Affiliation:
The Clinical Analysis Laboratory of the Virgen del Puerto Hospital of Plasencia (Spanish National Health Service), CP 10600 Plasencia, Spain
Email: javiermaron@gmail.com; martinlopezjavier@hotmail.com
ORCID: https://orcid.org/0000-0002-7729-8517
Explor Immunol. 2022;2:442–453 DOI: https://doi.org/10.37349/ei.2022.00061
Received: December 12, 2021 Accepted: April 12, 2022 Published: July 17, 2022
Academic Editor: Dominique J. Charron, Hospital Saint Louis, France
The article belongs to the special issue The Sepsis induced Immune Conundrum
This review pretends to shed light on the immune processes occurring in the coronavirus disease 2019 (COVID-19) from a perspective based on the antigens size, lower or larger than 70 kDa. This cutoff size point explains the host type of immune response against the antigenic proteins of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which may lead to the development of the memory B cells or, conversely, the immune suppression, apoptosis, viral escape, and sepsis. Here, based on previous experimental work and the review of related literature, the following is proposed: antigens < 70 kDa can access the germinal center through the follicular conduits, where the activated B cells can present the processed antigen to specific naive CD4+ T cells that, in interaction with the major histocompatibility complex class II (MHC-II), trigger the immune response T helper type 2 (Th2). Conversely, antigens > 70 kDa cannot circulate through the narrow follicular conduits network and might be captured within the subcapsular sinus by the macrophages and dendritic follicular cells. Then, these cognate antigens are presented, via complement receptors, to the B cells that acquire and present them through the MHC-II to the specific naive CD4+ T cells, triggering the immune response Th1. The sustained infected cells lysis can overfeed high levels of unassembled viral proteins < 70 kDa, which can lead to a strong and persistent B cell receptor (BCR) activation, enhancing the Th2 immune response, releasing interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) that may lead to the immune paralysis, apoptosis, sepsis, and death. Finally, it is suggested that the polymerization of the viral antigens < 70 kDa into an antigenic polymer > 70 kDa could shift the immune response type from Th2 to Th1, developing the memory B cells and immunoglobulin G2 (IgG2) production, and avoiding the sepsis.
The coronavirus disease (COVID-19) is a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and consists of severe involvement of the lower respiratory tract leading to an acute respiratory syndrome and, rapidly, to serious lung inflammation, with acute respiratory distress, cardiac damage, and renal injury, especially in patients with older age and comorbidities (diabetes mellitus, hypertension, and heart failure) [1, 2].
In January 2020, Huang et al. [3] reported the first data of patients infected with SARS-CoV-2 and suggested that, probably, they had high levels of inflammatory cytokines because of the activation of T helper type 1 (Th1). Those patients who required intensive care unit (ICU) admission had higher concentrations of inflammatory cytokines than those not required ICU admission. The Th1 pro-inflammatory immune response, followed by a Th2 deregulated phase, resulted in reduced peripheral lymphocyte counts; this apoptosis was associated with a high risk of developing a secondary bacterial infection [3]. The significant rise of the Th2 cytokines, interleukin-4 (IL-4), and IL-10 suppressed the inflammation and was correlated with the immune suppression and the deadly bacterial infection in patients [4]. The sepsis resulting from bacterial bloodstream infections remains a serious clinical concern and is currently defined as a life-threatening organ dysfunction caused by a dysregulated immune response that occurs as the result of an infection [5].
It is evident the abundance and the heterogeneity of the knowledge that has been gathered about the quality, dimension and persistence of the immune response against SARS-CoV-2. To date, the studies about COVID-19 are focused mainly on the efficacy of vaccines, comparing their results in the reduction of the number of symptomatic cases. Lately, the researchers are expanding the focus towards the possibility of reinfection, the maintenance of the vaccine effect over time, the need for a booster dose, the viral sepsis, and the detection of new virus variants [6, 7, 8].
