to the diversity of subtypes, the mechanisms of immune evasion also vary. In some patients, this is manifested by increased PD-L1 expression. PD-L1 on the surface of tumor cells binds to the PD-1 receptor on T cells, suppressing the cytotoxic activity of T cells. ICIs can block this interaction, alleviate immune suppression, and reactivate the immune system, thereby enhancing the anti-tumor activity of cytotoxic T lymphocytes (CTLs) (CD8+ T cells) and modulate the tumor microenvironments (TMEs) [16, 17]. This provides a potentially effective strategy for the immunotherapy of ovarian cancer. Although ICIs have demonstrated significant survival benefits in other malignancies, such as cervical and endometrial cancers [18], their efficacy in ovarian cancer remains limited. The median response rate to PD-1 and PD-L1 inhibitors as monotherapy is only 10–15% [19, 20]. However, combining ICIs with chemotherapy, anti-angiogenic agents, PARP inhibitors, ADCs, or other ICIs may significantly improve therapeutic outcomes. These combination strategies have the potential to enhance efficacy, extend the survival of ovarian cancer patients, and improve their quality of life [21, 22]. Currently, multiple clinical trials are underway to explore these approaches, and a detailed review is provided below (Figure 1).
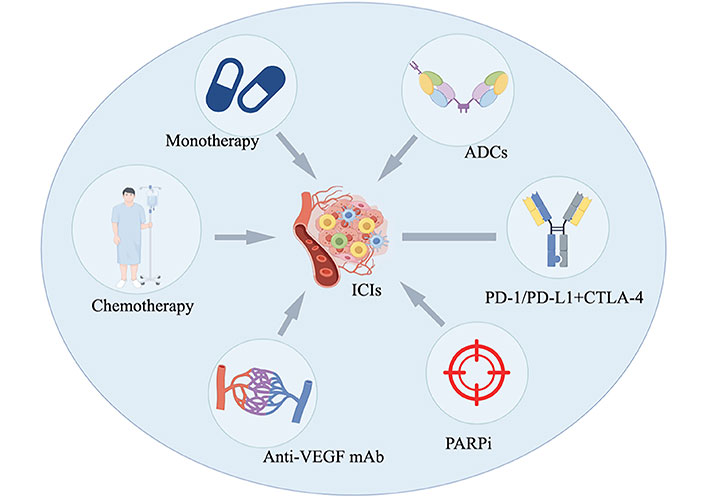
The application of ICIs in the treatment of ovarian cancer, including monotherapy, combination chemotherapy, combination with anti-angiogenic agents, combination with PARPi, combination with antibody-drug conjugates (ADCs), and combinations of different types of ICIs (Created by Figdraw ID: ROIPAa5363). ICIs: immune checkpoint inhibitors; VEGF: vascular endothelial growth factor; PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand 1; CTLA-4: cytotoxic T-lymphocyte antigen 4; PARPi: Poly(ADP-ribose) polymerase inhibitor
Immune checkpoint regulation is an important modulatory mechanism of the immune system, and these regulatory molecules play a central role in controlling self-tolerance, autoimmunity, and tissue damage caused by immune responses. The process of tumor elimination by the immune system involves several key steps: tumor cells generate and release tumor-associated antigens (TAAs), which are captured by mature dendritic cells (DCs; immature DCs mature in response to TAAs). These mature DCs migrate to the lymph nodes, where they present the captured TAAs to T cells, thereby activating effector T cells. Effector T cells exit the lymph nodes, enter the circulatory system, and infiltrate TME [23, 24]. They specifically recognize and bind to tumor cells through the interaction of the T cell receptor (TCR) with major histocompatibility complex (MHC) I molecules, directly killing tumor cells by releasing perforin and granzyme, or inducing tumor cell apoptosis through the regulation of the Fas/FasL pathway (Figure 2) [25, 26]. The immunotherapy strategies developed based on the cancer-immunity cycle are currently primarily focused on the research of ICIs, involving the PD-1/PD-L and CTLA-4/CD80/CD86 pathways [27]. PD-1 is an immune checkpoint receptor primarily expressed on the surface of activated T cells and is a member of the CD28/CTLA-4 family of co-inhibitory receptors, primarily functioning to inhibit the activity of effector T cells within tumor tissue [28, 29]. T cells can specifically recognize tumors through the MHC molecules on tumor cells. The TCR binds to the MHC-peptide complex, generating the first signal; meanwhile, the B7 molecule on antigen-presenting cells binds to CD28 on T cells, providing the second signal. The combination of these two signals activates T cells, leading to their proliferation and differentiation, ultimately promoting an immune response to attack tumor cells [30]. However, CD28 and CTLA-4 share the same B7 ligands (including CD80 and CD86), so the binding of CTLA-4 to B7 inhibits T cell activation. In the TME, high expression of CTLA-4 is typically associated with regulatory T cells (Tregs) [31], which can suppress the immune response, thereby limiting the attack on the tumor [29, 32, 33]. The most important peripheral regulatory pathway is the interaction between the PD-1 receptor expressed on T cells and PD-L1 and PD-L2 on the surface of tumor cells. The PD-1 receptor is also expressed on CD8-infiltrated macrophages, fibroblasts, and tumor cells [34]. When the PD-1 receptor binds to its ligands, it can inhibit the proliferation and activation of CD8+ T cells and regulate the production of interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-2 (IL-2). This is also one of the mechanisms by which tumor cells achieve immune evasion [35, 36].
