Abstract
In a number of malignancies, new immuno-oncology therapies that focus on the programmed cell death 1 (PD-1) have improvised the patient condition along with a positive aftereffect. Monoclonal antibodies (mAbs) directed against PD-1 and its ligand (PD-L1), have been widely used to treat a variety of malignancies, including melanoma, renal cancer, and non-small cell lung cancer (NSCLC). Dostarlimab, a therapeutic anti-PD-1 antibody, was authorised by the United States Food and Drug Administration (FDA) in April 2021 under the trade name JEMPERLI. It is a humanised contrary PD-1 immunoglobulin G 4 (IgG4) mAb, which successfully blocks interaction with PD-L1 and PD-L2 by binding tightly to the PD-1 receptor. This article summarizes the different aspects associated with the dostarlimab, including currently available anti-PD-1/PD-L1 antibodies, pharmacokinetics (PK), pharmacodynamics, adverse reaction, and mechanism of action of dostarlimab, as well as various reported clinical trials.
Keywords
Programmed cell death receptor 1, cancer therapy, monoclonal antibodies, programmed cell death 1 ligand 1, dostarlimab, clinical trialsIntroduction
The therapy options available to cancer patients have undergone a significant change as a result of programmed cell death 1 (PD-1) receptor and its ligand (PD-L1) inhibitors. PD-1 is an inhibitory immune checkpoint receptor, which is expressed by the activated T cells. The PD-1 receptor through interactions with PD-L1 and PD-L2 prevents activated effector T-cell cytokine production, proliferation, and cytotoxic activity [1, 2]. Numerous malignancies that upregulate PD-L1 are able to subvert the PD-1/PD-L1 pathway, which reduces the response of cytotoxic T-cells in the tumour microenvironment and has been linked to a poor prognosis [3, 4]. By blocking the binding of PD-1/PD-L1, immune evasion is reversed and an adaptive immune response against the tumour is restored [5]. Interestingly, PD-L1 monoclonal antibodies (mAbs) have shown anticancer efficacy in individuals with diverse solid tumours.
Currently, four anti-PD-1 antibodies got approval for use in cancer immunotherapy throughout the world. In 2014, pembrolizumab (KEYTRUDA®) and nivolumab (OPDIVO®) were approved by United States Food and Drug Administration (FDA) for treating non-small cell lung cancer (NSCLC) and melanoma. Since then, the antibodies’ indications have been increased to treat a variety of tumours such as breast cancer, classical Hodgkin lymphoma (CHL), urothelial carcinoma, head and neck squamous cell carcinoma (HNSCC), esophageal carcinoma, head carcinoma, endometrial cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma (HCC), either alone or in combination with other medications. In 2018, cemiplimab (LIBTAYO®) received approval from FDA for treating squamous cell carcinoma. Furthermore, the FDA has granted cemiplimab’s enlarged indication for the diagnosis of basal cell carcinoma as well as NSCLC. Dostarlimab (JEMPERLI), the fourth PD-1 mAb, was approved by the FDA in April 2021 for the cure of mismatch repair deficient (dMMR) solid tumours and dMMR endometrial cancer [6–12]. It is being developed by GlaxoSmithKline (GSK) under a license from AnaptysBio Inc. (AnaptysBio) to treat a variety of cancers, including ovarian cancer, NSCLC, pancreatic cancer, malignant melanoma, squamous cell cancer (SCC), peritoneal cancer, small-cell lung cancer (SCLC), endometrial cancer, and cancer of the neck and head. Dostarlimab is an immunoglobulin G 4 (IgG4) mAb that has been humanized and was produced from the hybridoma of a mouse. Dostarlimab is approved for the treatment of adult patients with advanced or recurrent endometrial cancer who have a dMMR mutation. Dostarlimab should be used in the following amounts: every three weeks 500 mg for the first four doses, followed by 1,000 mg every six weeks starting three weeks after dose four, or until the disease progresses or the side effects become intolerable. Numerous immune-related side effects have been linked to dostarlimab use, which may necessitate stopping the drug temporarily or permanently [13]. Two signalling motifs can be found in the cytoplasmic tail of PD-1. An immunoreceptor tyrosine-based switch motif (ITSM) and an immunoreceptor tyrosine-based inhibitory motif (ITIM) are the two types. The binding of PD-L1 or PD-L2 to PD-1 on activated T cells and T cell receptor (TCR) signalling result in the tyrosine phosphorylation of the cytoplasmic domain tyrosines and the recruitment of an Src homology 2-containing tyrosine phosphatase (SHP-2) to the ITSM. Downstream signalling is suppressed as a result of SHP-2’s dephosphorylation of TCR-associated CD-3z and zeta-chain-associated protein kinase 70 (ZAP-70). This includes the inhibition of Protein kinase B (Akt) and phosphoinositide 3-kinase (PI3K) activity, which impairs glucose metabolism and interleukin-2 (IL-2) release. The development of mAbs for cancer immunotherapy involves improving T cell activity by preventing the binding of PD-1 and PD-L1 or PD-L2 [14]. The currently available drugs that target PD-1/PD-L1 pathway and comparison are shown in Table 1.