The expansion of SARS-CoV-2 variants is associated with a faster transmission or greater pathogenicity, which could lead to evading the vaccine-mediated protection and serious reinfections, increasing the current problems in the world health systems [9].
To date, the COVID-19 clinical data remains unclear, and it does not adequately reflect the correlation between the seroconversion, the immune protection, and the fatal sepsis possibility. Although the immune response is not always protective, the long-lasting immune memory seems to play an important role in the control of SARS-CoV-2 infection, despite its diffuse definition, its apparent activity loss over time, and the data variability in the different published papers about the topic [10].
To summarize, the COVID-19 is a dysregulated immune response caused by the SARS-CoV-2 infection and the subsequent inflammatory response driven by proinflammatory cytokines [11]. The sepsis pathophysiology involves the immune response, Th1 or Th2, against the invading pathogen that, when the control of the host’s homeostasis fails, culminates in a pathological syndrome described by sustained excessive inflammation and immunity loss [11]. So, we can deduce that the host’s immune response is closely related to the prognosis during its infection development.
Unfortunately, the available data and the works published seem still insufficient to understand how the immune memory and the sepsis develop in COVID-19. We suggest that 70 kDa is the cutoff size point that defines the balance in the response type, Th1 or Th2, of the immune system and that it can explain the development of memory B cells and the risk of sepsis.
From this perspective, we pretend to analyze the responses of the vaccines available and the viability of new developments in the neutralization of the COVID-19 infection.
In October 2020, The Lancet Infection Disease published a paper written by the Oxford Vaccine Group asking about what defines an efficacious COVID-19 vaccine [12]. The article questioned the challenges faced when assessing the clinical efficacy of vaccines against SARS-CoV-2 and, among its answers, it posed another question: does this COVID-19 vaccine work?
One month later, in November 2020, The Lancet published an editorial warning that there is no time for complacency in COVID-19 vaccines [13], and proposed several questions: what does the long-term future look like? will SARS-CoV-2 become endemic in a post-pandemic phase? can infection provide sterilizing immunity? how quickly does protective immunity wane? will we have annual seasonal outbreaks? and how will health systems have to adapt accordingly?
Regarding the efficacy of vaccines, in The Lancet Microbe, July 2021, Olliaro et al. [14] expressed their reservations about the updated reports on the outcomes of the different vaccine trials; and they suggested that the different choice of working methods and dissimilar analysis of the results seem to introduce an interpretive bias. In this sense, they published a paper about the COVID-19 vaccine efficacy and effectiveness, where the interim results of seven vaccine efficacy studies were evaluated. The conclusion they arrived at is that certainty in the knowledge of the vaccine efficacy is difficult to achieve because the effects of the trials have been evaluated through different methods [14]. We cannot know whether a vaccine, with a given efficacy in a specific study population, will have the same efficacy in another population with different levels of background risk of COVID-19.
Currently, the reality is that in many of the vaccine trials reported so far, the antibody levels decline after vaccination without a clear explanation for those results; and the reports comparing the immune memory and the efficacy of vaccines based on the trials available cause confusion.
Why immunoglobulin G1 (IgG1) and IgG3 are the only “neutralizing antibodies” referred to in the initial COVID-19 vaccine trial reports? Why IgG2, which are the ones produced by the memory B cells, are not quantified in the vaccine trial reports?
In May 2020, Martín [15] published a mini review in Preprints based on his previous experimental works on the activation of B cells by proteins < 70 kDa and suggested that, in COVID-19 sepsis, the over activation of B cell receptor (BCR), by viral antigens size < 70 kDa, establishes the Th2 type immune response.
Six months later, in December 2020, in another article published by Martín Oncina [16] in Critical Reviews in Immunology, it was suggested the difficulty of COVID-19 vaccines to developing memory B cells, and argued that the activation of BCR by viral proteins < 70 kDa releases neutralizing IgG1 and IgG3 antibodies but does not promote IgG2 antibodies, so this pathway is not suitable for expanding memory B cells (IgG2+, CD27+, T-bet+) [17–20].