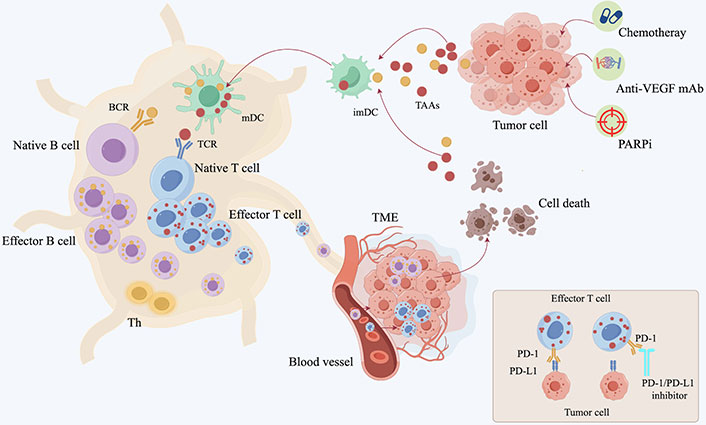
The process of the immune system eliminating tumors. 1. Treatments such as chemotherapy, anti-VEGF monoclonal antibodies, and PARPi lead to the release of antigens from tumor cells, promoting the maturation of DCs; 2. mature DCs capture the antigens and present them to T cells; 3. activation of effector T cells; 4. T cells exit the lymph nodes and enter the circulatory system; 5. T cells penetrate the vascular wall and infiltrate the TME; 6. the interaction of the TCR with MHC I specifically recognizes and binds to tumor cells, leading to their elimination. PD-1/PD-L1 inhibitors prevent the binding of effector T cells to tumor cells (Created by Figdraw ID: PSPUAc4caa). DCs: dendritic cells; TCR: T cell receptor; TME: tumor microenvironment; TAAs: tumor-associated antigens; VEGF: vascular endothelial growth factor; PARPi: Poly(ADP-ribose) polymerase inhibitor; PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand 1; BCR: B cell receptor; mDC: mature dendritic cell; imDC: immature dendritic cell
In ovarian cancer, high infiltration of effector CD8+ T cells may contribute to the survival of some patients with ovarian cancer. It has also been found that PD-L1 is often highly expressed on the surface of tumor-associated macrophages (TAMs) in primary and metastatic high-grade serous ovarian carcinoma patients [37]. Hamanishi et al. [34] observed a significant negative correlation between the count of CD8+ T lymphocytes within epithelial cells and the expression of PD-L1 in tumor cells, with patients having higher levels of PD-L1 in tumor cells having a poorer prognosis. Reddy et al. [38] observed the correlation between PD-L1 expression and malignant tumors as well as benign/borderline diseases, finding that the PD-L1 expression on monocytes in the ascites and blood of patients with malignant ovarian epithelial cancer is higher than that in patients with benign/borderline diseases. Therefore, inhibiting the binding between PD-1 and CTLA-4 and their receptors may enhance the effectiveness of cytotoxic CD8+ T cells, thereby generating higher antitumor activity [39].
