Affiliation:
1Dental Faculty, Urmia University of Medical Sciences, Urmia 5714783734, Iran
Email: Seyyediamir@yahoo.com
ORCID: https://orcid.org/0000-0003-0039-3855
Affiliation:
1Dental Faculty, Urmia University of Medical Sciences, Urmia 5714783734, Iran
ORCID: https://orcid.org/0000-0002-6112-4782
Affiliation:
2Department of Epidemiology, Medical Faculty, Urmia University of Medical Sciences, Urmia 5714783734, Iran
ORCID: https://orcid.org/0000-0001-7955-9891
Affiliation:
3Department of Biostatistics and Epidemiology, School of Medicine, Urmia University of Medical Sciences, Urmia 5714783734, Iran
Email: rohvali4@gmail.com
ORCID: https://orcid.org/0000-0002-6913-3210
Explor Med. 2022;3:451–460 DOI: https://doi.org/10.37349/emed.2022.00106
Received: May 13, 2022 Accepted: September 13, 2022 Published: October 27, 2022
Academic Editor: Lindsay A. Farrer, Boston University School of Medicine, USA
Aim: Wound healing is a complex phenomenon with various biological changes in tissue integrity, low-level laser therapy (LLLT) has acquired several unique components to help into accelerating tissue reconstruction and eventually wound healing. Thus, in the present systematic review and meta-analysis study, the role of LLLT in oral mucosal wound healing following surgical interventions was investigated.
Methods: The study databases, including PubMed, Web of Knowledge, Google Scholar, Scopus, and Cochrane, were searched by two blinded investigators considering eligible studies based on the following keywords: “Wound Healing”, “Oral Mucosal Wound Healing”, “Laser therapy”, “Low-level laser therapy”, “Oral Surgery”, “Photobiomodulation therapy”, among 88 screened, only 12 articles were eligible for the final analysis.
Results: There was a significant difference between control and laser group in all mentioned studies in the case of wound epithelialization in gingiva, with weighted mean difference (MD) of –0.28, [95% confidence interval (CI): –0.37, –0.19, P < 0.001], periodontium 1 day postoperative, with weighted MD of –0.56 (95% CI: –0.84, –0.27, P < 0.001) and 7 days postoperative, with weighted MD of –0.73 (95% CI: –0.97, –0.49, P < 0.001). In the cases of postoperative pain, LLLT has significantly declined pain in comparison with control group with weighted MD of –0.47 (95% CI: –0.69, –0.24, P < 0.001) for 7 days postoperative and –0.55 (95% CI: –0.96, –0.13, P = 0.005) 14 days postoperatively.
Conclusions: LLLT can be used as a promising tool in oral surgeries because of its inevitable capability in accelerating wound healing and reducing intraoperative pain.
The oral mucosa is highly susceptible to wounds because of several contributing factors including trauma, infection, and surgical and occlusal misalignments [1]. There are many models for oral mucosal wound healing in recently published literature. Following exposure to injury, the healing process starts which is composed of four overlapping stages: in the first phase, hemostasis; inflammation; proliferation, and maturation [2]. The first stage starts immediately after injury by activating the immune system and immune cell proliferation throughout the injured blood vessel endothelium, extra-cellular matrix exposed through injury activates platelets and starts the hemostasis process, and blood vessel constriction occurs induced by local chemokine agents [3, 4]. In the second phase, the inflammation process starts induced by local chemokines, this response gets to its maximum severity 24 h after injury and can last for 7 days to 10 days later [2, 5, 6]. At this phase, neutrophils as the first immune cells start injured tissue debridement using matrix metalloproteinases (MMPs) enzymes throughout the first debridement and also secreting cytokines to other immune cells including monocytes which phagocyte pathogen and injured cells to complement tissue re-epithelization [6]. In the third phase, cell proliferation starts induced by secreted growth factors and regenerative cytokines. The reconstruction of newly emerged blood vessels starts first [7, 8]. In the last phase, the tissue remodeling starts, and the fibroblasts and macrophages in wound bed tissue start to apoptosis, the secretion and regeneration of collagen bundles start throughout this stage and at the end, a well-functional, developed healed tissue is present at the former wound site [9–11].