In January 2021, Nature Nanotechnology published an editorial [21] suggesting that nano-vaccines might offer promising alternatives in vaccine formulation, and they positioned themselves with Singh’s paper [22] published in the same issue.
Singh [22] suggests that small nano-vaccines with a hydrodynamic radius of about 5 nm bypass the subcapsular sinus (SCS) macrophages and gain direct access to the B cells follicles through a network of collagen-rich fiber conduit and, although conduit openings are about 1 μm, collagen fibers have a spacing smaller than 10 nm, and therefore only allow passage of nano-vaccines with a dynamic radius of less than about 5 nm (~70 kDa). Singh [22] also proposes that polymeric nano-vaccines offer advantages as they can be characterized biochemically and can be purified to a high degree. He also suggests that nanomaterial chemistry offers a unique opportunity for antigen multimerization and precise dosing on a single particle, which can enable activation of low-to-high affinity B cells and the investigation of how immunogens interact with the BCR to induce strong immunogenic signals in B cells [22].
On July 10, 1996, this author registered the European Patent EP19960922046 titled Polymerized Vaccines claiming a procedure to obtain polymerized vaccines, obtained by polymerization, by gradual synthesis or polycondensation, of peptides or proteins less than 70 kDa, using polymerizing agents that result in a final protein greater than 70 kDa, which is a polymer of the starting proteins and induces an immune response of the Th1 type against those peptides [23, 24]. This patent is free (Figure 1).
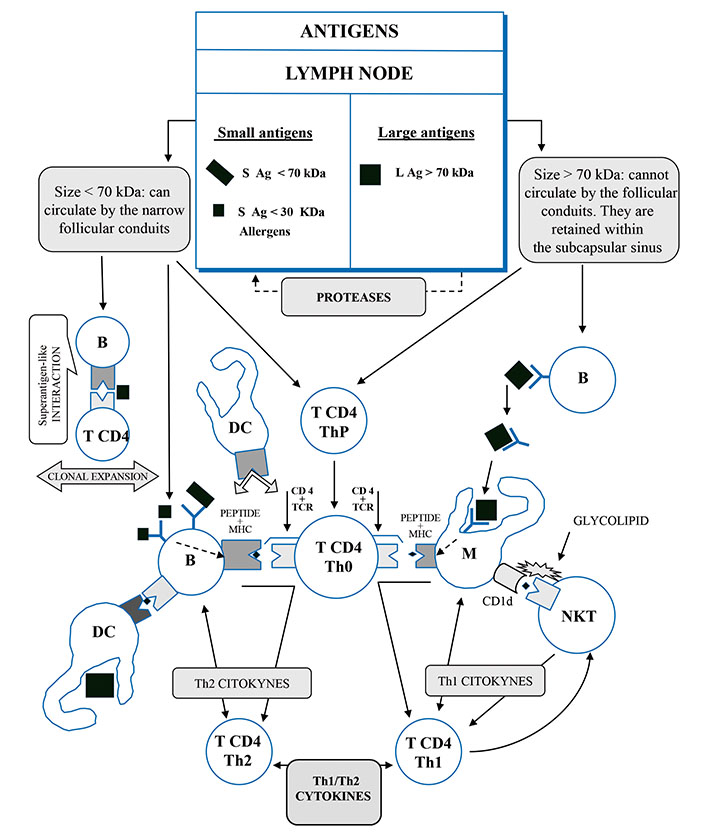
The antigens presentation pathways of different sizes. B: lymphocyte B; M: macrophage; DC: dendritic cell; CD4: cluster of quadruple differentiation; T CD4 ThP: lymphocyte T4 helper precursor; T CD4 Th0: naive lymphocyte T4 preactivated; T CD4 Th1: lymphocyte T4 helper 1 activated; T CD4 Th2: lymphocyte T4 helper 2 activated; CD1d: cluster differentiation 1d of macrophage receptor; NKT: invariant natural killer T cell; TCR: T cell receptor; MHC: major histocompatibility complex
Note. Adapted from “Polymerized vaccines,” by Martín Oncina FJ. European Patent Office EP0782860A1 (https://worldwide.espacenet.com/patent/search/family/008287454/publication/EP0782860A1?q=EP0782860A1). © Martín Oncina FJ.