In the treatment of ovarian cancer, the application of PD-L1/PD-1 inhibitors has made certain progress, primarily including anti-PD-1 monoclonal antibodies represented by nivolumab and pembrolizumab, as well as anti-PD-L1 monoclonal antibodies represented by atezolizumab and avelumab [40–42]. They restore the immune system’s ability to attack tumor cells by blocking the PD-1/PD-L1 pathway. The response rate in the treatment of ovarian cancer is approximately 10–15%. In terms of safety, most treatment-related adverse events (TRAEs) of PD-1/PD-L1 inhibitors are grade 1–2, with a lower incidence of grade 3–4 adverse reactions [41, 43, 44]. A phase I clinical trial evaluating the use of atezolizumab in 12 patients with recurrent ovarian cancer showed that 91.7% of patients experienced TRAEs of any grade, with two cases experiencing grade 3 events. The objective response rate (ORR) and disease control rate (DCR) were both 22.2%. Among the 8 patients with PD-L1 expression of ≥ 5%, 2 cases achieved objective responses [42]. In a phase Ib clinical trial involving 125 patients with recurrent or refractory ovarian epithelial cancer, the ORR of avelumab reached 9.6%, with only one patient achieving a complete response (CR). In this study, the one-year progression-free survival (PFS) rate for patients was 10.2%, and the median OS was 11.2 months. In terms of safety, 7.2% of patients experienced grade 3–4 TRAEs, and 16.8% of patients had any-grade immune-related events. Additionally, the study found no correlation between PD-L1 expression levels and clinical response rates [41].
In the KEYNOTE-028 study, a total of 26 patients with PD-L1-positive advanced ovarian cancer were enrolled, with an observed ORR of 11.5%, including 1 patient with a CR and 2 patients with partial response (PR). There were 7 patients with stable disease (SD), accounting for 26.9%. The median PFS (mPFS) was 1.9 months [95% confidence interval (CI): 1.8 months to 3.5 months], and the OS was 13.8 months (95% CI: 6.7 months to 18.8 months). TRAEs occurred in 19 patients, representing 73.1%, mainly grade 1–2, with the most common adverse events (AEs) including arthralgia (19.2%), nausea (15.4%), and pruritus (15.4%). One grade 3 AE was reported, but there were no deaths or discontinuations due to AEs [44]. In the KEYNOTE-100 trial, researchers deeply explored the clinical efficacy of pembrolizumab in patients with advanced ovarian cancer, using the PD-L1 combined positive score (CPS) as a predictive biomarker for treatment response. The results showed that for patients with CPS < 1, the ORR was 4.1%, for CPS ≥ 1 it was 5.7%, and for CPS ≥ 10 it was 10.0%. Therefore, patients with higher PD-L1 expression had an increased ORR and prolonged OS when treated with pembrolizumab. Additionally, the TRAEs observed in this trial were consistent with the performance of pembrolizumab in other studies [45]. The most common TRAEs include fatigue, gastrointestinal reactions, endocrine disorders, and skin issues. Although rare, toxicities affecting the nervous, cardiac, pulmonary, and renal systems have also been observed. A large retrospective analysis showed that 0.36% of patients treated with PD-1 inhibitors experienced fatal outcomes, while 1.23% of patients treated with immunotherapy combinations had fatal outcomes, with the highest mortality rates associated with adverse reactions being colitis, pneumonia, and myocarditis [46, 47]. Organ toxicity is a rare AEs during the treatment process with ICIs, but in clinical trial UMIN000005714, out of 20 patients, 6 experienced arrhythmias, with no reported cases of myocarditis [43].
Overall, ICIs have demonstrated limited efficacy as monotherapy in the treatment of ovarian cancer. Representative agents have been evaluated in multiple clinical trials, showing modest ORR and DCR. Despite the low overall response rates, ICIs are generally well-tolerated, with most TRAEs classified as grade 1–2. Serious AEs (grade 3–4) are relatively uncommon. In summary, while ICIs as monotherapy have not yet achieved significant success in ovarian cancer treatment, ongoing studies aim to enhance their efficacy through combination therapies and improved patient selection based on biomarkers such as PD-L1 expression. The following discussion will focus on the combination approaches of ICIs in treating ovarian cancer, with specific clinical studies detailed in Table 1.