There are many techniques to improve wound healing in recent literature including platelet-rich plasma injection in the wound site, which is highly technique sensitive and costly in comparison to other techniques [12]. Transcriptional genes and agents from allograft and xenograft donors are between other newly emerged techniques. The high technical and equipment limitations of transcriptional genes and agents challenged their clinical use. Also, there is a probability of cross-interactions between the donor and wound sites because of different leukocyte antigens in different people and races [13]. Also, wound healing using a scalpel is slow in different trials and causes postoperative pain and edema in patients because of the more invasive technique in surgical flaps [14, 15].
Low-level laser therapy (LLLT) has many advantages in comparison to other techniques in wound healing acceleration [16]. The wavelength range in LLLT is in infrared and visible light (400–900 nm) and 1–1,000 mW output power [17–19]. The mechanism of action in LLLT is biostimulation [20]. In this phenomenon, cellular proliferation and metabolism are activated and help tissue regeneration [21–23]. Despite the above-mentioned facts, LLLT is not applicable in the clinical field due to the little evidence available in the studies. In this regard, this review aimed to investigate the role of LLLT in oral mucosal wound healing in terms of a systematic review and meta-analysis of randomized clinical trials.
This systematic review study considered the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guideline [24] to answer the question: what is the clinical impact of LLLT in the wound healing process?
International databases consisting of PubMed, Scopus, Web of Knowledge, Google Scholar, and Cochrane were reviewed by January 3rd, 2022. The used keywords were “Wound Healing”, “Oral Mucosal Wound Healing”, “Laser therapy”, “Low-level laser therapy”, “Oral Surgery”, and “Photobiomodulation therapy” for just English articles. Clinical trials on humans evaluating pain in patients suffering from oral mucosal wounds induced by surgeries were included. Studies with unclear findings were excluded. Besides, case reports, case series, and review papers were not included in the meta-analysis. The screening process of the meta-analysis was presented in Figure 1 and 88 articles were collected by database searching in the primary step. There were 7 duplications, and then 81 records remained to be assessed further. After reviewing the title and abstract, 69 records were excluded as well. Finally, 12 articles were included in the meta-analysis [25–36] (Figure 1).
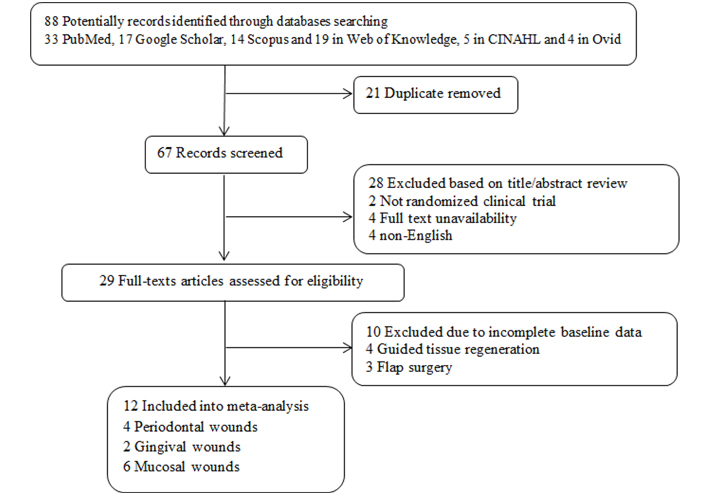
The flowchart of screening the eligible studies. CINAHL: Cumulative Index of Nursing and Allied Health
The primary outcome of the present study is the role of LLLT in postoperative wound remaining area 1 day and 7 days postoperatively and also the intensity of postoperative pain according to a visual analog scale, both in 7 days and 14 days postoperatively. Secondary outcomes were the role of LLLT in postoperative edema discomfort both in the baseline and 7 days postoperatively.