Four years later, in November 2000, Gretz et al. [25] established that 70 kDa is the antigen size exclusion limit in the access to the lymph node follicular conduits.
Today, the antigen nanoscale size range, lower or larger than 70 kDa, seems critical in the design of antigen multimerization to obtain polymeric nano-vaccines. The size of the nano-vaccines is critical for its specific location in the lymph node.
We suggest that, in the immune response, the antigens’ access to germinal centers (GCs) through different pathways regulates and releases the different patterns of the interleukins and Ig that modulate the development of memory B cells and the risk of sepsis.
In COVID-19 infection, the antigen binding to the BCR triggers its activation and starts a cascade of intracellular signals that lead to the internalization of the receptor/antigen complex and the IgM production. Then, the activated B cells migrate towards the border of the lymph nodes, at the T cell zone, for antigen processing and presentation. In the border of the lymph nodes, between the follicles of the B cell zone and the T cell zone, it is produced an interface where the B cells present fragments of an antigenic peptide to the naive CD4+ helper T cells. This B-T cell encounter occurs in the context of the major histocompatibility complex class II (MHC-II), providing them with survival and co-stimulatory signals [26–28]. Then, the activated B cells migrate to the GC to rapidly undergo somatic hypermutation and clonal expansion [27].
Unfortunately, it yet remains without consensus how the differentiation of activated B cells occurs in the immune memory B cells or the plasmatic B cells and, also, how those processes are sepsis related.
The B cells are able to directly bind an antigen < 70 kDa or bind an antigen > 70 kDa that previously has been presented to them on the surface of the dendritic cells (DCs), the follicular dendritic cells (FDCs), or on the macrophages [29].
But how do the antigens locate the specific places in the lymph nodes? The physical-chemical characteristics of the antigens, especially the molecular size lower or larger than 70 kDa [23, 25], establish how the BCR recognizes and internalizes them, releasing cytokines and Ig that define a type of immune response: Th1 or Th2 [30, 31].
On the other hand, the immune response magnitude is related both to the number and quantity of different antigens as with the affinity and avidity, which are modulated by the host’s genetic factors, particularly MHC [30, 31].
Here, we pretend to show how the antigen size > 70 kDa is related to the Th1 immune response and the development of memory B cells, which pertain to the adaptive immune system and circulate quiescent in the bloodstream, sometimes for decades [17–19]. We also pretend to expose how the antigen size < 70 kDa is related to the Th2 immune response and with the activation induced cell death (AICD), which it could accelerate the apoptosis and lymphopenia by the release of anti-inflammatory cytokines, such as IL-10, increasing the risk of immune suppression, sepsis, and death [32, 33].
We also suggest that the answer to this conundrum is within the narrow follicular conduits of the lymph nodes that provide an efficient and rapid mechanism for the delivery of small antigens to the B cells. Only the antigens < 70 kDa can directly circulate through those narrow follicular conduits and contact the B cells [34, 35]; this cut-off point size (70 kDa) conditions the different pathways for antigen processing and, consequently, the release of their different cytokine and immunoglobulin patterns. The antigens > 70 kDa cannot be processed on this pathway [23, 25].
The antigens > 70 kDa in size cannot circulate through the narrow follicular conduits of the lymph nodes [23, 25], and they are retained within the SCS, where the macrophages and the follicular dendritic cells (FDCs) capture them directly and present them to the B cells [30, 31]. The antigen presenting cells (APCs) expose the antigen on their surface where is recognized by the BCR through the formation of a macromolecular cluster of defined composition, named the immunological synapse (IS). Those proteolyzed antigens are presented to the B cells by the tethering complement (CD21 and CD35) or Fc gamma-receptor (FcγR). Then, these cognate proteolyzed antigens, obtained by the B cells, are presented to the specific naive CD4+ T cells, in the context of MHC-II [30, 31].