Several clinical trials are currently underway to this investigate
| Type | Study | Design | Population | Explorimental arm | ORR | DCR | mPFS (m) | OS (m) | AE/TEAE | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| ICIs monotherapy | JAVELIN | 1b, open label | n = 125 | Ave | 9.6% | 10.2% (y = 1) | 11.2 | 7.2% pts, 3–4 S | [41] | |
| KEYNOTE-028 | Age ≥ 18 y, advanced, PD-L1 positivity | n = 26 | Pembrolizumab | 11.5% | 1.9 m | 13.8 | 19 (73.1%) pts, one grade 3 S | [44] | ||
| KEYNOTE-100 | Phase II | Cohort A, n = 285Cohort A, n = 91 | Pembrlizumab | A 7.4%B 9.9%CPS+< 1 was 4.1%> 1 was 5.7%≥ 10 was 10.0% | A: 8.2 mB: not reached | 2.1 m | A: not reachedB: 17.6 | [45] | ||
| NANT study | Prospective, multicenter, phase II, single arm | n = 127, HRD pts n = 67 | Niraparibb | HRD pts: 62.5%BRCAm pts: 77.3% | HRD pts: 87.5%BRCAm pts: 100% | ≥ 3 S | [97] | |||
| Chemotherapy plus ICIs | JAVELIN-200 | An open-label, three-arm, randomized, phase 3 | n = 566 | Ave:Ave + PLD:PLD = 1:1:1 | Ave: 9 mAve + PLD: 3.7 mPLD: 3.5 m | Ave: 8.2Ave + PLD: 18.4PLD: 17.4 | ≥ 3 S | [53] | ||
| JAVELIN-100 | Global, open label, three-arm, parallel, randomized, phase 3 | Ave: n = 332Chemotherapy + Ave: n = 331Control: n = 335 | Ave:chemotherapy + Ave:control = 1:1:1 | Ave: 16.8 mChemotherapy + Ave: 18.1 mControl: NE | Interruption | [54] | ||||
| Combined pembrolizumab and PLD in PROC | Single arm, multi-center phase II | n = 26 | PLD + pembrolizumab | 26.1% | [55] | |||||
| Anti-angiogenic drugs plus ICIs | Assessment of combined Niv and Bev in ROC | Single-arm, phase 2 | n = 38PROC: n = 18PSOC: n = 20 | Niv + Bev | PROC: 16.7%PSOC: 40.0% | 8.1 m | ≥ 3 S | [71] | ||
| ATALANTE/ENGOT-ov29 | Randomized (2:1), double blinded, phase III, PSOC | n = 614Atezolizumab: n = 410Placebo: n = 204 | Cx + Bev + At; Cx + Bev + placebo | At: 13.5 mPlacebo: 11.3 mPD-L1-positive: 15.2 m vs. 13.1 m | At: 35.5 mPlacebo: 30.6 m | ≥ 3 S | [98] | |||
| PARP inhibitors plus ICIs | MEDIOLA | Multicenter, open-label, phase 1/2, basket trial | gBRCAm doublet: n = 51Non-gBRCAm doublet: n = 32Non-gBRCAm triplet cohorts: n = 31 | Olaparib plus durvalumab, with or without Bev | gBRCAm expansion doublet 92.2% | (at 24 weeks) non-gBRCAm doublet: 28.1%Non-gBRCAm triplet cohorts: 74.2% | ≥ 3 S | [81, 99] | ||
| Keynote-162 | Open-label, single-arm phases 1 and 2 study | n = 62 | Niraparib + pembrolizumab | 18% | 65% | 3 m | ≥ 3 S | [100, 101] | ||
| ADCs plus ICIs | FORWARDII | Ib | MIRV + pembrolizumab | 43% | mDOR 6.9 m | 5.2 m | [80] | |||
| PD-1 + CTLA-4 | NGR oncology study | Randomized phase II | Niv (n = 49)Niv and ipilimumab (n = 51) | Niv and ipilimumab | Niv: 12.2 mNiv and ipilimumab: 31.4 m | Niv: 2 mNiv + ipilimumab: 3.9 m | ≥ 3 SNiv: 3%Niv + ipilimumab: 49% | [88] | ||
| Anti-CD27 antibody + anti-PD-1 | Agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (Niv) | Phase 1/2 dose-escalation and expansion | n = 175 (phase 1 n = 36; phase 2 n = 139) | Varlilumab and Niv | Phase 1: uncertainPhase 2: 12.5% | Without significant toxicity | [90] |
pts: patients; PD-L1: programmed cell death ligand 1; mPFS: median progression-free survival; ORR: objective response rate; OS: overall survival; mOS: median OS; DCR: disease control rate; mDOR: median duration of response; AE: adverse event; TRAEs: treatment-related AEs; n: number; PSOC: platinum-sensitive recurrent ovarian cancer; PROC: platinum-resistant recurrent ovarian cancer; Cx: platinum-based chemotherapy; Bev: bevacizumab; MIRV: mirvetuximab soravtansine; ADCs: antibody-drug conjugates; ICIs: immune checkpoint inhibitors; GEM/PLD: gemcitabine or pegylated liposomal doxorubicin; Ave: avelumab; Niv: nivolumab; m: months; y: years; CPS: combined positive score; NE: not evaluated; CTLA-4: cytotoxic T-lymphocyte antigen 4; PD-1: programmed cell death protein 1; HRD: homologous recombination deficiency; BRCAm: BRCA mutated; gBRCAm: germline BRCAm