The data were extracted through the selected studies using the predetermined checklist [including sample size, type of epithelial tissue (like gingiva, periodontium, palatal mucosa), type of LLLT laser, wavelength, energy density, output power, and exposure time as independent variables and postoperative epithelialized wound surface and pain using a visual analogue scale (VAS) as dependent variables] by two independent researchers (Saman Taram, Mohammad Heydari) and in the case of disagreement; the third independent researcher (Seyyed Amir Seyyedi) solved the discrepancy. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the selected studies in which allocation concealment, random sequence generation, blinding of patients and outcome assessor, incomplete outcome data, and selective reporting were considered in this process.
In this review, the measure of effect was a mean difference (MD) and 95% confidence interval (CI) as summary statistics. The random effect was used to obtain pooled estimation using the MD and its P values. To assess heterogeneity, I2 statistic was calculated. Publication bias was checked through a funnel plot. Statistical analysis was done through comprehensive meta-analysis (CMA) version 3.
In this review, 88 studies were initially observed in different online databases [33 PubMed, 17 Google Scholar, 14 Scopus and 19 in Web of Knowledge, 5 in CINAHL, and 4 in Ovid]. In the second phase, after duplicate and irrelevant studies exclusion, the final 29 articles were chosen for full-text review and after excluding irrelevant and incomplete studies finally 12 randomized clinical trials in the different phases (phase II and III clinical trials), consisting of 240 cases and 168 controls, were included into our analysis. In all included studies the type of wound was surgical wound. Eight studies were parallel randomized controlled trials (RCTs) [25–27, 30, 31, 33, 35, 36] and the four others were split-mouth RCTs [28, 29, 32, 34]. Six studies were on mucosal wounds [25, 27, 30, 32, 33, 36], four on gingival wounds [28, 29, 34, 35], and two on periodontal wounds (gingiva + supporting alveolar bone) [26, 32]. All lasers are with a wavelength ranging from 680 nm to 810 nm and an output power of 30–100 mW. All the studies used LLLT immediately after the operation. The flowchart of the studies’ inclusion and exclusion details is shown in Figure 1. Inclusion criteria were including discussing periodontal, gingival, and mucosal wounds. Exclusion criteria were considered as case reports, animal studies, and studies with an incorrect value of the selected index or irrelevant interventions such as flap surgery. The risk of bias assessment can be seen in Table 1 through the NOS value.
Risk of bias assessment
| Study’s first author | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias |
|---|---|---|---|---|---|---|---|
| Isler et al. [25] |  |  |  |  |  |  |  |
| Dias et al. [26] |  |  |  |  |  |  |  |
| Ustaoglu et al. [27] |  |  |  |  |  |  |  |
| Metin et al. [28] |  |  |  |  |  |  |  |
| Pouremadi et al. [29] |  |  |  |  |  |  |  |
| Heidari et al. [30] |  |  |  |  |  |  |  |
| Yildiz and Gunpinar [31] |  |  |  |  |  |  |  |
| Ozcelik et al. [32] |  |  |  |  |  |  |  |
| Paschoal and Santos-Pinto [33] |  |  |  |  |  |  |  |
| Demirturk-Gocgun et al. [34] |  |  |  |  |  |  |  |
| da Silva Neves et al. [35] |  |  |  |  |  |  |  |
| Sadighi et al. [36] |  |  |  |  |  |  |  |
We assess post-surgical wound re-epithelialization and pain in all 12 studies. Four studies evaluated gingival wound healing. Two studies on periodontal wound healing. In six studies, another anatomic wound included palate mucosa and hard tissue of the alveolar region. The results of the meta-analysis showed that LLLT has a significant positive effect on post-surgical wound re-epithelialization in the gingiva, with weighted MD of –0.28 (–0.37, –0.19, 95% CI, P < 0.001), in periodontium 1 day postoperatively with weighted MD of –0.56 (–0.84, –0.27, 95% CI, P < 0.001) and in periodontium 7 days postoperatively with weighted MD of –0.73 (–0.97, –0.49, 95% CI, P < 0.001) (Figures 2 and 3).