Currently available drugs that target PD-1/PD-L1 pathway
| Drugs | Approved indication | Dosage | Route of administration | Side effects |
|---|---|---|---|---|
| Nivolumab (brand name “OPDIVO®”, fully human IgG4 mAb) | Metastatic NSCLC, metastatic melanoma, CHL, malignant pleural mesothelioma, RCC, HNSCC, urothelial carcinoma, HCC, esophageal cancer, dMMR, or MSI-H metastatic colorectal cancer, gastric cancer, and gastroesophageal junction cancer | For melanoma, RCC, HNSCC, NSCLC, urothelial carcinoma, CHL, esophageal cancer, and gastroesophageal junction cancer: every two weeks 240 mgPediatric patients with MSI-H or dMMR metastatic colorectal cancer: every two weeks 3 mg/kg | Intravenous (i.v.) | Back pain, blurred vision, change or loss of taste, depressed mood, cough, dry skin and hair, irregular heartbeat and pulse, loss of appetite, itching, nausea, muscle cramps and stiffness, red, irritated eyes, swelling, redness, the pain of the skin, weight gain, vomiting, chest pain, trouble sleeping, tenderness, bloody or cloudy urine, drowsiness, trouble breathing |
| Pembrolizumab (brand name “KEYTRUDA®”, humanized IgG4 mAb) | Metastatic NSCLC, Metastatic melanoma, urothelial carcinoma, esophageal cancer, PMBCL, HNSCC, MSI-H or dMMR cancer, HCC, gastric cancer, cervical cancer, CHL, RCC, MCC, endometrial carcinoma, TMB-H cancer, triple-negative breast cancer, and CSCC | For adult metastatic melanoma patients: every 3 weeks 200 mgAdult patients with HNSCC, NSCLC, PMBCL, esophageal cancer, dMMR or MSI-H cancer, cervical cancer, TMB-H, HCC, urothelial carcinoma, MCC, CHL, CSCC: every 3 weeks 200 mg or every 6 weeks 400 mgPediatric patients with PMBCL, MSI-H or dMMR cancer, CHL, TMB-H, MCC: every 3 weeks 2 mg/kgPediatric melanoma patients: every 3 weeks 2 mg/kg | i.v. | Bladder pain, blurred vision, itching, swelling of the face, hands, arms, lower legs, or feet, loss of voice, body aches or pain, confusion, decreased appetite, cough, difficulty with breathing, depressed mood, dry mouth, feeling cold, joint or bone pain, painful or difficult urination, lower back or side pain, slow or fast heartbeat, stomach cramps, vomiting, weakness in the arms, yellow eyes or skin, sneezing, muscle weakness, trouble sleeping, severe or sudden headache, drowsiness |
| Cemiplimab (brand name “LIBTAYO®”, humanized IgG4 mAb) | Metastatic or locally advanced CSCC | Until illness development or toxicity, 350 mg once every three weeks | i.v. | Diarrhea, fatigue, musculoskeletal pain, rash, nausea, constipation, pruritus, immune-mediated adverse reactions |
| Atezolizumab (brand name “TECENTRIQ®”, humanized IgG1 mAb) | SCLC, urothelial carcinoma, metastatic NSCLC, HCC, and melanoma | Every 3 weeks 1,200 mg | i.v | Nausea, lower back or side pain, difficulty in breathing, diarrhea, body aches or pain, slow and fast heartbeat, burning or painful urination, fever, stomach cramps, rapid weight gain, headache, unusual tiredness or weakness, vomiting, confusion, depressed mood, facial swelling, trouble sleeping, yellow eyes and skin, increased thirst, muscle pain, redness of the skin |