The following and reiterated signals for somatic hypermutation, affinity maturation, and isotype switching occur in the GCs, where the APCs remain and work through IS that includes activated B cells, macrophages, and DC [26]. The specialized secondary lymphoid tissues increase the likelihood of the B cells finding these cognate peptides of internalized and digested proteins to present them to the naive CD4+ Th, which are specific for the same peptide of antigen, previously presented by the APCs [30, 31, 36]. This APC activation of the CD4+ Th releases interferon gamma (IFN-γ), which triggers the expression of the cellular transcriptional factor T-bet and promotes Th1 immune response, releasing IL-2 and IL-12 and feeding back more the synthesis of IFN-γ. Simultaneously, IL-12 facilitates Th1 cell lineage differentiation [37–39].
This B cellular expression of the T-bet transcriptional factor promotes the Ig switch to isotype IgG2 and IgA. Also, indirectly, the IFN-γ enhances IgG2 production [20, 40].
In summarizing, the expression in the B cells of the T-bet transcriptional cellular factor promotes the Th1 immune response that switches the Ig response to isotype IgG2 and IgA production [20]. The IgG2, IgG4, and IgA are isotypes of B memory Ig expressed in the Th1 immune response [19, 41]. The activation of BCR by viral proteins > 70 kDa expands the true memory B cells (IgG2+, CD27+, T-bet+) [17–20, 41, 42].
The antigens < 70 kDa can circulate through the narrow follicular conduits, present in the lymph nodes and the spleen, providing an efficient and rapid mechanism for the delivery of small antigens to the B cells, which is facilitated by cytokines such as C-X-C motif chemokine ligand 13 (CXCL13) [26, 27, 30, 31]; such B cells can contact these narrow follicular conduits directly [34, 35], conditioning the different pathways for antigen processing and, consequently, the release of their different cytokine and immunoglobulin patterns.
These follicular conduits receive the small antigens on the lymphatic fluid originating from the peripheral tissues, and they are delivered to the lymph node through afferent vessels to access the cognate follicular B cells [30]. Alternatively, the extrafollicular DC may present peptides of digested antigens to the cognate B cells as they arrive in the lymph node through the high endothelial vessels (HEV). Then, the B cells present the peptides of the processed antigens, in the MHC-II context, to the cell receptor of the specific naive CD4+ T cells that transform themselves into CD4+ Th2 cells [31].
The interactions of the naive T lymphocytes with the B cells activate the expression of the transcriptional factor GATA binding protein 3 (GATA-3) that stimulates the Th2 pattern cytokines synthesis, mainly IL-4, IL-5, and IL-10, promoting the Ig switch to isotype IgG1 and IgG3, and increasing the generation of plasmatic B cells [17, 43–45].
The analysis of IgG subclasses has shown that COVID-19 patients almost exclusively produce specific IgG1 and IgG3 against the receptor binding domain (RBD) of the spike protein of SARS-CoV-2, which has a size established in 26 kDa. But those analyses have not detected the levels of IgG2 or IgG4, which are the Ig of the memory B cells. In the same sense, we must recall that the IgG1 and IgG3 are the “neutralizing antibodies” referred to in the majority of SARS-CoV-2 vaccine trials reports. These findings illustrate that this antibody class-switching to IgG1 and IgG3 is not related to the Th1 long-lasting memory B cells [17, 46].
On the other hand, in the course of the infection, the Th1 and Th2 immune responses cause lysis in the infected cells of the host, releasing great amounts of cytoplasmatic viral proteins, still not assembled, which number, size, and immunogenicity are critical in the immune response. Thus, the SARS-CoV-2 disassembled proteins > 70 kDa promotes inflammatory Th1 immune response via the macrophages in the SCS and, conversely, the SARS-CoV-2 < 70 kDa disassembled proteins, which are a majority, can activate BCR to trigger Th2 adaptive humoral response [30, 31]. In essence, the lysis of the infected cells releases mainly proteins < 70 kDa that induce the Th2 immune response.