Clinical study data indicate that while chemotherapy destroys cancer cells, it can enhance the antigen presentation capability of tumors, increase their immunogenicity, and prompt tumor cells to express PD-L1. A recent study analyzed samples from patients with high-grade serous ovarian cancer who received neoadjuvant chemotherapy with cisplatin or paclitaxel and found that chemotherapy could increase the infiltration of natural killer cells in the tumor site as well as the oligoclonal expansion of T cell subsets [48]. Additionally, research has shown that chemotherapy can enhance the immunogenicity of high-grade serous ovarian cancer, thereby increasing its sensitivity to immune attacks [49]. Some studies have also found that neoadjuvant chemotherapy can increase the number of TILs and the expression levels of PD-L1 in the tumor stroma of ovarian cancer patients, which significantly affects the balance between immune response and immune tolerance subsets [19, 50]. Specifically, the ratios of CD8+/FOXP3+, CD3+/FOXP3+, and CD68+/CD163+ have increased, and these changes are closely associated with significant improvements in patient prognosis [51, 52]. In the JAVELIN-200 trial, a total of 566 patients with platinum-resistant recurrent or refractory ovarian cancer were enrolled and randomly assigned in a 1:1:1 ratio to three treatment groups: avelumab monotherapy, avelumab in combination with pegylated liposomal doxorubicin (PLD), and PLD monotherapy. The study results showed that the mPFS was 3.7 months for the combination therapy group, 3.5 months for the PLD monotherapy group, and 1.9 months for the avelumab monotherapy group. The median OS was 11.8 months, 15.7 months, and 13.1 months for the three groups, respectively. Although the results did not meet expectations, neither the combination of avelumab and PLD nor avelumab monotherapy significantly improved patients’ PFS or OS [53, 54]. At the same time, another phase III clinical trial (JAVELIN-100) aimed to evaluate the effectiveness of avelumab as a first-line treatment in combination with carboplatin and paclitaxel. However, the trial was terminated early due to the interim analysis not meeting expected outcomes. The trial data showed that the mPFS in the avelumab maintenance group was 16.8 months, the mPFS in the avelumab plus chemotherapy group was 18.1 months, and in contrast, the mPFS in the chemotherapy-only group was not estimable [54].
In a phase II clinical trial, researchers evaluated the efficacy of pembrolizumab in combination with PLD for the treatment of platinum-resistant ovarian cancer. The trial included a total of 26 patients, with a clinical benefit rate (CBR) of 52.2%, including 5 patients who achieved PR (21.7%), and 1 patient who achieved CR (4.3%), resulting in an ORR of 26.1%. Additionally, 6 patients had SD lasting at least 24 weeks. Compared to historical data of using PLD alone or single anti-PD-1/PD-L1 therapy, the combination treatment showed its advantages in terms of ORR and mPFS, providing preliminary clinical evidence of the benefits of combining immunotherapy with chemotherapy for the treatment of platinum-resistant ovarian cancer [55]. In a randomized phase II clinical study (NCT03275506), researchers explored the efficacy of neoadjuvant and adjuvant pembrolizumab in patients with advanced high-grade serous cancer. The study targeted patients with stage IIIC/IV high-grade serous ovarian carcinoma who could not achieve complete surgical resection and assessed the effect of adding pembrolizumab (200 mg every 3 weeks) to the standard treatment regimen (carboplatin and paclitaxel). Among the 61 patients treated with pembrolizumab, the primary endpoint—CRR reached 74%, compared to a CRR of 70% in the control group that received only standard therapy [56]. Based on the results of pembrolizumab in the neoadjuvant chemotherapy for CRR, a phase II clinical trial investigated the efficacy and safety of pembrolizumab as an ICI in combination with chemotherapy. The study data showed that the mPFS was 14.88 months, and the median OS was 57.43 months. In the subgroup of patients with a PD-L1 CPS of ≥ 10, both mPFS and OS were not reached, demonstrating a significant therapeutic effect compared to the mPFS (10.5 months) and OS (30.90 months) of patients with CPS < 10 [57]. Integrating these study results provides valuable guidance for the selection of ICIs in the future treatment of patients with platinum-resistant or platinum-refractory ovarian cancer.
The combination of chemotherapy and ICIs shows potential synergistic effects in the treatment of ovarian cancer. Chemotherapy not only directly kills cancer cells but also enhances the immunogenicity of tumors, promoting the infiltration of immune cells and the expression of PD-L1. Clinical studies indicate that the combination of chemotherapy and ICIs can improve patient CBRs and survival, although some trials have not met expectations. Overall, the integration of chemotherapy and ICIs offers a new treatment strategy for platinum-resistant or platinum-refractory ovarian cancer patients, warranting further research and exploration.