| Avelumab (brand name “BAVENCIO®”, fully human IgG1 mAb) | MCC, RCC, Metastatic urothelial carcinoma | 10 mg/kg every two weeks until the condition progresses or there is toxicity | i.v. | Headache, muscle, bone, or joint pain, nausea, constipation, tiredness, vomiting, and weight loss |
| Durvalumab (brand name “IMFINZI®”, humanized IgG1 mAb) | Metastatic urothelial carcinoma, NSCLC, SCLC | Every 2 weeks 10 mg/kg | i.v. | Bladder pain, fever, dry skin and hair, slowed heartbeat, tenderness, stomach cramps, bloody or cloudy urine, burning, or painful urination, rapid weight gain, chest pain, cough, difficult breathing, sweating, trouble sleeping, blurred vision, headache, chest tightness, pale skin, redness of the eye |
| Dostarlimab (brand name “JEMPERLI”, humanized IgG4 mAb) | dMMR endometrial cancer and dMMR solid tumours | Every 3 weeks 500 mg | i.v. | Bladder pain, burning or painful urination, feeling cold, constipation, hair loss, muscle cramps and stiffness, trouble breathing, weight gain, unusual tiredness or weakness, pale skin, chest pain or tightness, confusion, coma, cough, fever, diarrhea, headache, irritability, nausea, trouble sleeping, sweating, anxiety, change in vision, dry mouth, joint pain, drowsiness |
CSCC: cutaneous squamous cell carcinoma; MCC: Merkel cell carcinoma; MSI-H: microsatellite instability-high; PMBCL: primary mediastinal large B-cell lymphoma; TMB-H: tumour mutational burden-high cancer
Pathophysiology
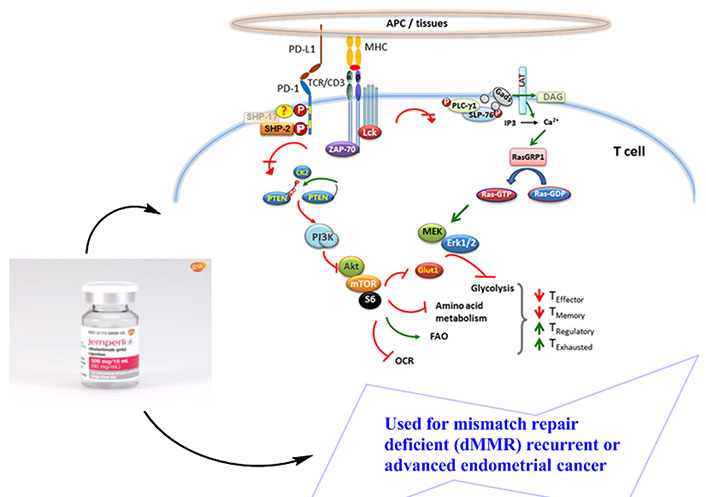
The T cell protein is phosphorylated as a result of TCR/CD3 chains oligomerizing (TCR) in response to a foreign antigen recognized by a T cell that has been presented by major histocompatibility complex (MHC) on the surface of an antigen-presenting cell (APC). This is followed by the recruitment of activated lymphocyte-specific protein tyrosine kinase (Lck) and zeta-chain-associated protein kinase 70 (Zap-70) to the phosphorylated immunoreceptor tyrosine-based activation motifs (ITAM) of the TCR tail, which starts the subsequent TCR-signaling cascade. SHP-2 is drawn to the ITSM by the phosphorylation of the two tyrosine residues on PD-1’s cytoplasmic tail when it engages with its ligands during TCR bridge (also possibly SHP-1). Zap-70 and Lck are thus dephosphorylated. Additionally, PD-1 ligation inhibits the PI3K/Akt/mammalian target of rapamycin (mTOR) and Ras/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk) pathways, which causes a decrease in metabolism of amino acids and glycolysis and an increase in fatty acid oxidation in T cells. The course of T cell development may be impacted by this alteration in metabolic reprogramming, which could reduce the formation of effector & memory T cells while promoting the production of regulatory T cells and weary T cells (Figure 1) [15].