The sustained overfeed of the viral particles, which have been released from the cells killed by the viral lysis, increases the levels of plasmatic viral antigen particles. Moreover, a powerful and persistent BCR activation by those antigens < 70 kDa releases high amounts of transforming growth factor-beta (TGF-β) and IL-10, which can deplete the immune cells and lead to apoptosis, immune suppression, and sepsis [32, 33].
The group of antigens < 70 kDa includes a subgroup of antigens < 30 kDa (approximate size) similar to the Fab fragment of the arm of Ig [47]. The peculiarity of these small antigens is that they can establish a divalent encounter with the IgM-BCR, but not with the membrane immune globulin D receptor (IgD-BCR) [48]. This double-bound can result in two antigens attached to one only membrane immune globulin M receptor (IgM-BCR) in which each antigen is bounded to a Fab fragment arm of the immune globulin receptor [47–49].
These small antigens (< 30 kDa) gain antigenicity because of their aggregation in the IgM-BCR and by the repeated exposition of their epitopes that bind efficiently to the BCR [50–51]. This bind triggers the activation signals and increases the release of Th2 interleukins, mainly IL-4, IL-6, and IL-10, but finds it difficult to develop an antibody response [52]. We must bear in mind that the host contact with an allergen triggers a Th2 immune response, limited in time to that contact with the allergen, which is not an infectious antigen.
In COVID-19, as in the infectious or allergic processes, the respiratory symptoms can be similar, but the damage extent is different, depending on the antigen affinity and avidity, the pathogen virulence, and the host’s genetic factors [53]. The different patterns of Ig and cytokines that display in the sepsis, asthmatic shock, cytokine storm, insufficiency lung, flu, cold, and allergy [54, 55], suggest different magnitudes of a Th2 immune response [56]. Also, these processes activated by small antigens (< 30 kDa), infectious or not, do not induce memory B cells and their immune response is not long-lasting.
The immune response patterns in respiratory infections can be revised from an antigen size perspective; as it happens with the antigenic proteins M (25 kDa) and RBD (26 kDa) in the coronaviruses; the antigenic viral protein (VP) [VP1 (32 kDa), VP2 (28 kDa) and VP3 (26 kDa)] in the rhinoviruses; the neuraminidase (60 kDa) matrix proteins M1 (25 kDa) and M2 (17 kDa) in influenza A; also the main allergens, which mostly have a size of 20–40 kDa [57], and other antigenic proteins overexpressed in the diseases process with respiratory symptoms [58–60]. The severe acute respiratory syndrome (SARS) and upper respiratory tract infections (URTIs) respiratory symptoms can be explained by the size of their structural proteins and Th2 immune response.
To summarize, we suggest that small antigens (< 30 kDa) can strongly modulate the B cell Th2 response, the patterns of interleukins and Ig, the tissular injury possibility, and the risk of sepsis.
We suggest that, in the SARS-CoV-2 infection, the difference in the size of the antigenic proteins, lower or larger than 70 kDa, is critical in the regulation and release of the different patterns of interleukins and in the switch of Ig isotype: Th1 or Th2. The strong and persistent BCR activation by antigens < 70 kDa drives to Th2 immune response, releasing high levels of TGF-β and IL-10, which produce immune cells depletion, apoptosis, immune suppression, and sepsis. Conversely, the true memory B cells (T-bet+, IgG2+, CD27+) are modulated by antigens > 70 kDa that induce the Th1 immune response, promoting IgG2, IgA, and IgG4.
It is known that in the reports of COVID-19 vaccine trials, the referred neutralizing Ig are mainly IgG1 and IgG3, which are promoted by antigens < 70 kDa, and do not generate long-term memory B cells.