Anti-angiogenic drugs, such as bevacizumab and sorafenib, play a role in cancer treatment through various mechanisms, including directly inhibiting tumor angiogenesis, improving the TME, and synergistic effects with immunotherapy, thereby showing significant efficacy in the treatment of various types of cancer [58]. During the process of tumor growth, various factors, such as the driving effect of vascular endothelial growth factor (VEGF), promote the expansion of the vascular bed, accompanied by the formation of new blood vessels [59]. Tumor neo vasculature, due to reduced perfusion and increased permeability, exacerbates hypoxia, acidosis, and necrosis within the tumor. These microenvironmental changes trigger a series of immunosuppressive mechanisms [60], leading to the suppression of effector T cell functions and impeding the infiltration of effector T cells into tumor tissues [61, 62]. Additionally, VEGF in the circulating blood can promote an increase in PD-1 expression on the surface of tumor CD8+ T cells, thereby inhibiting T cell activity [63]. It can also hinder the maturation and function of DCs, resulting in the evasion of tumor cells from immune surveillance [64]. Therefore, inhibiting the abnormal angiogenesis of tumors and preventing tumor cells from evading immune system surveillance is crucial for cancer treatment. It is widely believed that tumor cells coexist with a variety of immune cells [65, 66], some of which, such as innate immune cells, can release cytokines and chemokines like IL-12 or TNF, promoting the normalization of tumor vasculature from an abnormal state [67]. Additionally, adaptive immune cells, including CD8+ T cells and Th1 cells, can secrete the cytokine IFN-γ [68, 69]. The normalization of tumor vasculature can enhance blood perfusion in tumors, alleviate hypoxia, and thereby improve the distribution and therapeutic effects of antitumor drugs [70].
Currently, several clinical studies are actively advancing to evaluate the efficacy and safety of the combination therapy of anti-angiogenic drugs with ICIs. In a phase II clinical study targeting recurrent ovarian cancer, the combination treatment regimen of nivolumab and bevacizumab enrolled 38 patients, including 18 patients with platinum-resistant recurrence and 20 patients with platinum-sensitive recurrence. The study results showed that the ORR in platinum-sensitive patients reached 40.0%, while the ORR in platinum-resistant patients was 16.7%, with a mPFS of 8.1 months. Approximately 89% of patients experienced at least one TRAE of any grade, with 23.7% of patients experiencing AEs of grade 3 or higher. These results suggest that the combination therapy may offer greater benefits in treating PSROC, particularly with more pronounced effects in the platinum-sensitive patient population [71]. In a phase I clinical study exploring the combination therapy of durvalumab and cediranib for PRROS, a total of 14 patients participated. The study achieved an ORR of 50% and a DCR of 75%, with treatment responses being independent of tumor cell PD-L1 expression levels. However, this treatment regimen was also associated with more severe AEs such as hypertension and diarrhea [72]. In the phase III ATALANTE clinical trial, researchers evaluated the efficacy of atezolizumab combined with bevacizumab in 614 patients with platinum-resistant recurrent ovarian cancer (PROC). The results indicated that the mPFS was 13.5 months for the atezolizumab group, compared to 11.3 months for the placebo group. Both groups reported AEs of grade 3 or higher during the safety analysis. These findings suggest that the combination of atezolizumab and bevacizumab may offer certain clinical benefits for patients with PROC, but further research is needed to confirm its long-term efficacy and safety [73, 74].
ADCs are a novel class of anti-cancer drugs, consisting of three parts: monoclonal antibodies, linkers, and cytotoxic drugs. They recognize antigens on the surface of cancer cells and utilize linkers as carriers to facilitate the release of cytotoxic drugs within the cancer cells [75]. The released cytotoxic drugs have a potent cytotoxic effect on cancer cells, capable of inducing immunogenic cell death, thereby enhancing the body’s immune response to cancer. ADCs have been approved for the treatment of hematological malignancies, advanced breast cancer (HER2-positive breast cancer), FR-α-positive and platinum-resistant epithelial ovarian cancer, urothelial carcinoma, and other solid tumors [76, 77]. ADCs directly kill cancer cells by carrying cytotoxic drugs and may have a synergistic effect with ICIs by enhancing the immunogenicity of cancer cells and improving the TMEs. In addition, due to some normally active proliferating cells potentially expressing the same surface antigens as cancer cells, the drug may also act on these normal cells, leading to a range of AEs, such as gastrointestinal dysfunction (nausea, vomiting/diarrhea), hematological toxicity (leukopenia/anemia), hair loss, and so on. Based on these biological principles, the combined therapeutic strategy of ADCs with ICIs is currently under active investigation. In the field of ovarian cancer treatment, several clinical trials are also in progress [78].