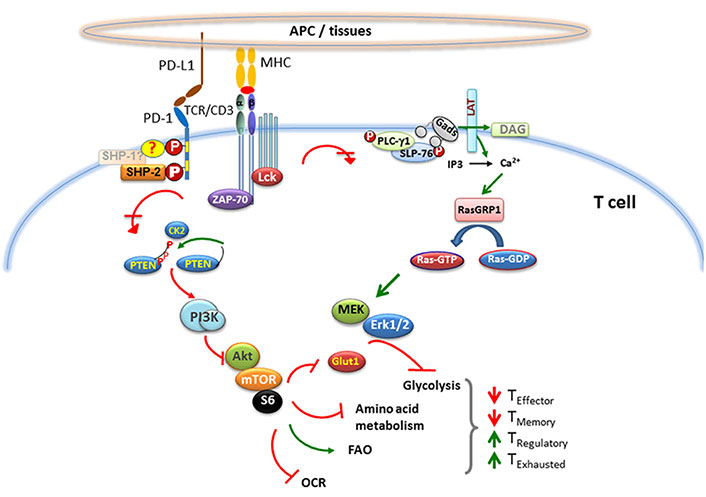
Pathophysiology of PD-1. CK2: casein kinase-2; DAG: diacylglycerol; FAO: fatty acid oxidation; Gads: GRB2-related adaptor downstream of Shc; GDP: guanosine diphosphate; Glut1: glucose transporter 1; GTP: guanosine triphosphate; IP3: inositol 1,4,5-trisphosphate; MEK: mitogen-activated protein kinase kinase; OCR: oxygen consumption rate; P: phosphorylated; PI3K: phosphoinositide 3-kinase; PLC-γ1: phospholipase C-gamma 1; PTEN: phosphatase and tensin homolog; RasGRP: Ras guanil releasing protein; LAT: linker for activation of T cells; SLP-76: SH2 domain-containing leukocyte phosphoprotein of 76kDa; ?: possibility of any of the options
Pharmacology
Mechanism of action
The PD-1 ligands PD-L1 and PD-L2 suppress cytokine production and T-cell proliferation when they attach to the PD-1 receptor on T cells. Some cancers that exhibit upregulation of PD-1 ligands and signaling via this route may help to reduce active T-cell immune monitoring of malignancies. Dostarlimab, a humanised IgG4 mAb, suppresses the immune response, as well as the anti-tumour immune response, by binding to the PD-1 protein and preventing it from binding with PD-L1 and PD-L2. In animal tumour models using genetically identical tissues, inhibiting PD-1 activation decreased tumour growth [12].
Pharmacodynamics
Dostarlimab binds with high affinity to both cynomolgus monkey and human PD-1, as per surface plasmon resonance, through cell lines that overexpress chimeric PD-1 in flow cytometry, or attaching to the natural protein on peripheral blood mononuclear cells (PBMC). Additionally, in order to bind by the receptor, PD-L1 and PD-L2 were inhibited through antibody. Dostarlimab exhibited potent functional antagonistic behaviour with mixed lymphocyte response assay (LRA) in a human CD4+, increasing the production of IL-2. Dostarlimab was more active in this experiment when anti-LAG3 or anti-TIM3 antibodies were present. Human PBMCs were treated with dostarlimab, although the release of cytokines was not noticeably increased.
Pharmacokinetics
Pharmacokinetics (PK) of dostarlimab in patients with various solid tumours, including 150 people with endometrial cancer (EC), were investigated. The dosing range was 1–10 mg/kg. AUC0-inf, the Cmax mean, and AUC0-tau all dose increased proportionally. Dostarlimab AUC0-tau and mean cycle Cmax are 35,730 μg × h/mL (20%) and 171 μg/mL (20%) at the 500 mg dose once every three weeks and 95,820 μg × h/mL (29%) and 309 μg/mL (31%) at the 1,000 mg dose once every six weeks, respectively [12].
Distribution: dostarlimab’s mean steady-state distribution volume is 5.3 L (12%).
Metabolism: catabolic mechanisms are anticipated to break down dostarlimab into minor amino acids and peptides.
Elimination: at a steady state, the mean maximal elimination half-life of dostarlimab is 25.4 days and a mean clearance of 0.007 L/h (31%).
Specific Populations: based on tumour kinds, age (24–86 years), sex (females made up 79%), race/ethnicity, or age, there were no clinically significant changes in the dostarlimab PK (78% White, 4% African American, 2% Asian, and 16% other), or estimated creatinine clearance (CLCR, severe: CLCR = 15–29 mL/min, n = 3; moderate: CLCR = 30–59 mL/min, n = 90; mild: CLCR = 60–89 mL/min, n = 210; normal: CLCR 90 mL/min, n = 173).
Adverse reactions
Immune-mediated adverse reactions
Dostarlimab is a mAb that belongs to a group of drugs that binds to either PD-L1 or PD-1, disrupting the PD-L1/PD-1 pathway, eliminating immune response suppression, potentially rupturing peripheral tolerance, and producing immunological-mediated side effects. Any tissue or organ system might have immune-mediated adverse effects that can be serious or lethal. Immune-mediated side effects might occur at any point after initiating use of PD-L1/PD-1 blocking antibody. Although immune-mediated side effects of PD-L1/PD-1 blocking antibody therapy may emerge at any point during or after treatment, they frequently do so during diagnosis. To ensure the safe use of blocking antibodies of PD-L1/PD-1, early detection, and control of immune-mediated negative effects are crucial. A close eye has to be kept out for any symptoms or indications that ought to be outward signals of an underlying immune-mediated adverse event. Screening for thyroid, liver, and creatinine function have to be done at the beginning of treatment and on a regular basis after that. There is a need to start the necessary workup in situations of suspected immune-mediated adverse reactions to rule out other causes, like an infection. Medical management should be implemented as soon as possible, along with specialist advice as necessary. The drug should be withheld or ceased permanently depending on the severity. In general, systemic corticosteroids (1–2 mg/kg per day prednisone or similar) should be given if dostarlimab needs to be interrupted or discontinued until improvement to Grade 1 or less. The corticosteroids should be tapered off for a month when the condition improves to Grade 1 or less. If corticosteroids are unable to treat an immune-mediated adverse effect in a patient, administration of additional systemic immunosuppressants could be considered. Patients on PD-L1/PD-1 inhibitors, such as dostarlimab, may have a higher risk of developing deadly immune-mediated pneumonitis if they had previously undergone thoracic radiation treatment [12].