In the cases of postoperative pain, the LLLT has significantly declined pain in comparison with the control group with weighted MD of –0.47 (–0.69, –0.24, 95% CI, P < 0.001) for 7 days postoperative and with weighted MD of –0.55 (–0.96, –0.13, 95% CI, P = 0.005) for 14 days postoperatively. In terms of heterogeneity between studies in post-operative re-epithelialization in gingival, periodontal, and mucosal wound healing, significant relevance with I2 values of 25.82, 1.71, 25.38, and 10.73, respectively (Figure 4).
The heterogeneity across the studies in assessing the efficacy of LLLT on post-operative wound re-epithelialization was insignificant with I2 values ranging from 0 to 82% and the Egger’s test was excluding non-significant publication bias in the analyses. The results of the analysis also showed that publication bias did not have an influence on the creation of negative results, which is shown as symmetry in the funnel plot. Meanwhile, no evidence of publication bias was detected using Egger’s test (Egger’s test P > 0.05).
Surgical intervention success highly depends on postoperative wound healing acceleration and pain control [1]. The LLLT has been introduced as a main or adjuvant therapeutic agent that exhibited promising results both in human and animal studies [20–22].
In the present study, the role of LLLT in wound re-epithelialization was fully approved in gingival, periodontal, and mucosal wounds. A similar study showed that there was LLLT stimulates tissue regenerating factors like transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) [37]. In the present study, there was no consensus on the role of low or high energy density on epithelized wound area or wound remaining area. In similar meta-analysis studies, also the results were controversial, in which some studies showed better healing on low energy density (< 4 J/cm2) [38, 39], while others preferred high ones (> 12 J/cm2) [40, 41]. The controversies could be related to the difference in donor sites in which radiation scatter and absorption is different but due to the low sample size and included studies, this finding is less accurate.
In the present study, the role of LLLT in lowering postoperative pain was significantly obvious, among 12 included studies, six studies report significant pain reduction in the laser group in comparison to the control group, 14 days postoperatively, while six reported no significant difference between the two groups. The same result has been reported also [42]. The recent literature revealed that LLLT induces endorphin secretion and inhibits bradykinin modulation in the inflammatory process [43, 44]. Also, it is reported that red and infra-red lasers induce beta-endorphin secretion which acts as an analgesic agent [45, 46]. Some heterogeneity found among studies can be attributed to some influencing factors such as different doses of postoperative analgesics and frequencies [23, 39]. The controversy between different studies in postoperative pain control could be related to the energy density of lasers, which was ranging from 0.5 J/cm2 to 16 J/cm2 with a mean of 8 J/cm2. A theory suggested that pain inhibiting efficacy of light waves is dose-dependent until a certain threshold, which further increases radiation energy and leads to negative results because of the saturation phenomenon [40, 47]. However, in the present study, there was no significant difference in different energy density values on postoperative pain control. In this study also, there was no significant effect of output power and energy density on postoperative pain which showed that there is still no effective and promising treatment plan for lowering postoperative pain in terms of laser source characteristics. However, in order to standardize results, a multicenter randomized clinical trial with postoperative analgesics prescription is needed.
Despite the abovementioned valuable findings, there are still some limitations, the included studies have not mentioned all laser data including exposure time per point or type of handpiece and there were some missing different variables, so it needed to further investigation on laser adjustment variables and their role on surgical wound healing.
In conclusion, according to the findings of the present systematic review study, LLLT can be used as an adjuvant or main tool in oral surgeries due to its promising role in postoperative wound epithelialization and pain control. Today, we cannot declare that specific output power or wavelength can influence the wound-healing process. There was a significant increase in wound regeneration on the 7th day and pain control on the 14th day postoperatively. Due to a smaller number of suitable studies, there was no strong data to support the role of LLLT on postoperative edema and discomfort. Further investigation in terms of multi-centered clinical trials is needed to reveal the role of different laser adjustments on postoperative wound healing and pain control.
CI: confidence interval
LLLT: low-level laser therapy
MD: mean difference
SAS, ST, RV, and MH: conceptualization, writing—original draft; RV: writing—review & editing. All authors contributed to manuscript writing, revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