Mirvetuximab soravtansine (MIRV), an ADC targeting the FR-α antigen, has been approved by FDA for the treatment of ovarian cancer [79]. In the phase Ib FORWARD II study, the drug, in combination with pembrolizumab and other medications, has shown preliminary efficacy in patients with platinum-resistant FR-α-positive ovarian cancer. Data indicates that the ORR of MIRV combined with pembrolizumab is 43%; the median duration of response (mDOR) is 6.9 months. Additionally, this combined therapy has demonstrated good tolerability, with the main AEs being grade 1–2, including symptoms such as blurred vision, nausea, and fatigue. Currently, this treatment regimen is still under further investigation [80]. Another ongoing study is about XMT-1536 (UpRi), an ADC targeting the sodium-dependent phosphate transporter IIb (NaPi2b), which is highly expressed in most ovarian cancers [81]. The study aims to evaluate the efficacy and safety of UpRi monotherapy in patients with recurrent platinum-resistant ovarian cancer. Preliminary results show that the ORR in NaPi2b-positive ovarian cancer patients has reached 34%, and most AEs are grade 3 or lower, primarily consisting of fatigue and nausea [82], and its clinical trials in combination with immunotherapy may still be in the early stages. In addition, an emerging ADCs therapy, the immunostimulatory antibody conjugate (ISAC), has demonstrated potent and durable anti-tumor effects mediated by the immune system [83]. Clinical trials are currently underway to assess the efficacy and safety of ISAC in combination with anti-PD-1/PD-L1 therapies, such as NCT04278144 and NCT04460456. In summary, the combination of ADCs and ICIs offers a new treatment option for ovarian cancer patients, particularly those with PROC. By integrating the targeted cytotoxic effects of ADCs with the immune activation properties of ICIs, this combined strategy holds promise for improving treatment efficacy and patient survival rates. Preliminary research results indicate that the use of ADCs such as MIRV and UpRi in conjunction with ICIs can significantly enhance ORRs while demonstrating good tolerability. However, it remains essential to monitor AEs during clinical trials to ensure patient safety. As more clinical trials are conducted, the prospects for the application of ADCs and ICIs in the field of ovarian cancer are promising, potentially providing patients with better treatment outcomes and quality of life.
In the research on combination therapies for ovarian cancer, both PD-1 and CTLA-4 are immune checkpoint, and due to their distinct mechanisms of anti-tumor action, the combination of their use in ovarian cancer has also attracted attention [57]. CTLA-4 and CD28 are two co-stimulatory receptors expressed on the surface of T cells, both capable of binding to B7 molecules on the surface of APCs. When CD28 binds to B7 molecules, it enhances T cell activation and promotes immune responses, whereas when CTLA-4 binds to B7 molecules, it induces immune suppression [84]. However, because CTLA-4 has a higher binding affinity for B7 molecules than CD28, it can competitively inhibit the binding of CD28 to B7 molecules, thereby generating a suppressive immune effect [85]. CTLA-4 primarily participates in the stages of antigen presentation and initial T cell activation within lymph nodes, regulating T cell activation by competitively inhibiting the binding of CD28 to B7. In contrast, PD-1 inhibits the immune response of already activated T cells against target cells in peripheral tissues, thereby maintaining immune tolerance [86]. By targeting CTLA-4 and PD-1/PD-L1 with ICIs, the immune tolerance of T cells to tumors can be reversed, thereby enhancing their ability to recognize and kill tumor cells [87].
In a randomized phase II clinical trial (NGR GY003), the efficacy of nivolumab in combination with ipilimumab (a fully human monoclonal antibody that targets CTLA-4) for the treatment of recurrent or persistent ovarian cancer was investigated. The results showed that 12.2% of patients who received nivolumab alone achieved ORR within 6 months, while the ORR in the combination therapy group reached 31.4%. The mPFS was 2 months in the nivolumab alone group and 3.9 months in the combination group, and the median OS was 21.8 months and 28.1 months, respectively. In terms of safety, 33% of patients in the nivolumab group experienced grade 3 or higher AEs, compared to 49% in the combination group. There were no treatment-related deaths during the entire study period. These findings suggest that combined immunotherapy may provide better efficacy and manageable safety for patients with ovarian cancer [88]. Varlilumab is a fully human IgG1 anti-CD27 monoclonal antibody that activates T cells by binding to CD27. The activation of CD27 not only enhances T cell proliferation, survival, and effector functions but also improves the immune system’s ability to recognize and attack tumor cells. Furthermore, the activation of CD27 promotes the formation of memory T cells, enabling the immune system to respond more quickly and effectively upon re-encountering tumor antigens, thereby reducing the risk of recurrence [89]. An analysis of the safety and efficacy of its combination with nivolumab for the treatment of advanced solid tumors showed an ORR of 12.5% in patients with ovarian cancer. An increase in the expression of PD-L1 and TILs was observed, with an increase of ≥ 5% correlating with better PFS. TRAEs mainly included fatigue (18%), itching (16%), and rash (15%) [90]. In summary, the aforementioned combinations show promising prospects in the treatment of ovarian cancer. These therapeutic approaches activate and enhance T cell function, reversing tumor immune tolerance, and may provide more effective treatment options for patients. Future research needs to further explore the long-term effects of these immunotherapy combinations and their applicability in different patient populations.