Hypophysitis
Dostarlimab may cause immune-mediated hypophysitis. Acute mass effect symptoms like headaches, photophobia, or visual field cuts can be a sign of hypophysitis. The hypophysitis may also result in hypopituitarism. Hormone replacement therapy should be stated if necessary. If dostarlimab is not clinically stable, it should be withheld.
Thyroid disorders
Immune-mediated thyroid diseases can be caused by dostarlimab. Endocrinopathy may or may not accompany thyroiditis in a patient. After hyperthyroidism, hypothyroidism can occur. As clinically warranted, the hormone replacement therapy should be started or the medical treatment of hyperthyroidism should be done. The drug dostarlimab should be withheld if not clinically stable.
Thyroiditis
Around 0.5% (2/444) of individuals on dostarlimab have been reported to experience Grade 2 thyroiditis. There were no discontinuations of dostarlimab as a result of thyroiditis in either of the two thyroiditis events that occurred.
Hypothyroidism
It was found that 5.6% (25/444) of the patients using dostarlimab had Grade 2 hypothyroidism. In 40% of the 25 individuals, hypothyroidism was cured without the need to stop taking dostarlimab. None of the 25 hypothyroid patients needed to take systemic corticosteroids [12].
Type 1 diabetes mellitus
Diabetic Ketoacidosis: dostarlimab may present this problem with Type 1 Diabetes Mellitus. Hyperglycaemia or other diabetes-related symptoms should be properly monitored in patients. Insulin therapy should be started as soon as it is clinically appropriate. Dostarlimab may be discontinued completely or temporarily depending on the severity.
Other immune-mediated adverse reactions
Incidences of lymphadenitis, immunological thrombocytopenia, sarcoidosis, and solid organ transplant rejection have all been reported as clinically significant immune-mediated adverse events.
Effects involving nervous system include encephalitis, meningitis, myasthenia gravis, myelitis and demyelination, nerve paresis, Guillain-Barre syndrome, and autoimmune neuropathy are among the illnesses that can affect people. The vascular/cardiac symptoms include vasculitis, pericarditis, and myocarditis. Ocular effects include iritis, uveitis, and other ocular inflammatory toxicities. Retinal detachment may be seen in some cases. If uveitis develops in conjunction with other immune-mediated adverse responses, it could result in Vogt-Koyanagi-Harada syndrome, which may need systemic steroids to lessen the risk of irreversible vision loss. The connective tissue and musculoskeletal effects include rhabdomyolysis, polymyositis/myositis, and associated sequelae including polymyalgia rheumatic, arthritis, and renal failure. The gastrointestinal effects may include gastritis, increase in the levels of serum amylase and lipase, pancreatitis, and duodenitis. Endocrine system may lead to hypoparathyroidism. Other (immune/hematologic) effects could be aplastic anemia, systemic inflammatory response syndrome, hemolytic anemia, hemophagocytic lymphohistiocytosis, histiocytic necrotizing lymphadenitis, solid organ transplant rejection, immune thrombocytopenia.
Infusion-related reactions
With PD-L1/PD-1 blocking antibodies, serious or life-threatening infusion-related events have been documented. 0.2% (1/444) of patients taking dostarlimab experienced severe infusion-related events (Grade 3). The infusion-related reactions were all overcome by the patients. Patients need to be monitored for indications of infusion-related reactions. Depending on the severity of the reaction, the rate of infusion of dostarlimab should be stopped or decreased, or even permanently withdrawn [12].