The RBD of SARS-CoV-2 spike protein is the key to entry into human cells by way of the angiotensin- converting enzyme 2 (ACE2) receptor; for that reason, the RBD is the antigen target for control of the infection but, due to its size of 26 kDa [46], it can also access to the node follicular conduits pathway that facilitates the traffic and the presentation of soluble antigens to B cells, giving rise to a Th2-type immune response, with an increase in neutralizing antibodies IgG1 and IgG3, but not producing IgG2 neutralizing antibodies and long-lasting memory B cells.
To date, in the vaccine trials reports, the response of neutralizing Ig begins to decline in the fourth or fifth month after vaccination and may even become residual or disappear in the eighth month after vaccination; so, it is necessary to administer new doses of vaccine periodically to maintain the immune response.
The repeated vaccination with RBD monomer (26 kDa) can lead to a Th2-type immune response, with an increase of the relation IgG1/IgG2 of neutralizing antibodies, depletion of clonal B cells, and the vaccine’s antigen tolerization.
Regardless of the recognized importance of early COVID-19 messenger RNA (mRNA) or DNA (viral vector) vaccines at the beginning of the pandemic, the type of antibodies response of those vaccines is short-lived and they do not produce long-lasting memory B cells, as it was predicted in May and December 2020 [15, 16].
The COVID-19 vaccine design that we suggest is a variation of the classic proteins subunits vaccine model. We propose to polymerize protein subunits until they reach a size greater than 70 kDa, preventing like that their access to the narrow follicular conduits of the lymph node. This molecular protein size over 70 kDa prevents the antigen from being presented to the BCR to induce a Th2 immune response. In contrast, the RBD polymer (trimer or tetramer), with a molecular mass over 70 kDa, is retained in the SCS of the lymph node, where it encounters the macrophages, and promotes a Th1-type immune response developing long-lasting memory B cells and IgG2 neutralizing antibodies.
There are several strategies for polymerizing antigens. The development of recombinant protein fusion processes is widely disseminated in the specialized literature as well as the marketing of equipment, culture media, and reagents.
Succinctly, the process involves the genetic manipulation of culture cells to produce proteins by recombinant engineering. For a vaccine against the SARS-CoV-2, the gene chosen to be cloned should be the RBD (residues Ser325–Ser530) of the spike protein. The fusion polymers by recombinant protein are formed from the translation of two or more protein genes by recombinant DNA fusion techniques. These protein genes must be subcloned into a suitable vector and adapted to transfer the chosen gene to the cell culture for its expression.
The techniques for genetic construction and transfer in the cells, as well as the isolation of the proteins in cell cultures and their stabilization (disulphide bonds), are commercially available and there exist a lot of specialized bibliographies.
We propose the design of COVID-19 vaccines by polymerizing the SARS-CoV-2 RBD protein into trimers, tetramers, or polymers with higher molecular weight, always with a size over 70 kDa. The COVID-19 vaccine design we propose should be able to block the RBD-ACE2 fusion, impede the infection, implement the Th1 immune response, and promote the generation of long-lasting memory B cells against the RBD antigen.
This COVID-19 vaccine design we propose should be safe, effective, and long-lasting; the technology to produce it is accessible and economical, and the recombinant protein it would produce should be stable and logistically more manageable.
APCs: antigen presenting cells
BCR: B cell receptor
COVID-19: coronavirus disease 2019
DCs: dendritic cells
GC: germinal center
IFN-γ: interferon gamma
Ig: immunoglobulin
IL-4: interleukin-4
MHC: major histocompatibility complex
MHC-II: major histocompatibility complex class II
RBD: receptor binding domain
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
SCS: subcapsular sinus
Th2: T helper type 2
VP: viral protein
The author contributed solely to the work.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3433
Download: 33
Times Cited: 0
Fei Pei ... on behalf of the China Critical Care Immunotherapy Research Group
Michael Bauer, Reinhard Wetzker
Markus Blaess ... Hans-Peter Deigner
Dablu Lal Gupta ... D. N. Rao
Nicholas Daering, Majdi N. Al-Hasan