In recent years, significant progress has been made in the application of ICIs in the treatment of ovarian cancer. Although the effectiveness of ICIs as monotherapy is limited, combination strategies with other treatments have shown greater potential, especially in patients with platinum-resistant relapsed ovarian cancer, with clinical research data indicating hope [91]. However, the TME in ovarian cancer is complex, and immunosuppressive factors such as Tregs and TAMs weaken the anti-tumor effects of effector T cells, leading to non-responsiveness or resistance to ICI treatment in some patients [92].
Specifically, the TME in ovarian cancer exhibits high heterogeneity, resulting in significant differences in responses to ICIs among patients. Some tumors may alter their microenvironment to suppress the activity and infiltration of T cells, thereby evading immune surveillance. Additionally, tumor cells and infiltrating immune cells (such as TAMs) often express high levels of PD-L1, which binds to PD-1 on T cells, inhibiting T cell proliferation and activity, further exacerbating immune tolerance [93]. Certain tumor cells may alter antigen expression through genetic mutations, reducing T cell recognition and further contributing to ICI resistance. At the same time, tumor cells may escape the effects of ICIs by upregulating other immunosuppressive pathways (such as CTLA-4 or other immune checkpoints) during ICI treatment [94]. Future strategies should include targeting immunosuppressive mechanisms, optimizing combination therapy regimens, and personalizing treatment. By targeting the immunosuppressive mechanisms within the TME, such as inhibiting VEGF signaling pathways, the effectiveness of immunotherapy can be improved, and the activity of Tregs and TAMs in the TME can be reduced to enhance the anti-tumor effects of effector T cells [95]. Personalized treatment will become an important direction for the future, as genomic analysis and TME feature assessments of patients can help develop tailored treatment plans to improve efficacy and safety.
Moreover, researching new biomarkers, such as PD-L1 expression levels, tumor mutational burden (TMB), TILs, and other immune-related markers [96], will aid in predicting treatment responses and guiding personalized immunotherapy strategies, ultimately improving survival rates and quality of life for ovarian cancer patients. Through these efforts, it is hoped that current treatment barriers can be overcome, providing more effective treatment options for ovarian cancer patients.
ADCs: antibody-drug conjugates
AE: adverse events
CPS: combined positive score
CR: complete response
CTLA-4: cytotoxic T-lymphocyte antigen 4
CTLs: cytotoxic T lymphocytes
DCR: disease control rate
DCs: dendritic cells
HR: homologous recombination
HRD: homologous recombination deficiency
ICIs: immune checkpoint inhibitors
MHC: major histocompatibility complex
MIRV: mirvetuximab soravtansine
mPFS: median progression-free survival
ORR: objective response rate
OS: overall survival
PARP: Poly(ADP-ribose) polymerase
PD-1: programmed cell death protein 1
PD-L1: programmed cell death ligand 1
PFS: progression-free survival
PLD: pegylated liposomal doxorubicin
PROC: platinum-resistant recurrent ovarian cancer
SD: stable disease
TAAs: tumor-associated antigens
TAMs: tumor-associated macrophages
TCR: T cell receptor
TILs: tumor-infiltrating lymphocytes
TME: tumor microenvironment
TRAEs: treatment-related adverse events
Tregs: regulatory T cells
VEGF: vascular endothelial growth factor
GN: Writing—review & editing, Conceptualization. LZ: Writing—original draft, Investigation, Conceptualization. YZ: Data curation.
We declare that we have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study was financially supported by grants from Science and Technology Tackling in Henan Province [242102310359]; and Henan Polytechnic University Youth Innovation Exploratory Fund [NSFRF230414]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Luis Cabezón-Gutiérrez ... Vilma Pacheco-Barcia
Raffaele Pellegrino ... Antonietta Gerarda Gravina
Rawaa AlChalabi ... Ahmed AbdulJabbar Suleiman
Qing Bao ... Hailin Tang
Fakher Rahim ... Issenova Balday
Neha Kannan ... Giuseppe Minervini