Clinical trials
In a preliminary examination of data from the single-group phase I GARNET trial’s dMMR EC cohort, dostarlimab as monotherapy was linked to significant and long-lasting clinical activity (NCT02715284). Dostarlimab was administered at the prescribed dosage to 104 participants with advanced or recurrent dMMR tumours who had developed on platinum-based doublet chemotherapy. Persons with a six-month follow-up (n = 71) showed an objective response in 30 participants in a brief study after 11.2 median months of follow-up (from 0.03 months to 22.11 months), including of 9 and 21 certified complete and partial response, respectively. At the time of the data cut-off (8 July 2019), all confirmed complete responses were still being processed, and the median response time had not been attained. The rates of disease control and median progression-free survival were, respectively, 57.7% and 8.1 months. In a more recent investigation, information was gathered from 103 and 142 participants, respectively, who had mismatch repair proficient (MMRp) and dMMR EC. The overall response rate was 44.7% [35 (34%) partial and 11 (10.7%) complete responses] and 13.4% [16 (11.3%) partial and 3 (2.1%) complete responses], after 16.3 and 11.5 months of follow-up. There was a continued response in 41 and 12 patients, respectively [16–18]. In a small trial carried out by the Memorial Sloan Kettering Cancer Center (MSKCC) in 2022, it was observed that all 18 patients with rectal cancer experienced complete recovery of their cancer, 6 months after taking an experimental dose of dostarlimab. For the first time in history, this clinical trials of dostarlimab demonstrated complete elimination of the cancer disease in patients [19]. The various reported clinical trials of dostarlimab are summarized in Table 2.
Trials of dostarlimab
| Phase | Trial code | Cancer type | Participants | Sponsor | Purpose of study | Current status | Completion date |
|---|---|---|---|---|---|---|---|
| Phase 2 | NCT04068753 | Recurrent cervix cancer | 65 | University of Oklahoma | To evaluate the safety of dostarlimab and niraparib used in combination therapy and determine the effects (both positive and negative) that this therapy has on individuals with progressive or recurrent cervix cancer | Recruiting | July 2025 |
| Phase 2 | NCT05405192 | GTN | 24 | University of Miami | To determine whether Dostarlimab is a successful therapy for GTN | Not yet recruiting | December 1, 2028 |
| Phase 2 | NCT04313504 | Head and neck cancer | 23 | Trisha Wise-Draper | To identify which niraparib and dostarlimab combination provides individuals with recurrent or metastatic HNSCC with the greatest overall outcome | Recruiting | June 1, 2027 |
| Phase 1 | NCT04544995 | Neoplasms | 116 | GSK | To identify the optimal Phase 2 dose and assess the safety, PK, and effectiveness of niraparib combined with dostarlimab in children with resistant or recurrent solid tumours | Recruiting | March 15, 2030 |
| Phase 2 | NCT04139902 | Melanoma stage III; melanoma stage IV | 56 | Diwakar Davar | To evaluate the efficacy of anti-PD-1/anti-TIM-3 (TSR-042/TSR-022) or anti-PI-1 inhibitor (TSR-042) combo in operable melanoma patients | Recruiting | October 2027 |
| Phase 2 | NCT04409002 | Pancreatic cancer; metastatic pancreatic cancer | 25 | Massachusetts General Hospital | To examines the efficacy of RT, niraparib, and dostarlimab in the treatment of metastatic pancreatic cancer | Active, not recruiting | October 1, 2026 |
| Phase 2 Phase 3 | NCT04655976 | Lung cancer, non-small cell | 250 | GSK | To see how cobolimab combined with docetaxel and dostarlimab works in individuals with NSCLC who have developed on the previous anti-PD-L1 treatment and chemotherapy | Recruiting | January 1, 2026 |
| Phase 2 | NCT04701307 | Lung small cell carcinoma | 48 | MD Anderson Cancer Center | To investigate the efficacy of dostarlimab and niraparib in the treatment of SCLC | Recruiting | May 30, 2025 |
| Phase 3 | NCT05201547 | Endometrial cancer | 142 | Arcagy-Gineco GROUP | To compare effectiveness of dostarlimab to carboplatin-paclitaxel in advanced endometrial cancer or dMMR relapse patients | Recruiting | May 2029 |
| Phase 2 | NCT03955471 | Ovarian neoplasms | 41 | Tesaro, Inc. | To assess the safety and efficacy of dostarlimab and niraparib in patients with relapsed, advanced, fallopian tube, endometrioid, high-grade ovarian, clear cell, or primary peritoneal cancer who do not have a known BRCA mutation, have a platinum-resistant disease, and have also undergone bevacizumab treatment in the past | Terminated | January 12, 2022 |
| Phase 2 | NCT05126342 | Recurrent ovarian cancer | 100 | AGO Research GmbH | To gather proof of the effectiveness of dostarlimab and niraparib in two experimental cohorts of relapsed ovarian cancer patients | Not yet recruiting | November 1, 2026 |
| Phase 2 | NCT04581824 | Lung cancer, non-small cell | 243 | GSK | To compare the safety and effectiveness of the PD-1 inhibitors pembrolizumab and dostarlimab when given along with chemotherapy to patients with non-squamous NSCLC who do not have a known sensitizing ALK, EGFR, ROS-1, BRAF V600E mutation | Active, not recruiting | October 20, 2025 |
| Phase 1 Phase 2 | NCT04926324 | Rectal neoplasm malignant | 38 | Joseph Caster, Ph.D., M.D. | To establish the maximum tolerable dose of niraparib for locally advanced rectal cancer when treated with dostarlimab and hypofractionated radiotherapy | Recruiting | December 31, 2026 |
| Phase 2 | NCT04983745 | Homologous recombination deficient solid tumours | 30 | West Cancer Center | To assess the dostarlimab and niraparib effectiveness and safety in metastatic, recurrent, or unresectable solid tumours patients that have a suspected pathogenic, pathogenic, somatic HRD gene mutation | Not yet recruiting | August 2025 |
| Phase 2 | NCT04493060 | Metastatic pancreatic ductal adenocarcinoma | 20 | Mayo Clinic | To evaluate the efficacy of dostarlimab and niraparib in the treatment of individuals with germline or somatic PALB2 and BRCA1/2 mutant pancreatic cancer that has progressed to other parts of the body | Recruiting | December 1, 2022 |
| Phase 3 | NCT04679064 | Ovarian cancer | 427 | Fondazione Policlinico Universitario Agostino Gemelli IRCCS | To compare chemotherapy given at the doctor’s discretion with niraparib plus dostarlimab in the treatment of patients with primary peritoneal cancer when platinum is not an option | Recruiting | January 1, 2025 |
| Phase 2 | NCT04895046 | HRD; cholangiocarcinoma; metastatic cancer | 47 | Walid Shaib, M.D. | To evaluate HRD patient selection in molecularly chosen immune-based combination therapy for advanced cholangiocarcinoma maintenance therapies | Withdrawn | September 2023 |
| Phase 2 | NCT04837209 | Breast cancer | 32 | Massachusetts General Hospital | To evaluate the safety and efficacy of niraparib and dostarlimab in combination with RT in patients with metastatic triple-negative breast cancer | Recruiting | December 1, 2029 |
| Phase 2 | NCT03680508 | Adult primary liver cancer | 42 | University of Hawaii | To examine the efficacy of dostarlimab and cobolimab in treating locally advanced or metastatic liver cancer patients | Recruiting | October 2023 |
| Phase 1 | NCT02715284 | Neoplasms | 740 | Tesaro, Inc. | To assess the dostarlimab in advanced solid tumour patients who have few other alternatives for therapy | Recruiting | July 30, 2024 |
| Phase 2 | NCT04681469 | HNSCC | 49 | Gruppo Oncologico del Nord-Ovest | To assess the effectiveness and safety of a short course of the drug combination dostarlimab and niraparib | Recruiting | June 2028 |
| Phase 1 | NCT03843359 | Neoplasms | 300 | GSK | To assess the tolerability, safety, and early clinical efficacy of GSK3745417 whether given alone (Part 1A) or in combination (Part 2A) with dostarlimab in refractory/relapsed solid tumours patients | Recruiting | May 22, 2025 |
| Phase 2 | NCT04584255 | Breast cancer | 62 | Dana-Farber Cancer Institute | To evaluate the safety and efficacy of using dostarlimab plus niraparib as a neoadjuvant treatment for BRCA-mutated breast cancer patients | Recruiting | July 17, 2029 |
| Phase 3 | NCT03602859 | Ovarian neoplasms | 1405 | Tesaro, Inc. | To compare the effectiveness of dostarlimab and niraparib for platinum-based therapy with platinum-based therapy as the standard of care for treating stage III or stage IV nonmucinous epithelial ovarian cancer | Active, not recruiting | June 22, 2026 |
| Phase 1 | NCT04446351 | Neoplasms | 178 | GSK | To assess GSK6097608’s safety, tolerability, PD, PK, and preliminary clinical efficacy in advanced solid tumour patients when it is administered alone and in combination with dostarlimab | Recruiting | August 29, 2024 |
| Phase 2 | NCT04165772 | Rectal adeno carcinoma | 30 | Memorial Sloan Kettering Cancer Center | To investigate the efficacy of dostarlimab as a treatment for advanced dMMR solid tumours in comparison to standard surgery and standard chemoradiotherapy (capecitabine + RT) | Recruiting | November 30, 2025 |
| Phase 2 | NCT03308942 | Neoplasms | 53 | Tesaro, Inc. | To assess the effectiveness and safety of niraparib in combination and alone with PD-1 inhibitors in patients with metastatic and locally advanced NSCLC | Completed | August 31, 2021 |
| Phase 1 | NCT05277051 | Neoplasms | 126 | GSK | To examine the PK, safety, immunogenicity, and tolerability of GSK4381562 in certain metastatic or locoregionally recurrent solid tumours patients for whom no other effective or conventional treatment choices remain | Recruiting | February 26, 2027 |
| Phase 1 | NCT03250832 | Neoplasms | 111 | Tesaro, Inc. | To assess the TSR-033 alone and in combination with dostarlimab and combination with FOL/leucovorin, dostarlimab, 5-fluorouracil, and OX and bevacizumab in an individual with advanced solid tumours | Active, not recruiting | November 14, 2022 |
| Phase 1 | NCT04673448 | Breast cancer | 18 | University of Washington | To assess the effectiveness of niraparib and TSR-042 in treating individuals with primary peritoneal, pancreas, fallopian tube BRCA-mutated breast, ovary cancer | Recruiting | March 30, 2026 |
| Phase 1 Phase 2 | NCT04126200 | Multiple myeloma | 464 | GSK | To assess the impact of belantamab mafodotin in conjunction with other anti-cancer medications in refractory/relapsed multiple myeloma patients | Recruiting | February 23, 2028 |
| Phase 2 | NCT03016338 | Endometrial cancer | 51 | University Health Network, Toronto | To determine the effectiveness of dostarlimab with the experimental medication niraparib in recurrent/advanced endometrial cancer patients | Active, not recruiting | December 2023 |
| Phase 1Phase 2 | NCT05060432 | Advanced cancer | 376 | iTeos Belgium SA | To assess EOS-448’s safety, RP2D, tolerability, pharmacodynamics, PK, and anticancer efficacy in patients with advanced solid tumours who are receiving routine medical treatment and experimental medicines | Recruiting | September 2024 |
BRAF: B-Raf proto-oncogene, serine/threonine kinase; BRCA: breast cancer; EGFR: epidermal growth factor receptor; GTN: gestational trophoblastic neoplasia; HRD: homologous recombination deficiency; OX: oxaliplatin; PALB: partner and localizer of breast cancer 2; FOL: folinic acid; ROS-1: c-ros oncogene 1; RP2D: recommended phase 2 dose; RT: radiation therapy; TSR: anticancer treatment being developed by Tesaro to treat various types of cancer
Conclusions
Dostarlimab is a novel humanized anti-PD-1 that is approved for the treatment of different types of cancers such as ovarian cancer, NSCLC, SCC, malignant melanoma, SCLC, and peritoneal cancer, endometrial cancer, pancreatic cancer, fallopian tube cancer, and cancer of the neck and head. For the first time, small clinical studies of dostarlimab demonstrated complete elimination of the cancer disease in patients. In this review, we have summarized all aspects of dostarlimab including PK, pharmacodynamics, adverse reaction, and mechanism of action of dostarlimab, as well as various reported clinical trials. Additionally, monotherapy of dostarlimab demonstrated encouraging anticancer efficacy in patients with advanced cancers, positive PK, proof-of-concept pharmacodynamics, and safety.
Abbreviations
| CHL: | classical Hodgkin lymphoma |
| CLCR: | creatinine clearance |
| dMMR: | mismatch repair deficient |
| FDA: | Food and Drug Administration |
| GSK: | GlaxoSmithKline |
| HCC: | hepatocellular carcinoma |
| HNSCC: | head and neck squamous cell carcinoma |
| IgG4: | immunoglobulin G 4 |
| ITSM: | immunoreceptor tyrosine-based switch motif |
| Lck: | lymphocyte-specific protein tyrosine kinase |
| mAbs: | monoclonal Antibodies |
| MCC: | Merkel cell carcinoma |
| MSI-H: | microsatellite instability-high |
| NSCLC: | non-small cell lung cancer |
| PD-1: | programmed cell death 1 |
| PD-L1: | programmed cell death ligand-1 |
| PK: | pharmacokinetics |
| PMBCL: | primary mediastinal large B-cell lymphoma |
| RCC: | renal cell carcinoma |
| SCLC: | small-cell lung cancer |
| TCR: | T cell Receptor |
| TMB-H: | tumour mutational burden-high cancer |
| ZAP-70: | zeta-chain-associated protein kinase 70 |
Declarations
Acknowledgments
The authors are very thankful to the management of ISF College of Pharmacy, Moga, Punjab, for being supportive.
Author contributions
RA: Conceptualization. SAV: Data curation. PAC: Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2022.